Petrogenesis of the Eudialyte Complex of the Lovozero Alkaline Massif (Kola Peninsula, Russia)
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
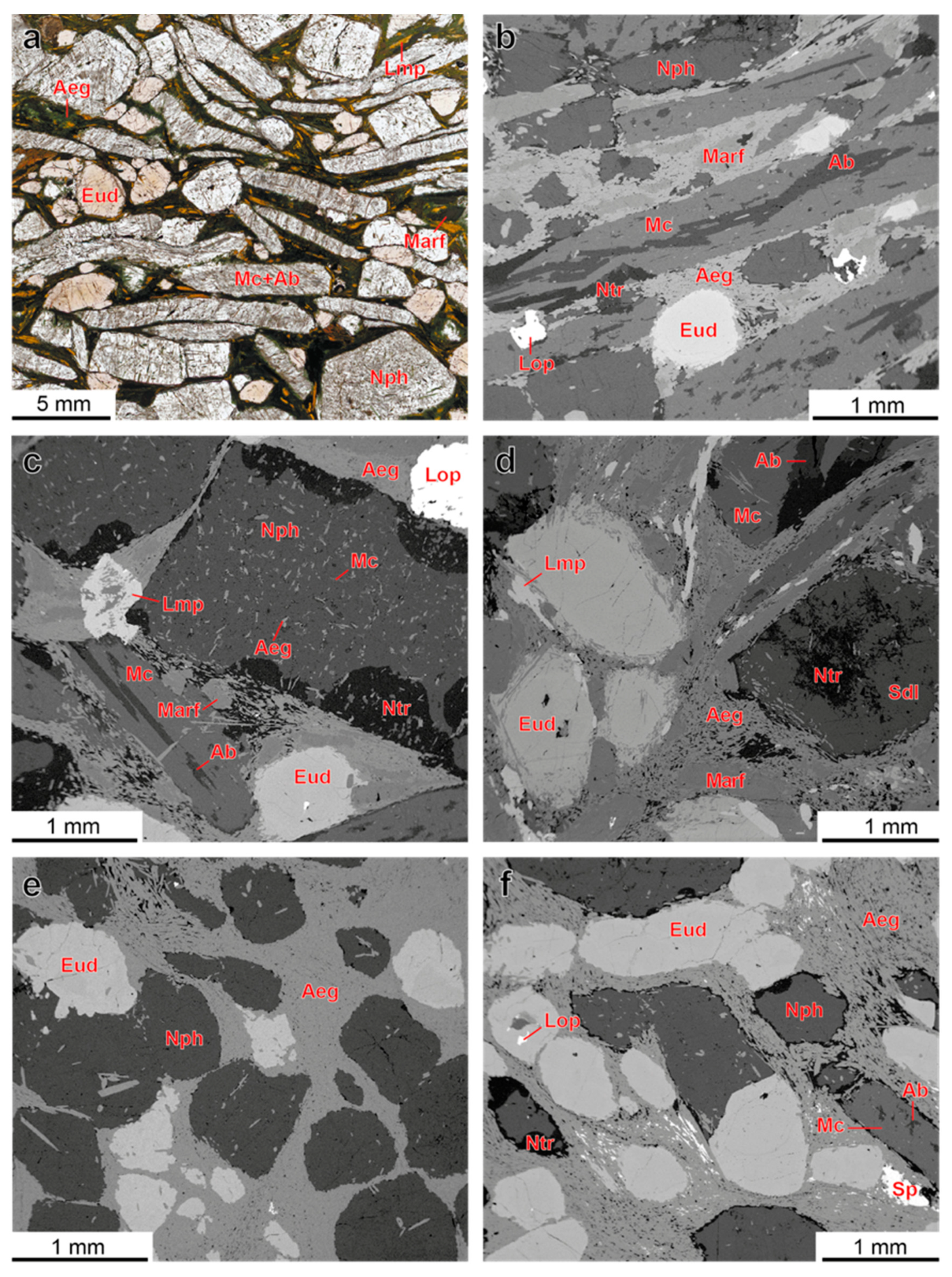
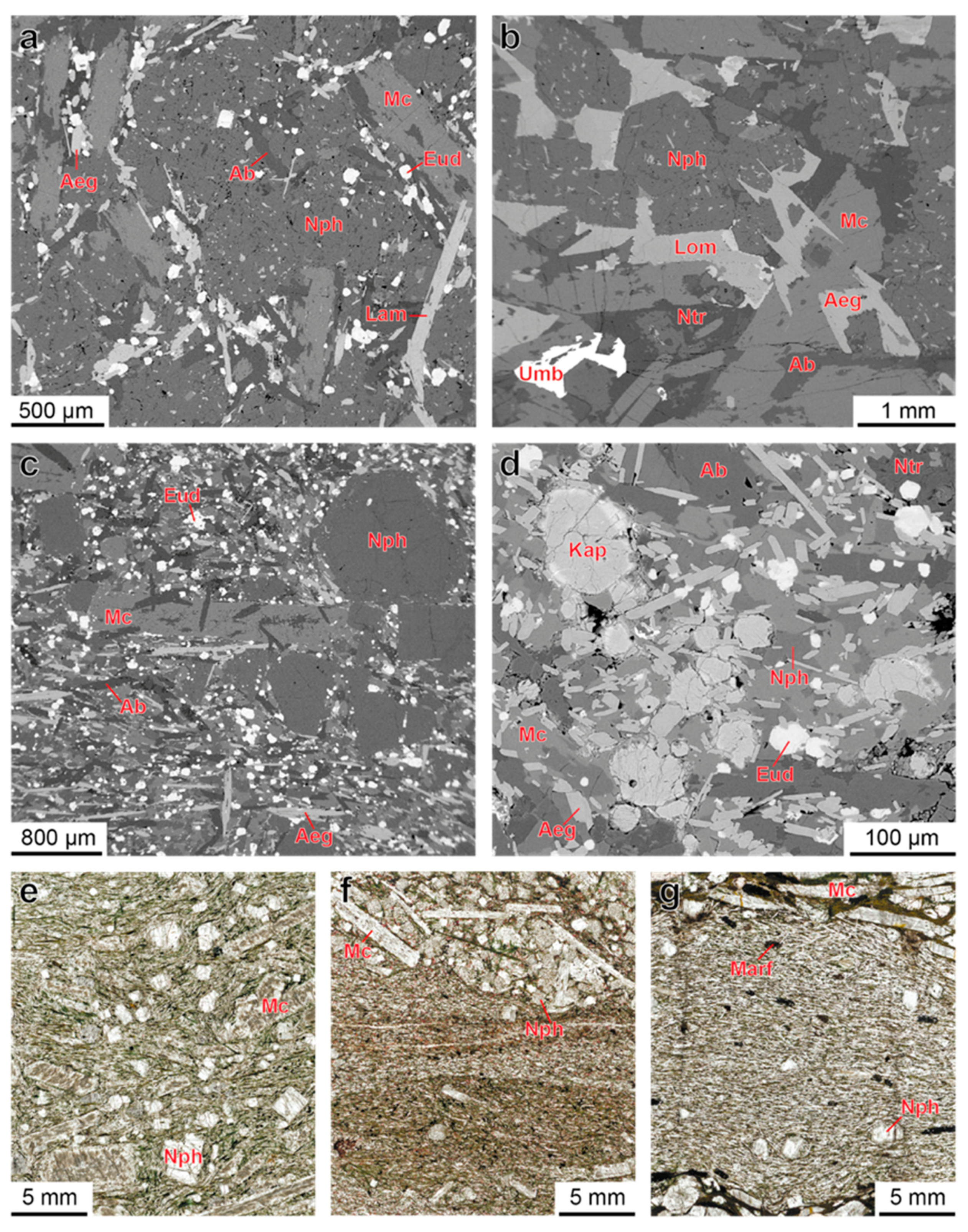
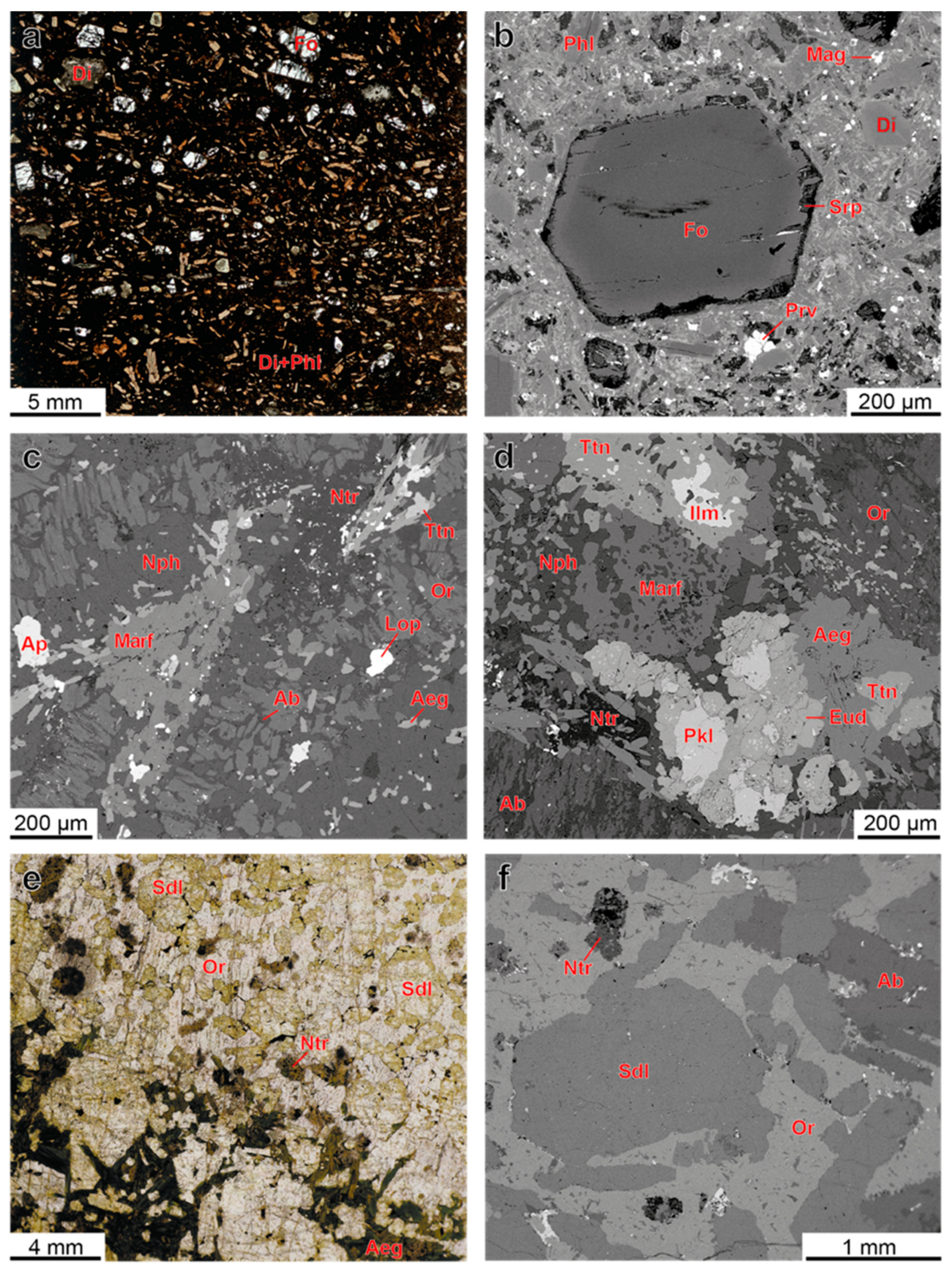
4.1. Petrography
4.2. Whole-Rock Chemistry
4.3. Mineral Chemistry
4.3.1. Alkali Feldspars
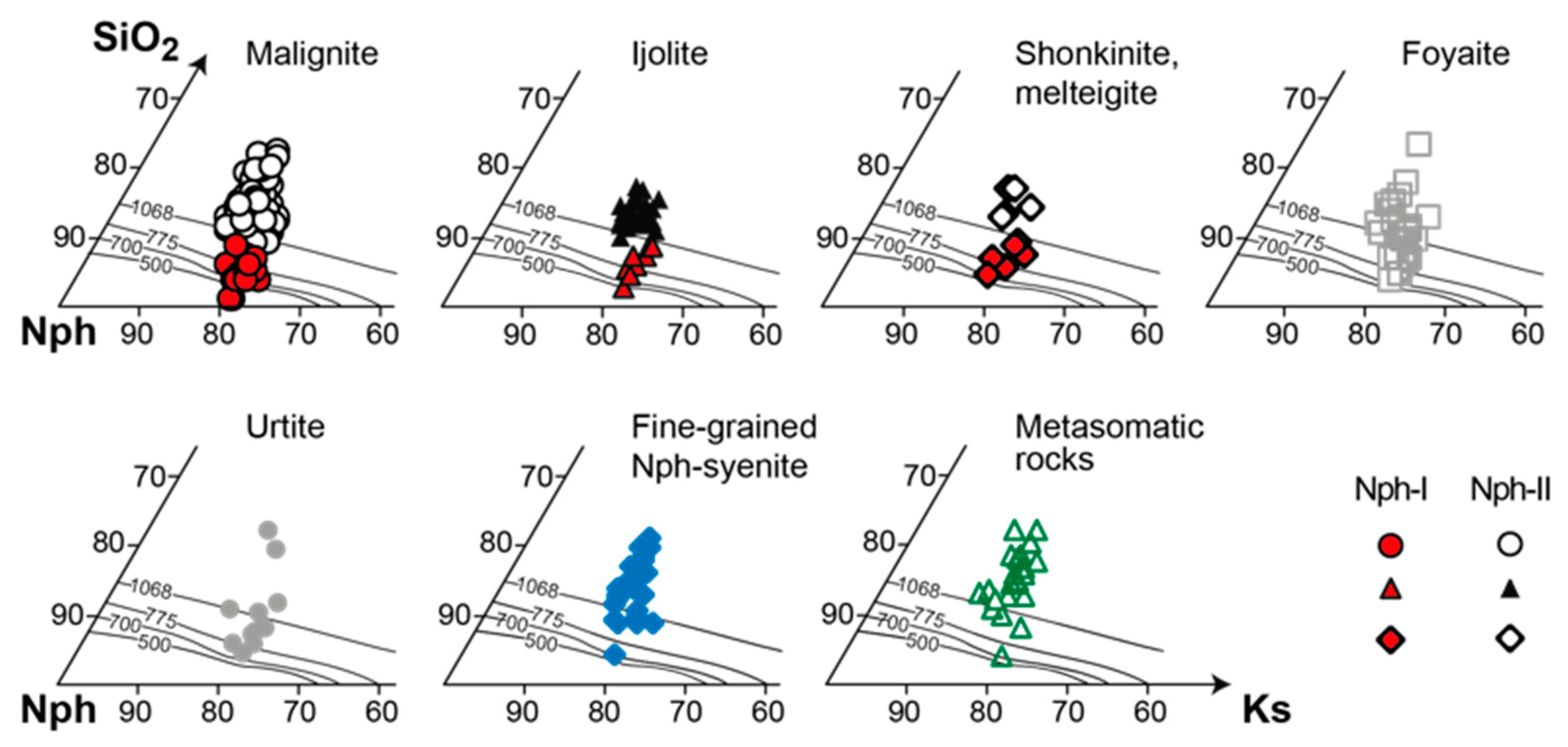
4.3.2. Nepheline
4.3.3. Clinopyroxenes
4.3.4. Amphiboles
4.3.5. Eudialyte-Group Minerals (EGM)
5. Discussion
6. Conclusions
- The Eudialyte Complex of the Lovozero Massif is indistinctly layered. Among the hypersolvus (meso)-melanocratic alkaline rocks (shonkinite, malignite, melteigite, ijolite) enriched with EGM, there are layers and lenses of subsolvus leucocratic rocks (foyaite, fine-grained foyaite and urtite) with phosphorus mineralization and primary lovozerite-group minerals.
- Leucocratic rocks were formed in the process of fractional crystallization of melanocratic melt enriched in Fe, HFSE, and halogens. The fractionation of the melanocratic melt proceeded in the direction of enrichment in nepheline and a decrease in the aegirine content. In a similar way, individual “rhythms” of the Layered Complex were probably differentiated.
- Hypersolvus meso-melanocratic rocks were formed in the temperature range from 650–700 °C to about 350 °C. As the rocks crystallized in this temperature range, a gradual transition from an almost anhydrous HSFE-, Fe3+-, halogen-rich alkaline melt to the Na(Cl, F)-rich water solution occurred. The temperature of crystallization of subsolvus leucocratic rocks was about 550 °C;
- If nepheline in alkaline rocks crystallizes simultaneously with aegirine, then it is enriched with Fe and Si according to the scheme ⬜︎B + (Si4+ + Fe3+)T ⇆ K+B + 2Al3+T. The composition of such nepheline cannot be used for thermometry purposes;
- Devonian volcaniclastic rocks played an important role in the giant eudialyte deposit formation. The relatively high fugacity of fluorine at first stage of the basalt fenitization causes formation of zircon in apo-basalt metasomatites. Following aegirine crystallization and the corresponding increase in Na/Si ratio led to parakeldyshite formation. At the final stage, EGM replaced parakeldyshite under the influence of Ca-rich solutions produced by the basalt fenitization.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Maitre, R.W. (Ed.) Igneous Rocks. A Classification and Glossary of Terms; Cambridge University Press: Cambridge, UK, 2002; ISBN 9780521662154. [Google Scholar]
- Frost, B.R.; Frost, C.D. A Geochemical Classification for Feldspathic Igneous Rocks. J. Petrol. 2008, 49, 1955–1969. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. A global review on agpaitic rocks. Earth-Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Kalashnikov, A.O.; Konopleva, N.G.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Rare earth deposits of the Murmansk Region, Russia—A review. Econ. Geol. 2016, 111, 1529–1559. [Google Scholar] [CrossRef]
- Krivovichev, S. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. Sect. A Found. Crystallogr. 2012, 68, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Markl, G.; Marks, M.A.W.; Frost, B.R. On the Controls of Oxygen Fugacity in the Generation and Crystallization of Peralkaline Melts. J. Petrol. 2010, 51, 1831–1847. [Google Scholar] [CrossRef] [Green Version]
- Nivin, V.A.; Treloar, P.J.; Konopleva, N.G.; Ikorsky, S.V. A review of the occurrence, form and origin of C-bearing species in the Khibiny Alkaline Igneous Complex, Kola Peninsula, NW Russia. Lithos 2005, 85, 93–112. [Google Scholar] [CrossRef]
- Beeskow, B.; Treloar, P.; Rankin, A.; Vennemann, T.; Spangenberg, J. A reassessment of models for hydrocarbon generation in the Khibiny nepheline syenite complex, Kola Peninsula, Russia. Lithos 2006, 91, 1–18. [Google Scholar] [CrossRef]
- Schönenberger, J.; Markl, G. The Magmatic and Fluid Evolution of the Motzfeldt Intrusion in South Greenland: Insights into the Formation of Agpaitic and Miaskitic Rocks. J. Petrol. 2008, 49, 1549–1577. [Google Scholar] [CrossRef]
- Chevychelov, V.Y.; Botcharnikov, R.E.; Holtz, F. Partitioning of Cl and F between fluid and hydrous phonolitic melt of Mt. Vesuvius at ~850–1000 °C and 200 MPa. Chem. Geol. 2008, 256, 172–184. [Google Scholar] [CrossRef]
- Dolejš, D.; Zajacz, Z. Halogens in Silicic Magmas and Their Hydrothermal Systems. In The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes; Harlov, D.E., Aranovich, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 431–543. ISBN 978-3-319-61665-0. [Google Scholar]
- Watson, E.B. Zircon saturation in felsic liquids: Experimental results and applications to trace element geochemistry. Contrib. Mineral. Petrol. 1979, 70, 407–419. [Google Scholar] [CrossRef]
- Keppler, H. Influence of fluorine on the enrichment of high field strength trace elements in granitic rocks. Contrib. Mineral. Petrol. 1993, 114, 479–488. [Google Scholar] [CrossRef]
- Gerasimovsky, V.I.; Volkov, V.P.; Kogarko, L.N.; Polyakov, A.I.; Saprykina, T.V.; Balashov, Y.A. Geochemistry of the Lovozero Alkaline Massif; Nauka: Moscow, Russia, 1966. (In Russian) [Google Scholar]
- Kramm, U.; Kogarko, L.N. Nd and Sr isotope signatures of the Khibina and Lovozero agpaitic centres, Kola Alkaline province, Russia. Lithos 1994, 32, 225–242. [Google Scholar] [CrossRef]
- Wu, F.-Y.; Yang, Y.-H.; Marks, M.A.W.; Liu, Z.-C.; Zhou, Q.; Ge, W.-C.; Yang, J.-S.; Zhao, Z.-F.; Mitchell, R.H.; Markl, G. In situ U–Pb, Sr, Nd and Hf isotopic analysis of eudialyte by LA-(MC)-ICP-MS. Chem. Geol. 2010, 273, 8–34. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Wu, F.-Y.; Yang, Y.-H. In situ U–Pb, Sr and Nd isotopic analysis of loparite by LA-(MC)-ICP-MS. Chem. Geol. 2011, 280, 191–199. [Google Scholar] [CrossRef]
- Korchak, Y.A.; Men’shikov, Y.P.; Pakhomovskii, Y.A.; Yakovenchuk, V.N.; Ivanyuk, G.Y. Trap formation of the Kola peninsula. Petrology 2011, 19, 87–101. [Google Scholar] [CrossRef]
- Shablinsky, G.N. On problem of deep structure of the Khibiny and Lovozero plutons. Trudy Leningradskogo Obshchestva Estestvoispytateley 1963, 74, 41–43. (In Russian) [Google Scholar]
- Bussen, I.V.; Sakharov, A.S. Petrology of the Lovozero Alkaline Massif; Nauka: Leningrad, Russia, 1972. (In Russian) [Google Scholar]
- Arzamastsev, A.A. Unique Paleozoic Intrusions of the Kola Peninsula; Geological Institute of the Kola Science Centre: Apatity, Russia, 1994. [Google Scholar]
- Pakhomovsky, Y.A.; Ivanyuk, G.Y.; Yakovenchuk, V.N. Loparite-(Ce) in rocks of the Lovozero layered complex at Mt. Karnasurt and Mt. Kedykvyrpakhk. Geol. Ore Depos. 2014, 56, 685–698. [Google Scholar] [CrossRef]
- Vlasov, K.A.; Kuzmenko, M.Z.; Eskova, E.M. The Lovozero Alkaline Massif; Izdatel’stvo Akademii Nauk: Moscow, Russia, 1959. (In Russian) [Google Scholar]
- Pekov, I.V. Lovozero Massif: History of Investigations, Pegmatites, Minerals; Ocean Pictures Ltd.: Moscow, Russia, 2001. [Google Scholar]
- Semenov, E.I. Mineralogy of the Lovozero alkaline massif; Nauka: Moscow, Russia, 1972. (In Russian) [Google Scholar]
- Dolivo-Dobrovolsky, D.D. MINAL, Free Software. Available online: http://www.dimadd.ru (accessed on 8 July 2013).
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Locock, A.J. An Excel spreadsheet to classify chemical analyses of amphiboles following the IMA 2012 recommendations. Comput. Geosci. 2014, 62, 1–11. [Google Scholar] [CrossRef]
- StatSoft Inc, Statistica 13. Available online: www.statsoft.ru (accessed on 19 June 2018).
- Systat Software Inc. TableCurve 2D. Available online: www.sigmaplot.co.uk/products/tablecurve2d/tablecurve2d.php (accessed on 19 June 2018).
- Hamilton, D.L. Nephelines as Crystallization Temperature Indicators. J. Geol. 1961, 69, 321–329. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Konoplyova, N.G.; Korchak, Y.A.; Pakhomovsky, Y.A. Nepheline of the Khibiny alkaline massif (Kola Peninsula). Proc. Rus. Mineral. Soc. 2010, 2, 80–91. (In Russian) [Google Scholar]
- Ivanyuk, G.; Yakovenchuk, V.; Pakhomovsky, Y.; Konoplyova, N.; Kalashnikov, A.; Mikhailova, J.; Goryainov, P. Self-Organization of the Khibiny Alkaline Massif (Kola Peninsula, Russia). In Earth Sciences; Dar, I.A., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-307-861-8. [Google Scholar] [Green Version]
- Pfaff, K.; Krumrei, T.; Marks, M.; Wenzel, T.; Rudolf, T.; Markl, G. Chemical and physical evolution of the ‘lower layered sequence’ from the nepheline syenitic Ilímaussaq intrusion, South Greenland: Implications for the origin of magmatic layering in peralkaline felsic liquids. Lithos 2008, 106, 280–296. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Ungaretti, L.; Oberti, R.; Bottazzi, P.; Czamanske, G.K. Li: An important component in igneous alkali amphiboles. Am. Mineral. 1993, 78, 733–745. [Google Scholar]
- Johnsen, O.; Grice, J.D. The crystal chemistry of the eudialyte group. Can. Mineral. 1999, 37, 865–891. [Google Scholar]
- Johnsen, O.; Ferraris, G.; Gault, R.A.; Grice, J.D.; Kampf, A.R.; Pekov, I.V. The nomenclature of eudialyte-group minerals. Can. Mineral. 2003, 41, 785–794. [Google Scholar] [CrossRef]
- Schilling, J.; Wu, F.-Y.; McCammon, C.; Wenzel, T.; Marks, M.A.W.; Pfaff, K.; Jacob, D.E.; Markl, G. The compositional variability of eudialyte-group minerals. Mineral. Mag. 2011, 75, 87–115. [Google Scholar] [CrossRef]
- Higgins, M.D. The origin of laminated and massive anorthosite, Sept Iles layered intrusion, Quebec, Canada. Contrib. Mineral. Petrol. 1991, 106, 340–354. [Google Scholar] [CrossRef]
- Paterson, S.R.; Vernon, R.H.; Tobisch, O.T. A review of criteria for the identification of magmatic and tectonic foliations in granitoids. J. Struct. Geol. 1989, 11, 349–363. [Google Scholar] [CrossRef]
- Bowen, N.L. Recent high-temperature research on silicates and its significance in igneous geology. Am. J. Sci. 1937, 193, 1–21. [Google Scholar] [CrossRef]
- Schairer, J.F. The alkali-feldspar join in the system NaAlSiO4-KAlSiO4-SiO2. J. Geol. 1950, 58, 512–517. [Google Scholar] [CrossRef]
- Schairer, J.F.; Bowen, N.L. Preliminary report on equilibrium-relations between feldspathoids, alkali-feldspars, and silica. Trans. Am. Geophys. Union 1935, 16, 325. [Google Scholar] [CrossRef]
- Hamilton, D.L.; MacKenzie, W.S. Phase-equilibrium studies in the system NaAlSiO4 (nepheline)–KAlSiO4(kalsilite)–SiO2-H2O. Mineral. Mag. 1965, 34, 214–231. [Google Scholar] [CrossRef]
- Davidson, A. Nepheline-feldspar intergrowth from Kaminak lake, Northwest Territories. Can. Mineral. 1970, 10, 191–206. [Google Scholar]
- Carmichael, I.S.E.; Nicholls, J. Iron-titanium oxides and oxygen fugacities in volcanic rocks. J. Geophys. Res. 1967, 72, 4665–4687. [Google Scholar] [CrossRef]
- Bowen, N.L.; Tuttle, O.F. The System NaAlSi3O8-KAlSi3O8-H2O. J. Geol. 1950, 58, 489–511. [Google Scholar] [CrossRef]
- Elkins, L.T.; Grove, T.L. Ternary feldspar experiments and thermodynamic models. Am. Mineral. 1990, 75, 544–559. [Google Scholar]
- Mitchell, R.H.; Vladykin, N.V. Compositional Variation of pyroxene and mica from the Little Murun ultrapotassic complex, Aldan Shield, Russia. Mineral. Mag. 1996, 60, 907–925. [Google Scholar] [CrossRef]
- Korobeynikov, A.N.; Laaioki, K. Petrological Aspects of the Evolution of clinopyroxene Composition in the Intrusive Rocks of the Lovozero Alkali Massif. Geochem. Int. 1994, 31, 69–76. [Google Scholar]
- Tyler, R.C.; King, B.C. The pyroxenes of the alkaline igneous complexes of eastern Uganda. Mineral. Mag. 1967, 36, 5–21. [Google Scholar] [CrossRef]
- Stephenson, D. Alkali clinopyroxenes from nepheline syenites of the South Qôroq Centre, South Greenland. Lithos 1972, 5, 187–201. [Google Scholar] [CrossRef]
- Larsen, L.M. Clinopyroxenes and coexisting mafic minerals from the alkaline Ilimaussaq intrusion, South Greenland. J. Petrol. 1976, 17, 258–290. [Google Scholar] [CrossRef]
- Bailey, D.K. The stability of acmite in the presence of H2O. Am. J. Sci. 1969, 267, 1–16. [Google Scholar]
- Nolan, J. Melting-relations in the system NaA1Si3O8–NaA1SiO4–NaFeSi2O6–CaMgSi2O6–H2O, and their bearing on the genesis of alkaline undersaturated rocks. Q. J. Geol. Soc. 1966, 122, 119–155. [Google Scholar] [CrossRef]
- Coish, R.A.; Taylor, L.A. The effects of cooling rate on texture and pyroxene chemistry in DSDP Leg 34 basalt: A microprobe study. Earth. Planet. Sci. Lett. 1979, 42, 389–398. [Google Scholar] [CrossRef]
- Mollo, S.; Blundy, J.D.; Iezzi, G.; Scarlato, P.; Langone, A. The partitioning of trace elements between clinopyroxene and trachybasaltic melt during rapid cooling and crystal growth. Contrib. Mineral. Petrol. 2013, 166, 1633–1654. [Google Scholar] [CrossRef]
- Jones, A.P.; Peckett, A. Zirconium-bearing aegirines from Motzfeldt, South Greenland. Contrib. Mineral. Petrol. 1981, 75, 251–255. [Google Scholar] [CrossRef]
- Piilonen, P.C.; McDonald, A.M.; Lalonde, A.E. The crystal chemistry of aegirine from Mont Saint-Hilaire, Quebec. Can. Mineral. 1998, 36, 779–791. [Google Scholar]
- Larsen, L.M. Sector zoned aegirine from the Ilímaussaq alkaline intrusion, South Greenland. Contrib. Mineral. Petrol. 1981, 76, 285–291. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Lazutkina, L.N.; Krigman, L.D. Conditions of Zirconium Concentraton in Magmatic Processes; Nauka: Moscow, Russia, 1988. (In Russian) [Google Scholar]
- Ernst, W.G. Synthesis, Stability Relations, and Occurrence of Riebeckite and Riebeckite-Arfvedsonite Solid Solutions. J. Geol. 1962, 70, 689–736. [Google Scholar] [CrossRef]
- Mitchell, R.H. A review of the compositional variation of amphiboles in alkaline plutonic complexes. Lithos 1990, 26, 135–156. [Google Scholar] [CrossRef]
- Cruciani, G. Zeolites upon heating: Factors governing their thermal stability and structural changes. J. Phys. Chem. Solids 2006, 67, 1973–1994. [Google Scholar] [CrossRef]
- Martin, R.F.; Bonin, B. Water and magma genesis; the association hypersolvus granite-subsolvus granite. Can. Mineral. 1976, 14, 228–237. [Google Scholar]
- Brown, W.L.; Parsons, I. Towards a more practical two-feldspar geothermometer. Contrib. Mineral. Petrol. 1981, 76, 369–377. [Google Scholar] [CrossRef]
- Guarino, V.; Gennaro, R.D.; Melluso, L.; Ruberti, E.; Azzone, R.G. The Transition from Miaskitic to Agpaitic Rocks, as Highlighted by the Accessory Phase Assemblages in the Passa Quatro Alkaline Complex (SouthEastern Brazil). Can. Mineral. 2019, 57, 339–361. [Google Scholar] [CrossRef]
- Bailey, D.K.; Schairer, J.F. The System Na2O−Al2O3−Fe2O3−SiO2 at 1 atmosphere, and the petrogenesis of alkaline rocks. J. Petrol. 1966, 7, 114–170. [Google Scholar] [CrossRef]
- Melluso, L.; Guarino, V.; Lustrino, M.; Morra, V.; De’Gennaro, R. The REE-and HFSE-bearing phases in the Itatiaia alkaline complex (Brazil) and geochemical evolution of feldspar-rich felsic melts. Mineral. Mag. 2017, 81, 217–250. [Google Scholar] [CrossRef]
- Farges, F.; Ponader, C.W.; Brown, G.E. Structural environments of incompatible elements in silicate glass/melt systems: I. Zirconium at trace levels. Geochim. Cosmochim. Acta 1991, 55, 1563–1574. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y.; Bazai, A.V.; Yakovenchuk, V.N.; Elizarova, I.R.; Kalashnikov, A.O. REE mineralogy and geochemistry of the Western Keivy peralkaline granite massif, Kola Peninsula, Russia. Ore Geol. Rev. 2017, 82, 181–197. [Google Scholar] [CrossRef]






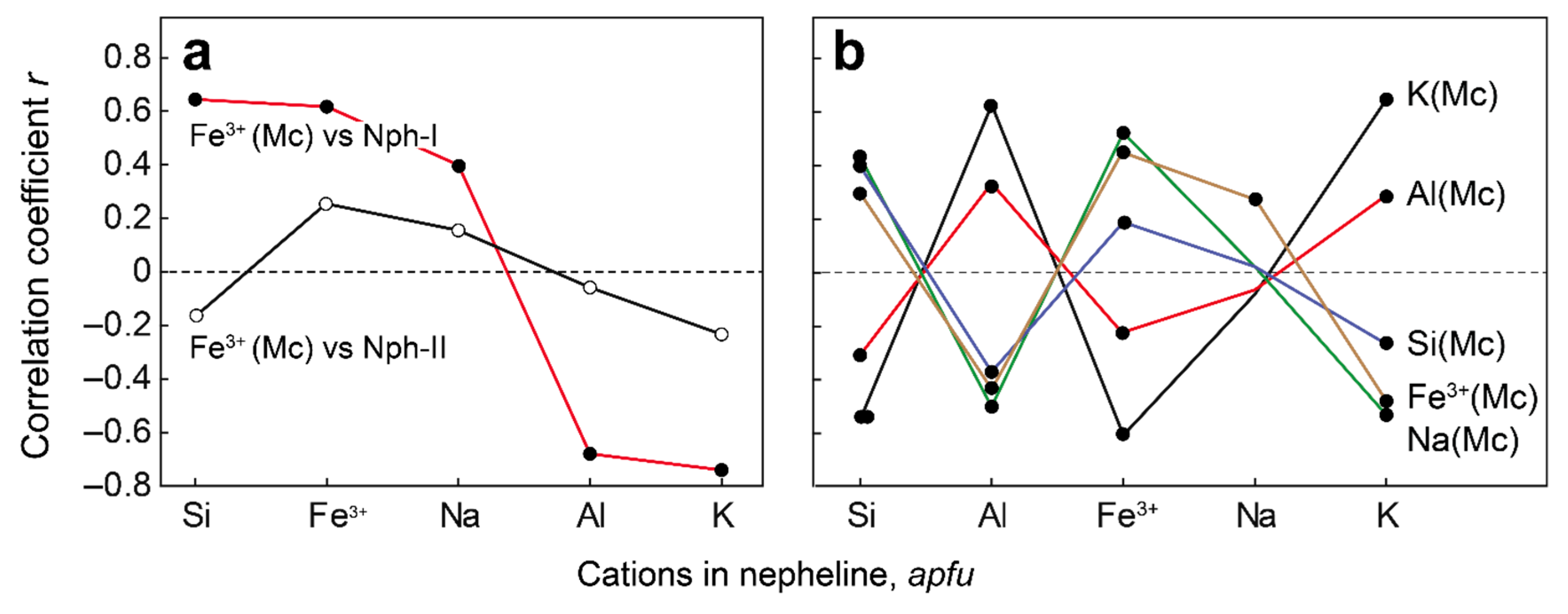

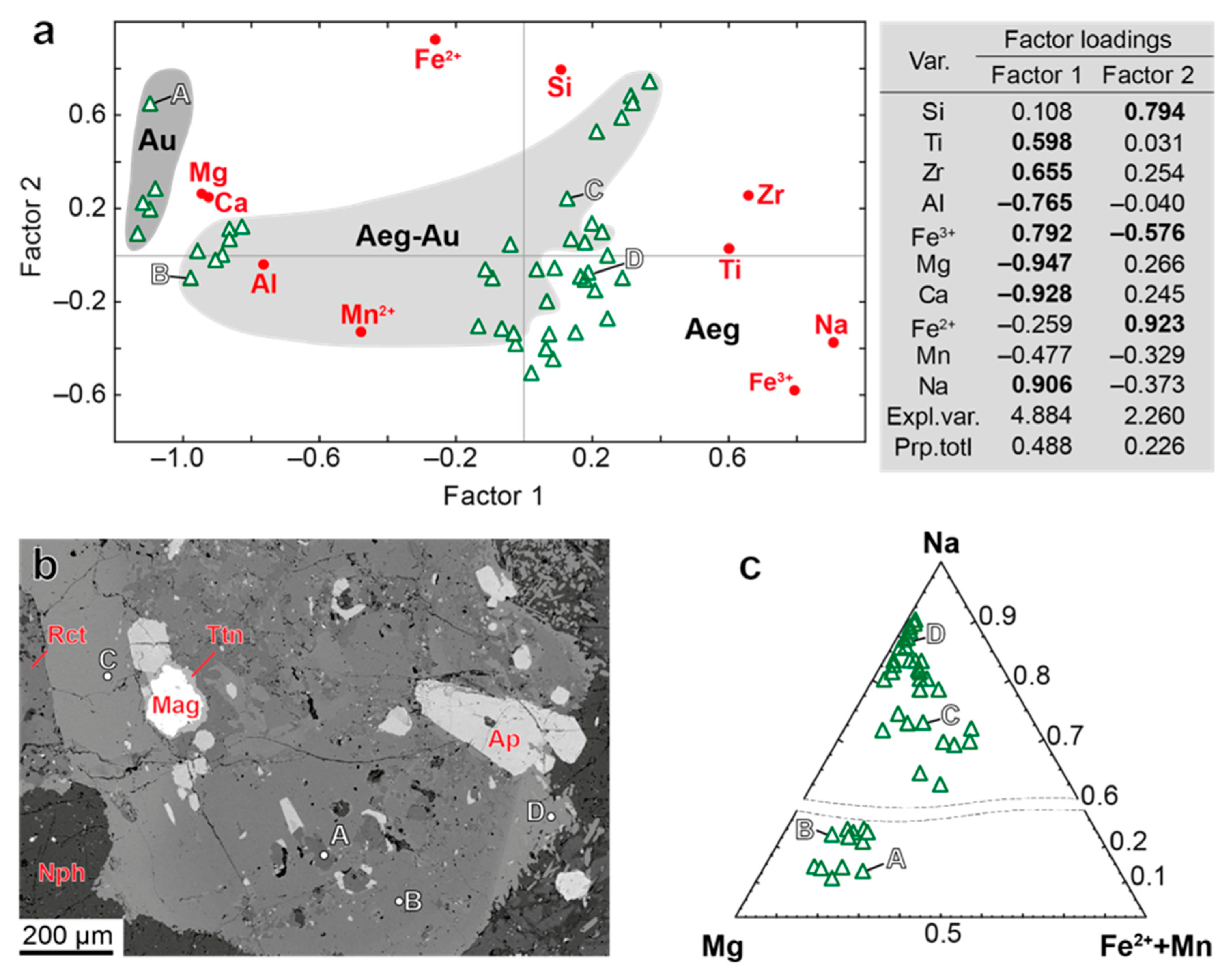
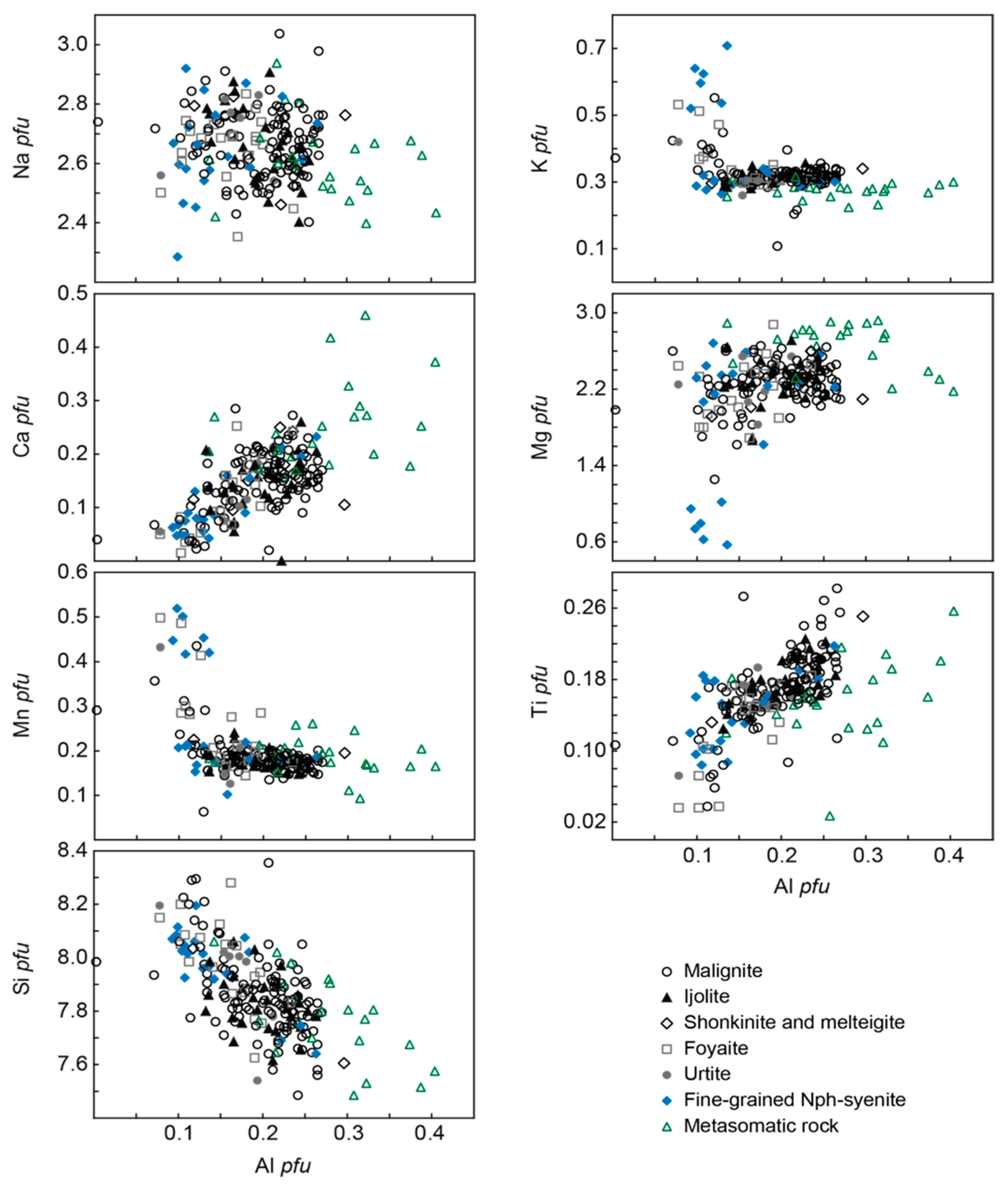
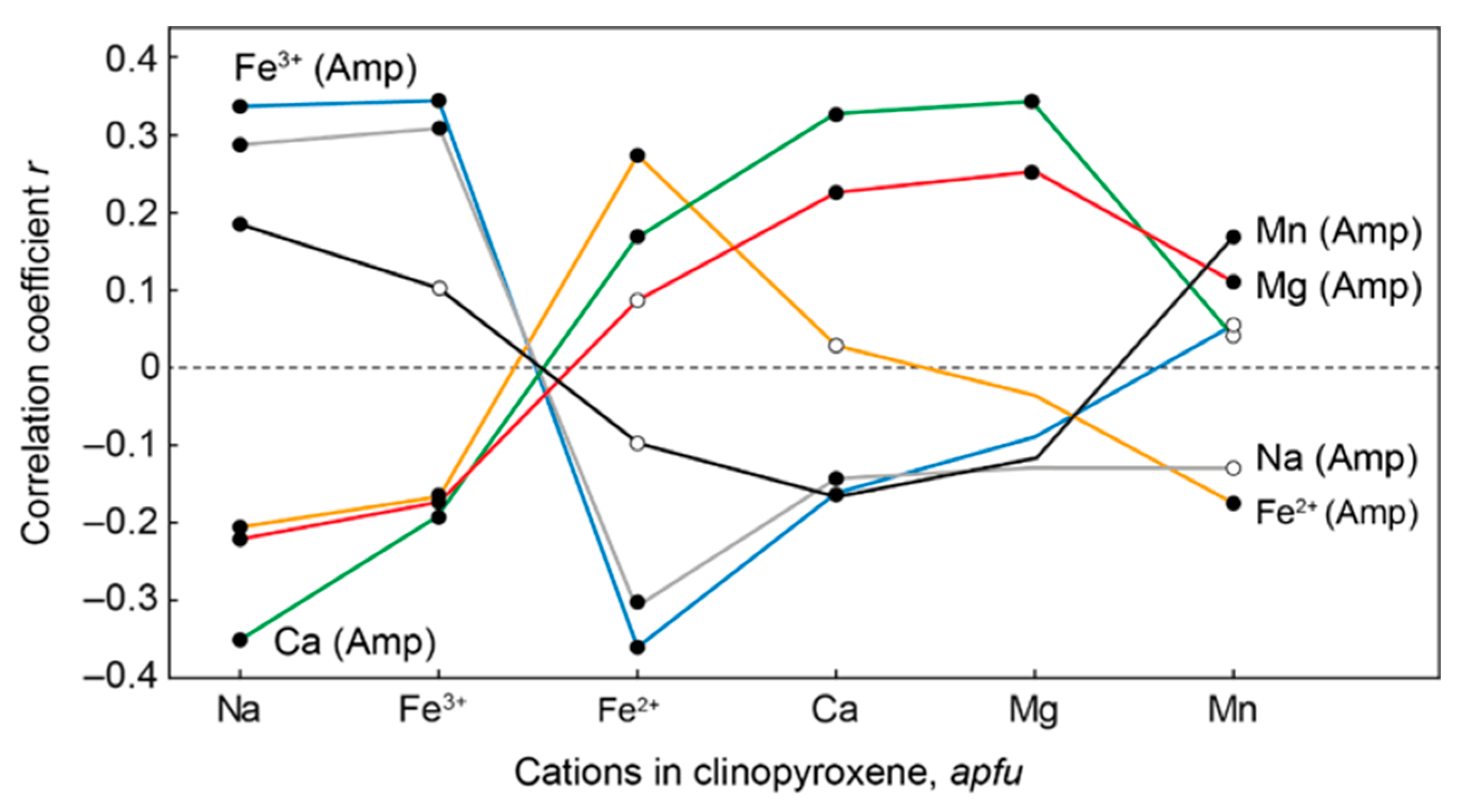
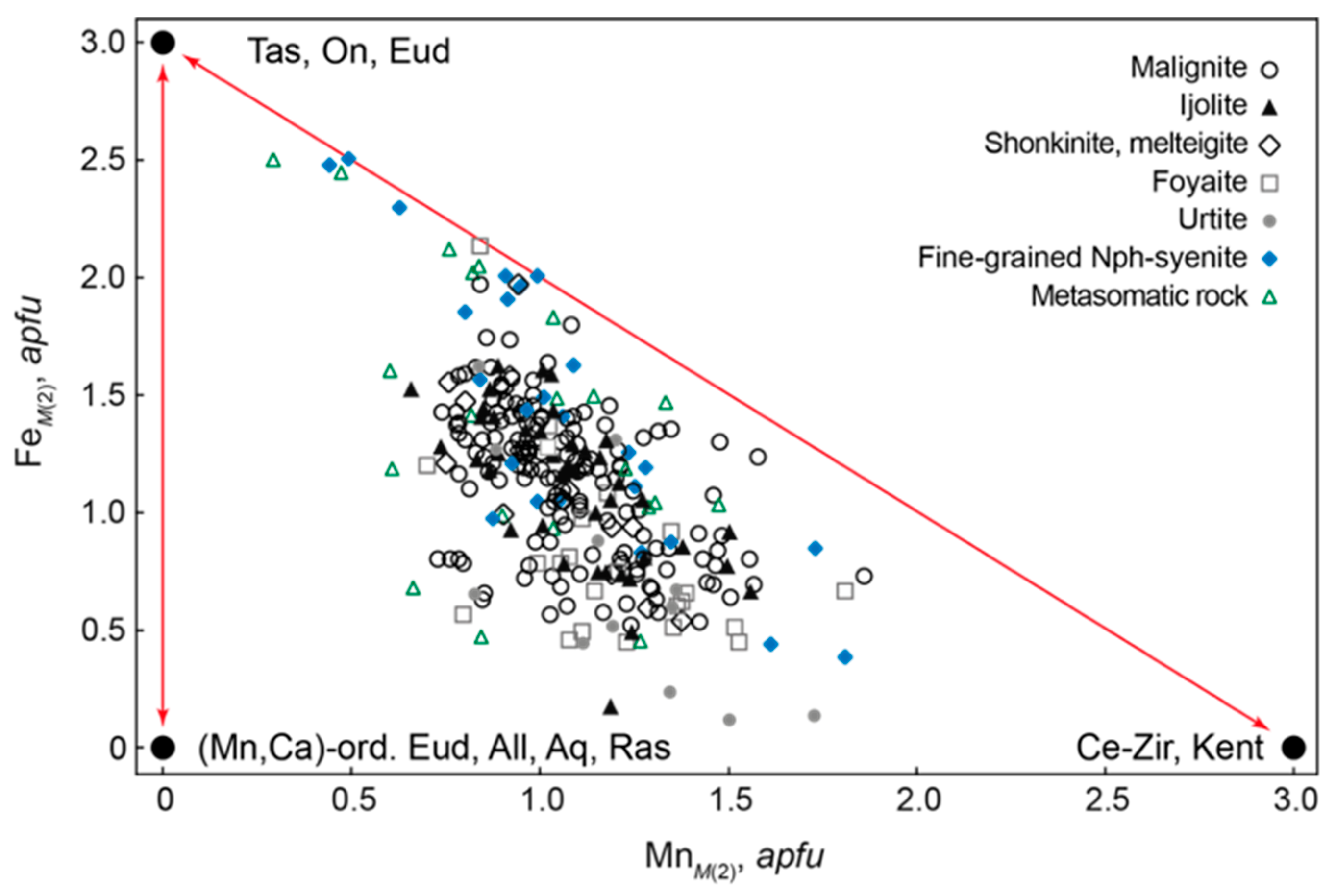

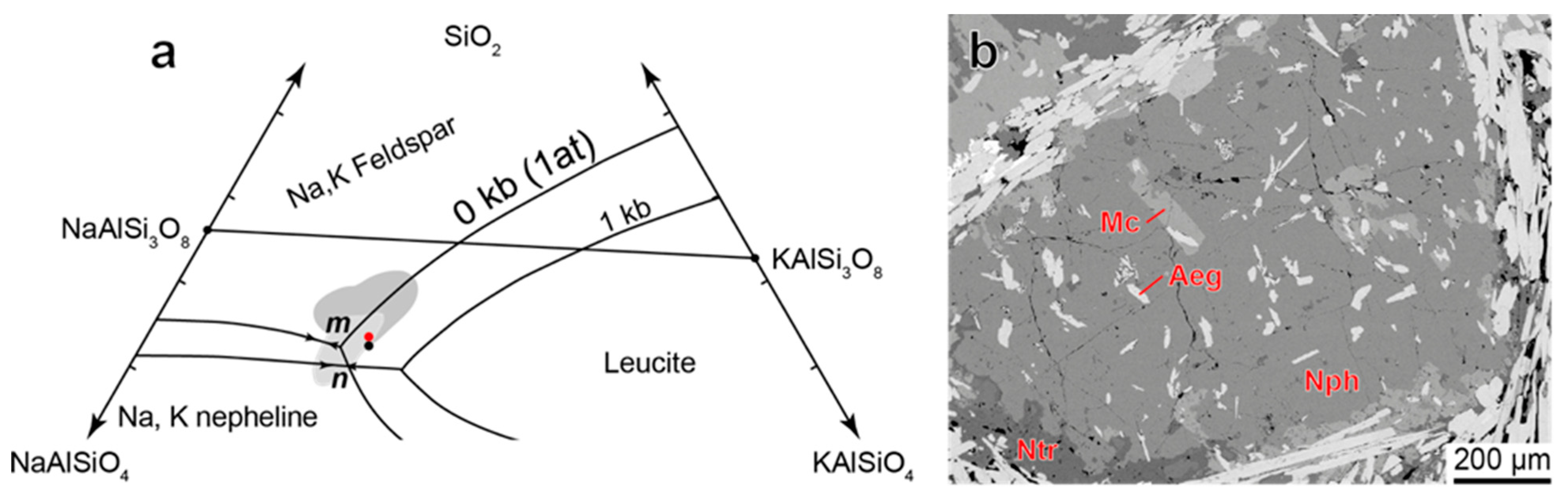
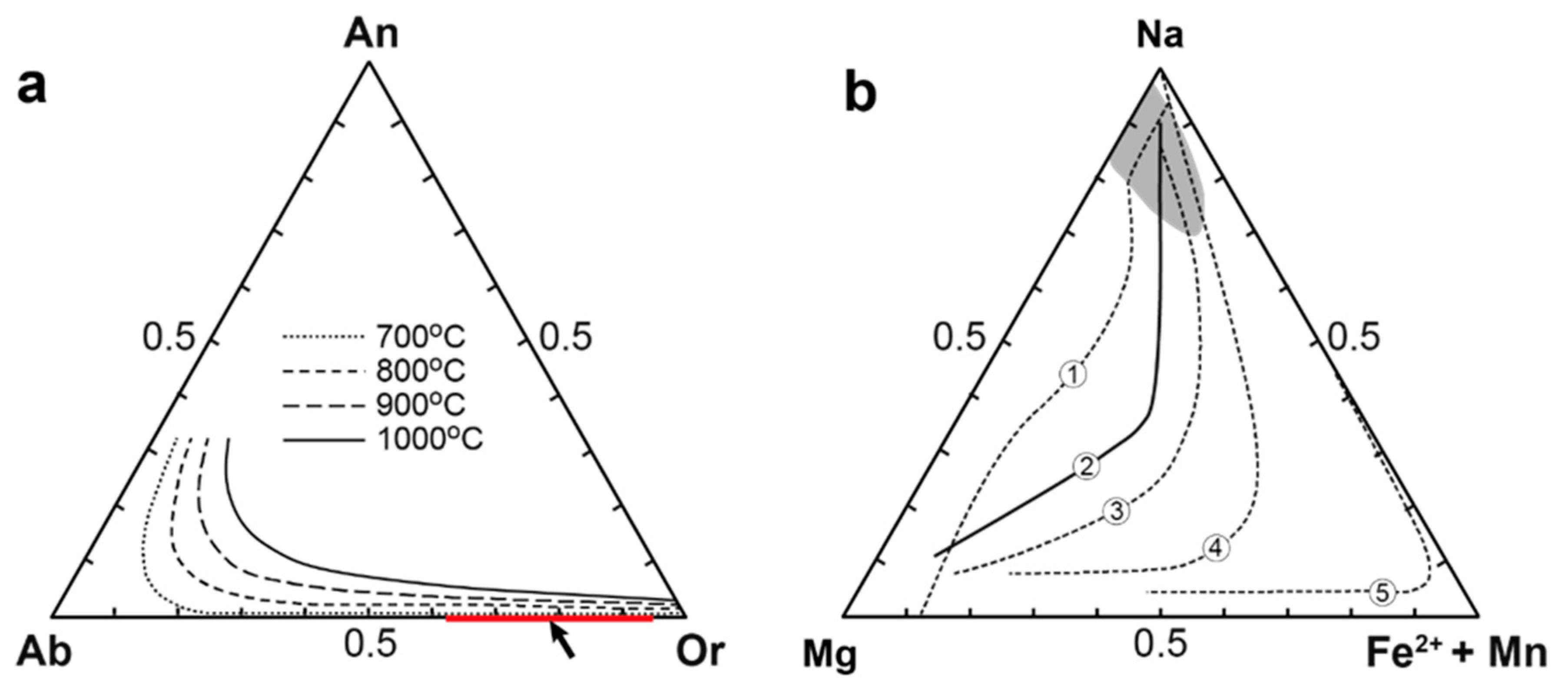
| Rock | Malignite | Ijolite | Shonkinite, Melteigite | Foyaite | Urtite | Fine-Grained Nph-Syenite | Metasomatic Rock | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drill hole | 29 | 117 | 153 | 160 | 153 | 222 | 154 | 224 | 117 | 147 | 160 | 157 | 156 | 156 |
| Deep, m | 37.5 | 102 | 143 | 120 | 36 | 123 | 271 | 102 | 208 | 85 | 172 | 36 | 77 * | 77 ** |
| SiO2 | 63.98 | 64.90 | 65.32 | 65.88 | 65.28 | 65.08 | 64.24 | 65.14 | 63.92 | 65.74 | 65.53 | 63.80 | 64.98 | 64.40 |
| Al2O3 | 16.95 | 18.04 | 18.32 | 18.43 | 17.34 | 18.03 | 18.38 | 18.33 | 17.88 | 18.18 | 17.61 | 17.54 | 19.62 | 19.15 |
| Fe2O3 | 0.05 | 0.06 | b.d. | 0.03 | b.d. | 0.09 | 0.02 | 0.04 | b.d. | 0.08 | 0.51 | 0.65 | 0.38 | 0.45 |
| BaO | 0.14 | b.d. | b.d. | b.d. | b.d. | 0.12 | b.d. | 0.08 | 0.13 | b.d. | 0.12 | b.d. | 1.60 | 1.32 |
| Na2O | 0.30 | 0.26 | 0.35 | 0.38 | 0.30 | 0.32 | 0.43 | 0.34 | 0.39 | 0.39 | 1.74 | 0.64 | 5.00 | 4.41 |
| K2O | 16.72 | 16.19 | 16.43 | 15.89 | 16.89 | 16.32 | 16.15 | 16.07 | 16.04 | 16.20 | 14.05 | 15.72 | 6.75 | 7.52 |
| Sum | 98.14 | 99.45 | 100.42 | 100.61 | 99.81 | 99.96 | 99.22 | 100.00 | 98.36 | 100.59 | 99.56 | 98.35 | 98.33 | 97.25 |
| Formula based on 8 oxygen atoms, apfu | ||||||||||||||
| Si | 3.03 | 3.01 | 3.01 | 3.01 | 3.03 | 3.01 | 2.99 | 3.01 | 3.01 | 3.02 | 3.02 | 3.00 | 2.97 | 2.98 |
| Al | 0.95 | 0.99 | 0.99 | 0.99 | 0.95 | 0.98 | 1.01 | 1.00 | 0.99 | 0.98 | 0.96 | 0.97 | 1.06 | 1.04 |
| Fe3+ | - | - | - | - | - | - | - | - | - | - | 0.02 | 0.02 | 0.01 | 0.02 |
| Ba | - | - | - | - | - | - | - | - | - | - | - | - | 0.03 | 0.02 |
| Na | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.04 | 0.03 | 0.16 | 0.06 | 0.44 | 0.39 |
| K | 1.01 | 0.96 | 0.97 | 0.93 | 1.00 | 0.96 | 0.96 | 0.95 | 0.96 | 0.95 | 0.83 | 0.94 | 0.39 | 0.44 |
| Sum | 5.02 | 4.98 | 5.00 | 4.96 | 5.01 | 4.98 | 5.00 | 4.99 | 5.00 | 4.98 | 4.99 | 4.99 | 4.90 | 4.89 |
| Mol. % endmembers | ||||||||||||||
| Or | 97 | 98 | 97 | 96 | 97 | 97 | 96 | 97 | 96 | 96 | 82 | 92 | 44 | 50 |
| Ab | 3 | 2 | 3 | 4 | 3 | 3 | 4 | 3 | 4 | 4 | 16 | 6 | 51 | 45 |
| Csn | - | - | - | - | - | - | - | - | - | - | - | - | 3 | 3 |
| For | - | - | - | - | - | - | - | - | - | - | 2 | 2 | 2 | 2 |
| Rock | Malignite | Ijolite | Shonkinite, Melteigite | Foyaite | Urtite | Fine-Grained Nph-syenite | Metasomatic Rock | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drill hole | 117 | 154 | 153 | 44 | 117 | 153 | 117 | 157 | 230 | 117 | 147 | 160 | 156 | 156 |
| Deep, m | 121 | 85 | 178 | 108 | 111 | 36 | 217 | 164 | 239 | 208 | 1 | 148 | 137 | 157 |
| SiO2 | 44.76 | 41.79 | 42.71 | 45.72 | 43.14 | 44.58 | 42.38 | 44.74 | 43.12 | 41.45 | 42.20 | 43.61 | 44.35 | 45.81 |
| Al2O3 | 30.65 | 32.38 | 32.73 | 31.08 | 31.15 | 30.78 | 32.79 | 29.88 | 33.38 | 32.78 | 33.62 | 29.40 | 31.69 | 30.78 |
| Fe2O3 | 1.44 | 0.16 | 0.18 | 1.63 | 1.53 | 1.25 | 0.16 | 1.87 | 0.26 | 0.15 | 0.12 | 1.91 | 1.77 | 1.09 |
| CaO | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.03 |
| K2O | 5.44 | 6.63 | 6.71 | 6.08 | 5.85 | 5.81 | 6.51 | 5.42 | 6.62 | 6.67 | 7.25 | 5.36 | 5.47 | 5.35 |
| Na2O | 16.23 | 16.23 | 16.36 | 15.95 | 16.54 | 16.16 | 15.23 | 16.13 | 15.56 | 15.27 | 15.14 | 15.69 | 15.91 | 15.17 |
| Sum | 98.52 | 97.19 | 98.68 | 100.45 | 98.21 | 98.58 | 97.08 | 98.04 | 98.93 | 96.31 | 98.32 | 95.97 | 99.20 | 98.24 |
| Formula based on 8 oxygen atoms, apfu | ||||||||||||||
| Si | 4.35 | 4.15 | 4.18 | 4.37 | 4.24 | 4.34 | 4.19 | 4.38 | 4.18 | 4.14 | 4.14 | 4.36 | 4.28 | 4.43 |
| Al | 3.51 | 3.79 | 3.77 | 3.50 | 3.61 | 3.53 | 3.82 | 3.45 | 3.82 | 3.86 | 3.88 | 3.47 | 3.61 | 3.51 |
| Fe3+ | 0.11 | 0.01 | 0.01 | 0.12 | 0.11 | 0.09 | 0.01 | 0.14 | 0.02 | 0.01 | 0.01 | 0.14 | 0.13 | 0.08 |
| K | 0.67 | 0.84 | 0.84 | 0.74 | 0.73 | 0.72 | 0.82 | 0.68 | 0.82 | 0.85 | 0.91 | 0.68 | 0.67 | 0.66 |
| Na | 3.06 | 3.13 | 3.10 | 2.95 | 3.15 | 3.05 | 2.92 | 3.06 | 2.93 | 2.96 | 2.88 | 3.04 | 2.98 | 2.85 |
| Sum | 11.71 | 11.93 | 11.90 | 11.67 | 11.84 | 11.73 | 11.76 | 11.70 | 11.77 | 11.82 | 11.81 | 11.70 | 11.67 | 11.53 |
| Mol. % endmembers | ||||||||||||||
| Nph | 70 | 75 | 74 | 68 | 74 | 70 | 70 | 70 | 70 | 71 | 69 | 70 | 69 | 64 |
| Ks | 16 | 20 | 20 | 17 | 17 | 17 | 19 | 15 | 20 | 21 | 22 | 16 | 16 | 15 |
| Qtz | 14 | 5 | 6 | 15 | 9 | 13 | 11 | 15 | 10 | 8 | 9 | 14 | 15 | 21 |
| Rock | Malignite | Ijolite | Shonkinite, Melteigite | Foyaite | Urtite | Fine-Grained Nph-Syenite | Metasomatic Rock | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drill hole | 117 | 228 | 33 | 160 | 153 | 222 | 154 | 157 | 117 | 156 | 154 | 157 | 156 | 156 | 156 | 156 |
| Deep, m | 121 | 84 | 211 | 120 | 8 | 177 | 35 | 155 | 235 | 56 | 226 | 36 | 89 A** | 89 B | 89 C | 89 D |
| SiO2 | 52.12 | 52.66 | 50.68 | 52.54 | 49.96 | 53.23 | 51.75 | 52.36 | 51.97 | 51.49 | 51.34 | 52.12 | 51.91 | 50.61 | 51.46 | 51.78 |
| ZrO2 | 0.74 | 0.75 | 0.71 | 0.61 | 0.53 | 0.41 | 0.78 | 0.70 | 0.76 | 0.61 | 0.98 | 0.64 | b.d. | 0.27 | 1.05 | 0.90 |
| TiO2 | 3.06 | 2.97 | 3.06 | 2.54 | 2.74 | 3.84 | 2.70 | 2.35 | 2.77 | 4.26 | 2.97 | 2.74 | 0.97 | 1.32 | 3.17 | 4.01 |
| Al2O3 | 0.79 | 0.89 | 0.71 | 0.80 | 0.87 | 0.80 | 0.77 | 0.97 | 0.86 | 0.89 | 0.76 | 0.78 | 1.33 | 1.12 | 0.90 | 0.87 |
| V2O3 | 0.05 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| CaO | 2.67 | 2.69 | 2.74 | 3.04 | 1.68 | 1.59 | 2.57 | 3.78 | 1.90 | 2.49 | 3.17 | 2.71 | 17.58 | 19.96 | 5.71 | 3.46 |
| MgO | 1.54 | 1.90 | 1.79 | 2.11 | 1.12 | 1.22 | 1.64 | 2.17 | 1.46 | 1.47 | 1.72 | 1.70 | 12.59 | 10.77 | 3.03 | 2.12 |
| FeO | 23.60 | 23.37 | 23.61 | 23.13 | 24.91 | 24.87 | 24.13 | 24.13 | 23.70 | 24.43 | 23.49 | 24.76 | 8.32 | 10.08 | 20.85 | 22.53 |
| MnO | 0.40 | 0.57 | 0.45 | 0.53 | 0.40 | 0.48 | 0.48 | 0.44 | 0.33 | 0.46 | 0.45 | 0.41 | 0.66 | 0.65 | 0.47 | 0.62 |
| ZnO | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.08 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Na2O | 12.28 | 12.57 | 12.05 | 12.44 | 13.41 | 13.40 | 12.60 | 12.20 | 13.15 | 12.16 | 12.59 | 12.79 | 1.85 | 2.91 | 10.48 | 12.04 |
| K2O | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.10 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Sum | 97.25 | 98.37 | 95.81 | 97.72 | 95.61 | 99.83 | 97.50 | 99.08 | 96.97 | 98.27 | 97.47 | 98.62 | 95.21 | 97.69 | 97.12 | 98.32 |
| Formula based on 4 cations and 6 oxygen atoms, apfu | ||||||||||||||||
| Si | 1.99 | 1.98 | 1.96 | 1.98 | 1.92 | 1.97 | 1.96 | 1.96 | 1.97 | 1.96 | 1.95 | 1.95 | 2.02 | 1.93 | 1.98 | 1.96 |
| Zr | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | – | 0.01 | 0.02 | 0.02 |
| Ti | 0.09 | 0.08 | 0.09 | 0.07 | 0.08 | 0.11 | 0.08 | 0.07 | 0.08 | 0.12 | 0.09 | 0.08 | 0.03 | 0.04 | 0.09 | 0.11 |
| Al | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 | 0.04 | 0.03 | 0.03 | 0.06 | 0.05 | 0.04 | 0.04 |
| Ca | 0.11 | 0.11 | 0.11 | 0.12 | 0.07 | 0.06 | 0.11 | 0.15 | 0.08 | 0.10 | 0.13 | 0.11 | 0.73 | 0.81 | 0.24 | 0.14 |
| Mg | 0.09 | 0.11 | 0.10 | 0.12 | 0.06 | 0.07 | 0.09 | 0.12 | 0.08 | 0.08 | 0.10 | 0.10 | 0.73 | 0.61 | 0.17 | 0.12 |
| Fe2+ | 0.06 | 0.01 | 0.02 | - | - | 0.02 | - | - | - | 0.10 | - | - | 0.27 | 0.09 | 0.10 | 0.04 |
| Fe3+ | 0.69 | 0.72 | 0.74 | 0.73 | 0.80 | 0.76 | 0.77 | 0.75 | 0.75 | 0.68 | 0.75 | 0.78 | - | 0.23 | 0.57 | 0.67 |
| Mn | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| Na | 0.91 | 0.92 | 0.91 | 0.91 | 1.00 | 0.96 | 0.93 | 0.88 | 0.97 | 0.90 | 0.93 | 0.93 | 0.14 | 0.22 | 0.78 | 0.88 |
| K | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - |
| Mol % endmembers | ||||||||||||||||
| Q | 13 | 11 | 12 | 12 | 6 | 7 | 10 | 13 | 8 | 14 | 11 | 10 | 100 | 78 | 25 | 15 |
| Aeg | 83 | 84 | 84 | 84 | 89 | 89 | 86 | 82 | 88 | 81 | 85 | 86 | - | 18 | 70 | 81 |
| Jd | 4 | 5 | 4 | 4 | 5 | 4 | 4 | 5 | 4 | 5 | 4 | 4 | - | 4 | 5 | 4 |
| Rock | Malignite | Ijolite | Shonkinite, Melteigite | Foyaite | Urtite | Fine-Grained Nph-Syenite | Metasomatic Rock | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drill hole | 117 | 147 | 231 | 117 | 153 | 222 | 147 | 154 | 147 | 156 | 154 | 154 | 156 | 231 |
| Deep, m | 4 | 194 | 176 | 93 | 36 | 231 | 275 | 172 | 85 | 56 | 245 * | 245 ** | 89 *** | 146 |
| SiO2 | 51.81 | 53.78 | 53.10 | 53.32 | 52.33 | 52.50 | 52.30 | 53.82 | 52.90 | 52.44 | 53.12 | 51.10 | 52.56 | 54.65 |
| TiO2 | 1.76 | 1.50 | 1.36 | 1.54 | 1.63 | 1.77 | 1.56 | 1.02 | 1.47 | 1.31 | 1.64 | 0.86 | 0.56 | 2.01 |
| Al2O3 | 1.49 | 0.77 | 0.78 | 1.25 | 1.33 | 1.29 | 1.14 | 1.09 | 1.22 | 1.00 | 0.62 | 0.58 | 1.99 | 1.61 |
| FeO | 17.54 | 17.19 | 17.56 | 17.00 | 18.16 | 16.79 | 17.75 | 12.62 | 16.78 | 18.00 | 17.42 | 26.17 | 10.95 | 14.24 |
| MnO | 1.39 | 1.50 | 1.49 | 1.20 | 1.52 | 1.51 | 1.49 | 1.68 | 1.37 | 1.38 | 1.66 | 3.13 | 1.29 | 1.61 |
| MgO | 9.36 | 10.45 | 10.03 | 10.37 | 9.75 | 10.29 | 10.52 | 13.08 | 11.62 | 9.61 | 9.32 | 2.64 | 14.00 | 12.98 |
| CaO | 0.94 | 0.83 | 0.59 | 1.15 | 1.02 | 1.13 | 1.20 | 0.91 | 1.35 | 0.71 | 0.48 | 0.29 | 5.66 | 1.65 |
| ZnO | 0.07 | 0.05 | 0.06 | b.d. | 0.08 | 0.05 | b.d. | 0.05 | b.d. | b.d. | 0.08 | 0.07 | 0.07 | b.d. |
| Na2O | 9.30 | 9.50 | 9.17 | 9.26 | 8.90 | 9.37 | 9.26 | 9.41 | 8.92 | 8.78 | 10.10 | 8.46 | 6.87 | 9.12 |
| K2O | 1.65 | 1.55 | 1.56 | 1.68 | 1.76 | 1.67 | 1.61 | 1.53 | 1.64 | 1.67 | 1.68 | 3.11 | 1.40 | 1.57 |
| F | b.d. | b.d. | 2.10 | b.d. | b.d. | 1.50 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 1.70 |
| О=F | 0.00 | 0.00 | −0.88 | 0.00 | 0.00 | −0.63 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −0.72 |
| Sum | 95.31 | 97.12 | 96.92 | 96.77 | 96.48 | 97.24 | 96.83 | 95.21 | 97.27 | 94.90 | 96.11 | 96.41 | 95.35 | 100.42 |
| Formula based on O + OH + F = 24 apfu and OH = 2 – 2Ti | ||||||||||||||
| Si (T) | 7.83 | 7.94 | 7.99 | 7.90 | 7.84 | 7.81 | 7.76 | 7.93 | 7.78 | 7.99 | 7.93 | 8.04 | 7.84 | 7.80 |
| Al (T) | 0.17 | 0.05 | 0.01 | 0.10 | 0.16 | 0.19 | 0.20 | 0.07 | 0.21 | 0.01 | 0.07 | - | 0.16 | 0.20 |
| Ti (T) | - | - | - | - | - | - | 0.04 | - | 0.01 | - | - | - | - | - |
| Sum T | 8.00 | 7.99 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.04 | 8.00 | 8.00 |
| Ti (C) | 0.20 | 0.17 | 0.15 | 0.17 | 0.18 | 0.20 | 0.13 | 0.11 | 0.15 | 0.15 | 0.18 | 0.10 | 0.06 | 0.22 |
| Al (C) | 0.09 | 0.08 | 0.12 | 0.12 | 0.08 | 0.04 | - | 0.12 | - | 0.16 | 0.03 | 0.11 | 0.19 | 0.07 |
| Fe3+ (C) | 0.72 | 0.66 | 0.56 | 0.62 | 0.64 | 0.78 | 0.91 | 0.70 | 0.75 | 0.47 | 0.92 | 0.81 | - | 0.51 |
| Mn (C) | 0.18 | 0.19 | 0.19 | 0.15 | 0.19 | 0.19 | 0.19 | 0.21 | 0.17 | 0.18 | 0.21 | 0.42 | 0.16 | 0.19 |
| Fe2+ (C) | 1.49 | 1.46 | 1.65 | 1.49 | 1.64 | 1.31 | 1.29 | 0.86 | 1.31 | 1.83 | 1.26 | 2.64 | 1.36 | 1.19 |
| Mg (C) | 2.11 | 2.30 | 2.25 | 2.29 | 2.18 | 2.28 | 2.33 | 2.87 | 2.55 | 2.18 | 2.07 | 0.62 | 3.11 | 2.76 |
| Zn (C) | 0.01 | - | 0.01 | - | 0.01 | - | - | 0.01 | - | - | 0.01 | 0.01 | 0.01 | - |
| Sum C | 4.80 | 4.86 | 4.93 | 4.84 | 4.92 | 4.80 | 4.85 | 4.88 | 4.93 | 4.97 | 4.68 | 4.71 | 4.89 | 4.94 |
| Ca (B) | 0.15 | 0.13 | 0.09 | 0.18 | 0.16 | 0.18 | 0.19 | 0.14 | 0.21 | 0.12 | 0.08 | 0.05 | 0.90 | 0.25 |
| Na (B) | 1.85 | 1.87 | 1.91 | 1.82 | 1.84 | 1.82 | 1.81 | 1.86 | 1.79 | 1.88 | 1.92 | 1.95 | 1.10 | 1.75 |
| Sum B | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Na (A) | 0.88 | 0.85 | 0.77 | 0.84 | 0.74 | 0.88 | 0.85 | 0.83 | 0.76 | 0.71 | 1.00 | 0.63 | 0.89 | 0.77 |
| K (A) | 0.32 | 0.30 | 0.30 | 0.32 | 0.34 | 0.32 | 0.30 | 0.29 | 0.31 | 0.32 | 0.32 | 0.62 | 0.27 | 0.29 |
| Sum A | 1.20 | 1.15 | 1.07 | 1.16 | 1.08 | 1.20 | 1.15 | 1.12 | 1.07 | 1.03 | 1.30 | 1.25 | 1.16 | 1.06 |
| OH (W) | 2.00 | 2.00 | 1.00 | 2.00 | 2.00 | 1.29 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 1.23 |
| F (W) | - | - | 1.00 | - | - | 0.71 | - | - | - | - | - | - | - | 0.77 |
| Sum W | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Rock. | Malignite | Ijolite | Shonkinite | Foyaite | Urtite | Fine-grained Nph- Syenite | Metasomatic Rock | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drill hole | 28 | 28 | 117 | 117 | 44 | 44 | 117 | 156 | 147 | 154 | 154 | 160 | 154 | 156 |
| Deep, m | 153 * | 153 ** | 47 * | 47 ** | 50 * | 50 ** | 215 | 216 | 85 | 144 | 226 | 148 | 197 | 118 |
| Nb2O5 | 0.49 | 0.39 | 0.67 | 0.73 | 0.72 | 0.71 | 0.35 | 1.22 | 0.81 | 0.71 | 0.36 | 1.11 | 0.64 | 1.62 |
| SiO2 | 49.99 | 51.75 | 49.45 | 50.97 | 53.07 | 50.44 | 51.81 | 49.92 | 49.34 | 51.24 | 53.51 | 49.48 | 51.02 | 49.33 |
| ZrO2 | 12.54 | 11.69 | 13.95 | 12.79 | 12.04 | 13.90 | 13.03 | 11.78 | 12.90 | 11.20 | 14.71 | 11.65 | 12.93 | 12.80 |
| TiO2 | 0.66 | 0.46 | 0.61 | 0.36 | 0.38 | 0.66 | 0.65 | 0.52 | 0.70 | 0.75 | 1.01 | 0.56 | 0.65 | 0.29 |
| Al2O3 | 0.22 | 0.15 | 0.16 | 0.10 | 0.07 | 0.22 | 0.17 | 0.23 | 0.22 | 0.10 | 0.14 | 0.21 | 0.24 | 0.16 |
| Y2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.84 |
| La2O3 | 0.19 | 0.40 | 0.29 | 0.27 | 0.30 | 0.25 | 0.46 | 0.42 | 0.25 | 0.61 | 0.37 | 0.33 | b.d. | 0.25 |
| Ce2O3 | 0.53 | 0.85 | 0.57 | 0.69 | 0.58 | 0.74 | 0.98 | 0.94 | 0.62 | 1.73 | 1.24 | 0.94 | 0.51 | 0.56 |
| Nd2O3 | 0.32 | 0.33 | 0.37 | 0.39 | 0.17 | 0.19 | 0.33 | 0.39 | 0.19 | 0.68 | 0.39 | 0.32 | b.d. | 0.25 |
| CaO | 7.48 | 8.71 | 6.27 | 7.19 | 9.04 | 6.20 | 6.92 | 7.96 | 6.34 | 7.33 | 6.49 | 8.09 | 10.31 | 8.54 |
| MgO | 0.16 | b.d. | 0.08 | 0.03 | 0.09 | 0.12 | b.d. | b.d. | 0.07 | b.d. | b.d. | b.d. | b.d. | 0.20 |
| FeO | 3.42 | 1.87 | 2.90 | 3.00 | 3.11 | 3.73 | 1.07 | 5.00 | 3.03 | 0.32 | 2.66 | 4.54 | 1.64 | 4.73 |
| MnO | 2.29 | 2.89 | 1.95 | 2.10 | 2.31 | 1.93 | 2.96 | 1.95 | 2.75 | 4.06 | 2.64 | 2.18 | 1.58 | 2.44 |
| SrO | 1.30 | 1.28 | 2.05 | 1.97 | 1.84 | 1.55 | 3.13 | 2.17 | 1.48 | 2.20 | 2.28 | 2.32 | 1.28 | 3.70 |
| BaO | 0.18 | 0.22 | b.d. | b.d. | 0.15 | b.d. | 0.76 | b.d. | 0.26 | 0.62 | 0.20 | 0.17 | 0.19 | 0.14 |
| Na2O | 16.02 | 15.97 | 15.32 | 13.19 | 12.46 | 16.14 | 13.39 | 15.51 | 14.68 | 15.47 | 9.49 | 14.69 | 12.50 | 11.35 |
| K2O | 0.24 | 0.18 | 0.29 | 0.22 | 0.19 | 0.29 | 0.23 | 0.27 | 0.25 | 0.47 | 0.19 | 0.22 | 0.28 | 0.32 |
| Cl | 1.51 | 1.17 | 1.30 | 1.08 | 1.04 | 1.27 | 1.46 | 1.56 | 1.27 | 0.98 | 1.67 | 1.44 | 1.27 | 1.58 |
| О=Cl | –0.34 | –0.26 | –0.29 | –0.24 | –0.23 | –0.29 | –0.33 | –0.35 | –0.29 | –0.22 | –0.38 | –0.33 | –0.29 | –0.36 |
| Total | 95.71 | 96.86 | 94.63 | 93.75 | 96.27 | 96.76 | 95.91 | 97.93 | 93.58 | 97.26 | 95.28 | 96.47 | 93.48 | 97.15 |
| Formula based on Si + Zr + Ti + Nb + Al + Hf = 29 apfu | ||||||||||||||
| Nb | 0.11 | 0.09 | 0.15 | 0.16 | 0.16 | 0.16 | 0.08 | 0.28 | 0.19 | 0.16 | 0.08 | 0.26 | 0.14 | 0.37 |
| Si | 25.40 | 25.81 | 25.07 | 25.52 | 25.81 | 25.10 | 25.46 | 25.45 | 25.20 | 25.75 | 25.13 | 25.48 | 25.34 | 25.23 |
| Zr | 3.11 | 2.84 | 3.45 | 3.12 | 2.85 | 3.37 | 3.12 | 2.93 | 3.21 | 2.75 | 3.37 | 2.92 | 3.13 | 3.19 |
| Ti | 0.25 | 0.17 | 0.23 | 0.13 | 0.14 | 0.25 | 0.24 | 0.20 | 0.27 | 0.28 | 0.36 | 0.22 | 0.24 | 0.11 |
| Al | 0.13 | 0.09 | 0.09 | 0.06 | 0.04 | 0.13 | 0.10 | 0.14 | 0.13 | 0.06 | 0.08 | 0.13 | 0.14 | 0.10 |
| Y | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.23 |
| La | 0.04 | 0.07 | 0.05 | 0.05 | 0.05 | 0.05 | 0.08 | 0.08 | 0.05 | 0.11 | 0.06 | 0.06 | 0.00 | 0.05 |
| Ce | 0.10 | 0.16 | 0.11 | 0.13 | 0.10 | 0.14 | 0.18 | 0.18 | 0.12 | 0.32 | 0.21 | 0.18 | 0.09 | 0.11 |
| Nd | 0.06 | 0.06 | 0.07 | 0.07 | 0.03 | 0.03 | 0.06 | 0.07 | 0.04 | 0.12 | 0.07 | 0.06 | 0.00 | 0.05 |
| Ca | 4.07 | 4.65 | 3.41 | 3.86 | 4.71 | 3.30 | 3.64 | 4.35 | 3.47 | 3.95 | 3.27 | 4.46 | 5.49 | 4.68 |
| Mg | 0.12 | 0.00 | 0.06 | 0.02 | 0.06 | 0.09 | - | - | 0.05 | - | - | - | - | 0.15 |
| Fe2+ | 1.45 | 0.78 | 1.23 | 1.25 | 1.26 | 1.55 | 0.44 | 2.13 | 1.29 | 0.13 | 1.04 | 1.95 | 0.68 | 2.02 |
| Mn | 0.99 | 1.22 | 0.84 | 0.89 | 0.95 | 0.81 | 1.23 | 0.84 | 1.19 | 1.73 | 1.05 | 0.95 | 0.67 | 1.05 |
| Sr | 0.38 | 0.37 | 0.60 | 0.57 | 0.52 | 0.45 | 0.89 | 0.64 | 0.44 | 0.64 | 0.62 | 0.69 | 0.37 | 1.10 |
| Ba | 0.04 | 0.04 | - | - | 0.03 | - | 0.15 | - | 0.05 | 0.12 | 0.04 | 0.03 | 0.04 | 0.03 |
| Na | 15.77 | 15.44 | 15.06 | 12.81 | 11.75 | 15.57 | 12.76 | 15.33 | 14.54 | 15.08 | 8.64 | 14.67 | 12.03 | 11.25 |
| K | 0.16 | 0.12 | 0.19 | 0.14 | 0.12 | 0.18 | 0.14 | 0.18 | 0.16 | 0.30 | 0.12 | 0.15 | 0.18 | 0.21 |
| Cl | 1.30 | 0.99 | 1.12 | 0.91 | 0.86 | 1.07 | 1.22 | 1.35 | 1.10 | 0.83 | 1.33 | 1.25 | 1.07 | 1.37 |
| Sum | 52.18 | 51.91 | 50.61 | 48.78 | 48.58 | 51.17 | 48.58 | 52.79 | 50.40 | 51.50 | 44.11 | 52.21 | 48.55 | 49.92 |
| Rock | Malignite | Ijolite | Shonkinite | Foyaite | Urtite | Fine-grained Nph-Syenite | Metasomatic Rock | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drill hole | 28 | 28 | 117 | 117 | 44 | 44 | 117 | 156 | 147 | 154 | 154 | 160 | 154 | 156 |
| Interval, m | 153 * | 153 ** | 47 * | 47 ** | 50 * | 50 ** | 215 | 216 | 85 | 144 | 226 | 148 | 197 | 118 |
| Al(M4) | 0.13 | 0.09 | 0.09 | 0.06 | 0.04 | 0.13 | 0.10 | 0.14 | 0.13 | 0.06 | 0.08 | 0.13 | 0.14 | 0.10 |
| Si (M4) | 0.87 | 0.91 | 0.91 | 0.94 | 0.96 | 0.87 | 0.90 | 0.86 | 0.87 | 0.94 | 0.92 | 0.87 | 0.86 | 0.90 |
| Sum М4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Zr (Z) | 3.00 | 2.84 | 3.00 | 3.00 | 2.85 | 3.00 | 3.00 | 2.93 | 3.00 | 2.75 | 3.00 | 2.92 | 3.00 | 3.00 |
| Ti (Z) | - | 0.16 | - | - | 0.14 | - | - | 0.07 | - | 0.25 | - | 0.08 | - | - |
| Nb (Z) | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - |
| Sum Z | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Nb (M3) | 0.11 | 0.09 | 0.15 | 0.16 | 0.15 | 0.16 | 0.08 | 0.28 | 0.19 | 0.16 | 0.08 | 0.26 | 0.14 | 0.37 |
| Si (M3) | 0.53 | 0.90 | 0.17 | 0.58 | 0.85 | 0.22 | 0.56 | 0.59 | 0.33 | 0.81 | 0.20 | 0.60 | 0.48 | 0.32 |
| Ti(M3) | 0.25 | 0.01 | 0.23 | 0.13 | - | 0.25 | 0.24 | 0.13 | 0.27 | 0.03 | 0.36 | 0.14 | 0.24 | 0.11 |
| Zr (M3) | 0.11 | - | 0.45 | 0.12 | - | 0.37 | 0.12 | - | 0.21 | - | 0.37 | - | 0.13 | 0.19 |
| Sum M3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Fe (M2) | 1.45 | 0.78 | 1.23 | 1.25 | 1.26 | 1.55 | 0.44 | 2.13 | 1.29 | 0.13 | 1.04 | 1.95 | 0.68 | 2.02 |
| Mn (M2) | 0.99 | 1.22 | 0.84 | 0.89 | 0.95 | 0.81 | 1.23 | 0.84 | 1.19 | 1.73 | 1.05 | 0.95 | 0.67 | 0.82 |
| Mg (М2) | 0.12 | - | 0.06 | 0.02 | 0.06 | 0.09 | - | - | 0.05 | - | - | - | - | 0.15 |
| Sum М2 | 2.56 | 2.00 | 2.13 | 2.16 | 2.28 | 2.45 | 1.67 | 2.97 | 2.54 | 1.86 | 2.10 | 2.90 | 1.35 | 3.00 |
| Mn (M1) | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.23 |
| Ca (M1) | 4.07 | 4.65 | 3.41 | 3.86 | 4.71 | 3.30 | 3.64 | 4.35 | 3.47 | 3.95 | 3.27 | 4.46 | 5.49 | 4.68 |
| REE (M1) | 0.19 | 0.29 | 0.23 | 0.25 | 0.19 | 0.22 | 0.32 | 0.33 | 0.20 | 0.55 | 0.34 | 0.30 | 0.09 | 0.20 |
| Na (M1) | 1.74 | 1.06 | 2.36 | 1.90 | 1.11 | 2.48 | 2.04 | 1.33 | 2.33 | 1.50 | 2.39 | 1.24 | 0.42 | 0.67 |
| Y (M1) | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.23 |
| Sum М1 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Na (N) | 14.04 | 14.38 | 12.69 | 10.91 | 10.64 | 13.09 | 10.72 | 14.00 | 12.21 | 13.58 | 6.24 | 13.43 | 11.62 | 10.59 |
| K(N) | 0.16 | 0.12 | 0.19 | 0.14 | 0.12 | 0.18 | 0.14 | 0.18 | 0.16 | 0.30 | 0.12 | 0.15 | 0.18 | 0.21 |
| Ba(N) | 0.04 | 0.04 | - | - | 0.03 | - | 0.15 | - | 0.05 | 0.12 | 0.04 | 0.03 | 0.04 | 0.03 |
| Sr (N) | 0.38 | 0.37 | 0.60 | 0.57 | 0.52 | 0.45 | 0.89 | 0.64 | 0.44 | 0.64 | 0.62 | 0.69 | 0.37 | 1.10 |
| Sum N | 14.61 | 14.91 | 13.48 | 11.62 | 11.30 | 13.72 | 11.90 | 14.82 | 12.86 | 14.64 | 7.02 | 14.30 | 12.20 | 11.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, J.A.; Ivanyuk, G.Y.; Kalashnikov, A.O.; Pakhomovsky, Y.A.; Bazai, A.V.; Yakovenchuk, V.N. Petrogenesis of the Eudialyte Complex of the Lovozero Alkaline Massif (Kola Peninsula, Russia). Minerals 2019, 9, 581. https://doi.org/10.3390/min9100581
Mikhailova JA, Ivanyuk GY, Kalashnikov AO, Pakhomovsky YA, Bazai AV, Yakovenchuk VN. Petrogenesis of the Eudialyte Complex of the Lovozero Alkaline Massif (Kola Peninsula, Russia). Minerals. 2019; 9(10):581. https://doi.org/10.3390/min9100581
Chicago/Turabian StyleMikhailova, Julia A., Gregory Yu. Ivanyuk, Andrey O. Kalashnikov, Yakov A. Pakhomovsky, Ayya V. Bazai, and Victor N. Yakovenchuk. 2019. "Petrogenesis of the Eudialyte Complex of the Lovozero Alkaline Massif (Kola Peninsula, Russia)" Minerals 9, no. 10: 581. https://doi.org/10.3390/min9100581
APA StyleMikhailova, J. A., Ivanyuk, G. Y., Kalashnikov, A. O., Pakhomovsky, Y. A., Bazai, A. V., & Yakovenchuk, V. N. (2019). Petrogenesis of the Eudialyte Complex of the Lovozero Alkaline Massif (Kola Peninsula, Russia). Minerals, 9(10), 581. https://doi.org/10.3390/min9100581







