Further Characterization of the RW-1 Monazite: A New Working Reference Material for Oxygen and Neodymium Isotopic Microanalysis
Abstract
1. Introduction
2. Sample Descriptions
3. Analytical Methods
3.1. Spectroscopic and Image Investigations
3.2. Major and Trace Element Compositions
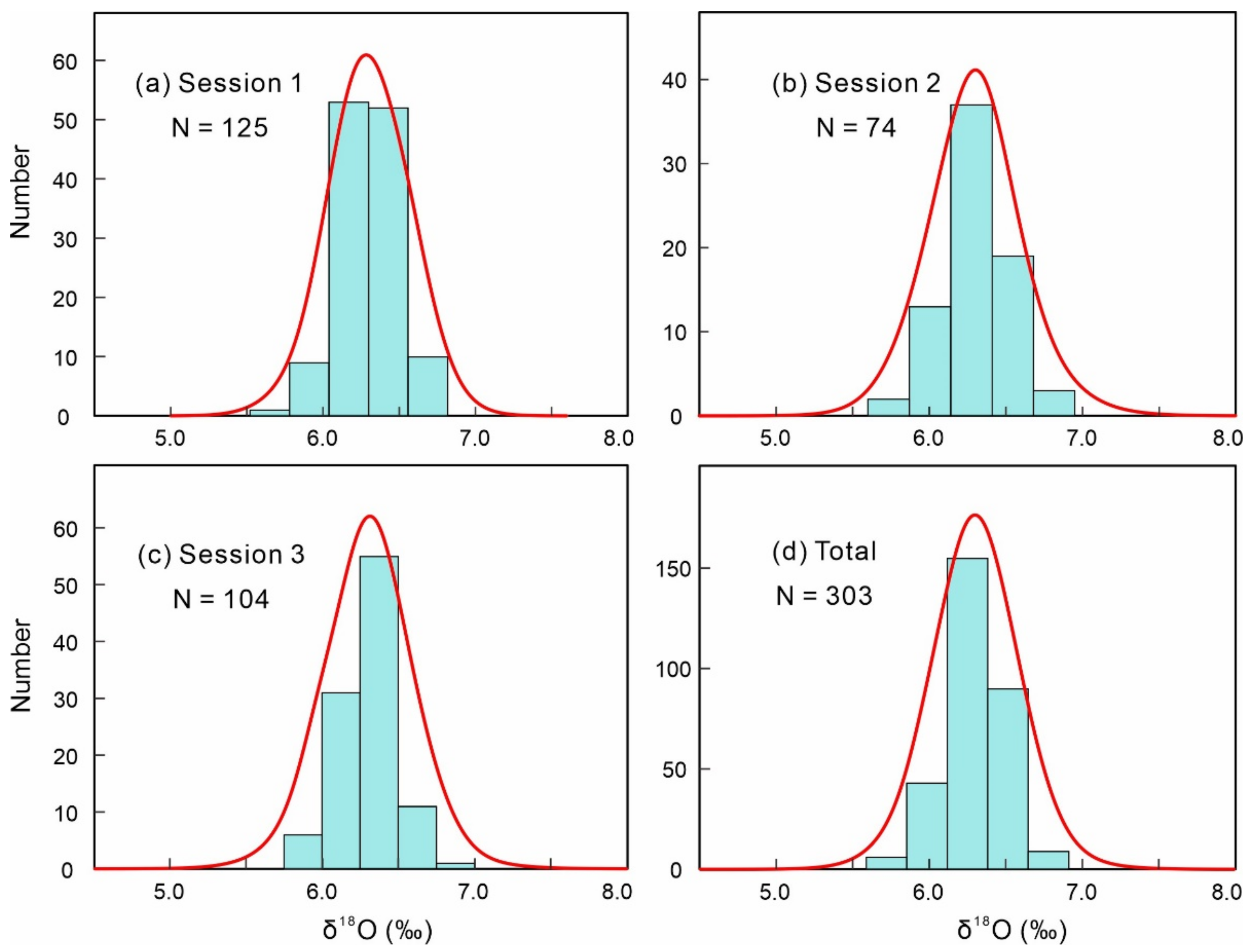
3.3. SIMS Oxygen Isotopic Analysis
3.4. LF-IRMS Oxygen Isotopic Analysis
3.5. Sm–Nd Isotopic Composition
3.6. TIMS Nd Isotopic Analysis
4. Results and Discussion
4.1. RW-1 Mineralogical Features and Elemental Compositions
4.2. Oxygen Isotopic Compositions
4.3. Nd Isotopic Compositions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



References
- Williams, M.L.; Jercinovic, M.J.; Hetherington, C.J. Microprobe monazite geochronology: Understanding geologic processes by integrating composition and chronology. Annu. Rev. Earth Planet. Sci. 2007, 35, 137–175. [Google Scholar] [CrossRef]
- Franz, G.; Andrehs, G.; Rhede, D. Crystal chemistry of monazite and xenotime from Saxothuringian–Moldanubian metapelites, NE Bavaria, Germany. Eur. J. Mineral. 1996, 8, 1097–1118. [Google Scholar] [CrossRef]
- Catlos, E.J.; Miller, N.R. Speculations linking monazite compositions to origin: Llallagua Tin ore deposit (Bolivia). Resources 2017, 6, 36. [Google Scholar] [CrossRef]
- Meldrum, A.; Boatner, L.A.; Weber, W.J.; Ewing, R.C. Radiation damage in zircon and monazite. Geochim. Cosmochim. Acta 1998, 62, 2509–2520. [Google Scholar] [CrossRef]
- Parrish, R.R. U–Pb dating of monazite and its application to geological problems. Can. J. Earth Sci. 1990, 27, 1431–1450. [Google Scholar] [CrossRef]
- Perumalsamy, C.; Bhadra, S.; Balakrishnan, S. Decoding evolutionary history of provenance from beach placer monazites: A case study from Kanyakumari coast, southwest India. Chem. Geol. 2016, 427, 83–97. [Google Scholar] [CrossRef]
- Ayers, J.C.; Loflin, M.; Miller, C.F.; Barton, M.D.; Coath, C.D. In situ oxygen isotope analysis of monazite as a monitor of fluid infiltration during contact metamorphism: Birch Creek Pluton aureole, White Mountains, eastern California. Geology 2006, 34, 653–656. [Google Scholar] [CrossRef]
- Pyle, J.M.; Spear, F.S.; Rudnick, R.L.; McDonough, W.F. Monazite–xenotime–garnet equilibrium in metapelites and a new monazite–garnet thermometer. J. Petrol. 2001, 42, 2083–2107. [Google Scholar] [CrossRef]
- Gratz, R.; Heinrich, W. Monazite–xenotime thermobarometry; experimental calibration of the miscibility gap in the binary system CePO4–YPO4. Am. Mineral. 1997, 82, 772–780. [Google Scholar] [CrossRef]
- Gauthiez-Putallaz, L.; Rubatto, D.; Hermann, J. Dating prograde fluid pulses during subduction by in situ U–Pb and oxygen isotope analysis. Contrib. Mineral. Petrol. 2016, 171, 15. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Lan, Z.; Guo, C.; Yang, Y.; Liu, Y.; Tang, G. Monazite and xenotime U–Th–Pb geochronology by ion microprobe: Dating highly fractionated granites at Xihuashan tungsten mine, SE China. Contrib. Mineral. Petrol. 2013, 166, 65–80. [Google Scholar] [CrossRef]
- Liu, Z.-C.; Wu, F.-Y.; Yang, Y.-H.; Yang, J.-H.; Wilde, S.A. Neodymium isotopic compositions of the standard monazites used in U–Th–Pb geochronology. Chem. Geol. 2012, 334, 221–239. [Google Scholar] [CrossRef]
- Breecker, D.O.; Sharp, Z.D. A monazite oxygen isotope thermometer. Am. Mineral. 2007, 92, 1561–1572. [Google Scholar] [CrossRef]
- Rubatto, D.; Putlitz, B.; Gauthiez-Putallaz, L.; Crépisson, C.; Buick, I.S.; Zheng, Y.F. Measurement of in-situ oxygen isotope ratios in monazite by SHRIMP ion microprobe: Standards, protocols and implications. Chem. Geol. 2014, 380, 84–96. [Google Scholar] [CrossRef]
- Didier, A.; Putlitz, B.; Baumgartner, L.P.; Bouvier, A.-S.; Vennemann, T.W. Evaluation of potential monazite reference materials for oxygen isotope analyses by SIMS and laser assisted fluorination. Chem. Geol. 2017, 450, 199–209. [Google Scholar] [CrossRef]
- Förster, H.-J. The chemical composition of REE-Y-Th-U-rich accessory minerals in peraluminous granites of the Erzgebirge–Fichtelgebirge region, Germany, Part I: The monazite-(Ce)–brabantite solid solution series. Am. Mineral. 1998, 83, 259–272. [Google Scholar] [CrossRef]
- Ling, X.-X.; Huyskens, M.H.; Li, Q.-L.; Yin, Q.-Z.; Werner, R.; Liu, Y.; Tang, G.-Q.; Yang, Y.-N.; Li, X.-H. Monazite RW-1: A homogeneous natural reference material for SIMS U–Pb and Th–Pb isotopic analysis. Mineral. Petrol. 2017, 111, 163–172. [Google Scholar] [CrossRef]
- Cherniak, D.J.; Zhang, X.Y.; Nakamura, M.; Watson, E.B. Oxygen diffusion in monazite. Earth Planet. Sci. Lett. 2004, 226, 161–174. [Google Scholar] [CrossRef]
- Gonçalves, G.; Lana, C.; Scholz, R.; Buick, I.; Gerdes, A.; Kamo, S.L.; Corfu, F.; Rubatto, D.; Wiedenbeck, M.; Nalini, H.A.; et al. The Diamantina monazite: A new low-Th reference material for microanalysis. Geostand. Geoanal. Res. 2017, 42, 25–47. [Google Scholar] [CrossRef]
- Aleinikoff, J.N.; Schenck, W.S.; Plank, M.O.; Srogi, L.; Fanning, C.M.; Kamo, S.L.; Bosbyshell, H. Deciphering igneous and metamorphic events in high-grade rocks of the Wilmington Complex, Delaware: Morphology, cathodoluminescence and backscattered electron zoning, and SHRIMP U-Pb geochronology of zircon and monazite. Geol. Soc. Am. Bull. 2006, 118, 39–64. [Google Scholar] [CrossRef]
- Harrison, M.T.; Grove, M.; McKeegan, K.D.; Coath, C.D.; Lovera, O.M.; Fort, P.L. Origin and episodic emplacement of the Manaslu Intrusive Complex, central Himalaya. J. Petrol. 1999, 40, 3–19. [Google Scholar] [CrossRef]
- Gasquet, D.; Bertrand, J.-M.; Paquette, J.-L.; Lehmann, J.; Ratzov, G.; De Ascencão Guedes, R.; Tiepolo, M.; Boullier, A.-M.; Scaillet, S.; Nomade, S. Miocene to Messinian deformation and hydrothermal activity in a pre-Alpine basement massif of the French western Alps: New U–Th–Pb and argon ages from the Lauzière massif. Bull. Soc. Geol. Fr. 2010, 181, 227–241. [Google Scholar] [CrossRef]
- Paquette, J.L.; Tiepolo, M. High resolution (5 μm) U–Th–Pb isotope dating of monazite with excimer laser ablation (ELA)-ICPMS. Chem. Geol. 2007, 240, 222–237. [Google Scholar] [CrossRef]
- Knoper, M.; Armstrong, R.; Andreoli, M.; Ashwal, L. The Steenkampskraal monazite vein: A subhorizontal stretching shear zone indicating extensional collapse of Namaqualand at 1033 Ma? J. Afr. Earth Sci. 2000, 31, 38. [Google Scholar]
- Ayers, J.C.; Miller, C.F.; Loflin, M.; Barton, M.D.; Coath, C. Dating fluid infiltration using monazite. In Proceedings of the Eleventh International Symposium on Water–Rock Interaction, Saratoga Springs, NY, USA, 27 June 2004; pp. 247–251. [Google Scholar]
- Wu, S.; Karius, V.; Schmidt, B.C.; Simon, K.; Wörner, G. Comparison of ultrafine powder pellet and flux-free fusion glass for bulk analysis of granitoids by laser ablation–inductively coupled plasma–mass spectrometry. Geostand. Geoanal. Res. 2018, 42, 575–591. [Google Scholar] [CrossRef]
- Griffin, W.L. GLITTER: Data reduction software for laser ablation ICP-MS. In Laser Ablation ICP–MS in the Earth Sciences: Current Practices and Outstanding Issues; Mineralogical Association of Canada: Toronto, QC, Canada, 2008; pp. 308–311. [Google Scholar]
- Li, X.-H.; Li, W.-X.; Li, Q.-L.; Wang, X.-C.; Liu, Y.; Yang, Y.-H. Petrogenesis and tectonic significance of the ∼850 Ma Gangbian alkaline complex in South China: Evidence from in situ zircon U–Pb dating, Hf–O isotopes and whole-rock geochemistry. Lithos 2010, 114, 1–15. [Google Scholar] [CrossRef]
- Tang, G.-Q.; Li, X.-H.; Li, Q.-L.; Liu, Y.; Ling, X.-X.; Yin, Q.-Z. Deciphering the physical mechanism of the topography effect for oxygen isotope measurements using a Cameca IMS-1280 SIMS. J. Anal. At. Spectrom. 2015, 30, 950–956. [Google Scholar] [CrossRef]
- Coplen, T.B.; Kendall, C.; Hopple, J. Comparison of stable isotope reference samples. Nature 1983, 302, 236–238. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Xie, L.; Fan, H.; Wu, F. In situ Nd isotopic measurement of natural geological materials by LA-MC-ICPMS. Chin. Sci. Bull. 2008, 53, 1062–1070. [Google Scholar] [CrossRef]
- Dubois, J.C.; Retali, G.; Cesario, J. Isotopic analysis of rare earth elements by total vaporization of samples in thermal ionization mass spectrometry. Int. J. Mass Spectrom. 1992, 120, 163–177. [Google Scholar] [CrossRef]
- Isnard, H.; Brennetot, R.; Caussignac, C.; Caussignac, N.; Chartier, F. Investigations for determination of Gd and Sm isotopic compositions in spent nuclear fuels samples by MC–ICPMS. Int. J. Mass Spectrom. 2005, 246, 66–73. [Google Scholar] [CrossRef]
- Li, C.-F.; Chen, F.; Li, X.-H. Precise isotopic measurements of sub-nanogram Nd of standard reference material by thermal ionization mass spectrometry using the NdO+ technique. Int. J. Mass Spectrom. 2007, 266, 34–41. [Google Scholar] [CrossRef]
- Tanaka, T.; Togashi, S.; Kamioka, H.; Amakawa, H.; Kagami, H.; Hamamoto, T.; Yuhara, M.; Orihashi, Y.; Yoneda, S.; Shimizu, H.; et al. JNdi-1: A neodymium isotopic reference in consistency with LaJolla neodymium. Chem. Geol. 2000, 168, 279–281. [Google Scholar] [CrossRef]
- Silva, E.N.; Ayala, A.P.; Guedes, I.; Paschoal, C.W.A.; Moreira, R.L.; Loong, C.K.; Boatner, L.A. Vibrational spectra of monazite-type rare-earth orthophosphates. Opt. Mater. 2006, 29, 224–230. [Google Scholar] [CrossRef]
- Huittinen, N.; Arinicheva, Y.; Kowalski, P.M.; Vinograd, V.L.; Neumeier, S.; Bosbach, D. Probing structural homogeneity of La1−xGdxPO4 monazite-type solid solutions by combined spectroscopic and computational studies. J. Nucl. Mater. 2017, 486, 148–157. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Itano, K.; Iizuka, T.; Chang, Q.; Kimura, J.I.; Maruyama, S. U–Pb chronology and geochemistry of detrital monazites from major African rivers: Constraints on the timing and nature of the Pan-African Orogeny. Precambrian Res. 2016, 282, 139–156. [Google Scholar] [CrossRef]
- York, D.; Evensen, N.M.; Martínez, M.L.; Delgado, J.D.B. Unified equations for the slope, intercept, and standard errors of the best straight line. Am. J. Phys. 2004, 72, 367–375. [Google Scholar] [CrossRef]






| Name | Th Content (wt.%) | 2SD a | δ18OV-SMOW (‰) b | 2SD | Reference Age (Ma) | 2SD | 143Nd/144Nd | 2SD | Host Lithology (Location) | Quantity | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RW-1 | 8.3 | 0.4 | 6.30 | 0.16 | 904.2 | 0.3 | 0.512282 | 0.000011 | pegmatite (Norway) | limited | this study, [17] |
| eBay | 0.02–0.34 (heterogeneous) | - | 9.34 (heterogeneous) | 0.62 | - | - | - | - | unknown (Hiddenite, North Carolina) | limited | [7,13,18] |
| 9.52 | - | ||||||||||
| Diamantina | 0.2 | 0.1 | - | - | 495.3 | 0.5 | 0.511427 | 0.000023 | hydrothermal quartz vein (Brazil) | unlimited | [19] |
| UNIL1-Mnz1 | 0.6 | 0.3 | 8.45 | 0.38 | - | - | - | - | carbonatite (Namibia) | limited | [15] |
| USGS-44069 | 2.1–5.5 (heterogeneous) | - | 7.67 | 0.52 | 424.9 | 0.4 | 0.512175 | 0.000040 | metapsamittic layer (USA) | unlimited | [12,14,15,20] |
| 554 | 3.3 | - | 7.54 (heterogeneous) | 0.24 | 45.3 | 1.4 | 0.512075 | 0.000041 | granite (Santa Catalina, Mountains, Arizona) | unlimited | [7,12,21] |
| Itambe | 6.3 (heterogeneous) | 1.2 | 0.46 | 0.42 | ~509–514 | - | - | - | pegmatite (Brazil) | limited | [14] |
| Moacyr | 6.5 | 0.4 | 1.45 | 0.10 | 506.4 | 0.7 | 0.512421 | 0.000011 | pegmatite (Brazil) | unlimited | [15,22] |
| UNIL-Mnz2 | 9.4 (heterogeneous) | 1 | 9.51 | 0.54 | - | - | - | - | pegmatite (Madagascar) | limited | [15] |
| Manangoutry | 12.1 | 0.2 | 10.19 | 0.16 | 555 | 2 | 0.511044 | 0.000022 | charnockite (Madagascar) | limited | [12,15,23] |
| Namaqualand | 8.3 | 0.2 | - | - | ~1033 | - | 0.511894 | 0.000025 | monazite vein (South Africa) | unlimited | [12,24] |
| Brazil | 6.1 | - | 1.43 | 0.16 | - | - | - | - | unknown | limited | [7,25] |
| Brazil c | 13.3 | 0.5 | 7.89 | 0.28 | - | - | - | - | unknown | limited | [13] |
| M1 | 7.9–14.3 (heterogeneous) | - | - | - | ~535 | - | 0.511716 | 0.000052 | unknown | limited | [12] |
| M4 | 6.4–8.5 (heterogeneous) | - | - | - | ~525 | - | 0.511761 | 0.000020 | unknown | limited | [12] |
| Methods | EPMA, wt.% * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | P2O5 | Y2O3 | ThO2 | UO2 | PbO | La2O3 | Ce2O3 | Pr2O3 | Nd2O3 | |
| Average | 1.81 | 0.66 | 28.03 | 2.39 | 9.48 | 0.23 | 0.39 | 7.85 | 25.05 | 3.68 | 14.26 |
| 2SD | 0.20 | 0.06 | 0.76 | 0.17 | 0.50 | 0.05 | 0.03 | 0.28 | 0.60 | 0.51 | 0.28 |
| 2RSD (%) | 10.77 | 8.51 | 2.73 | 7.21 | 5.32 | 22.39 | 8.62 | 3.53 | 2.41 | 13.92 | 1.98 |
| Methods | EPMA, wt.% | LA, ppm, N = 23 | |||||||||
| Sm2O3 | Gd2O3 | Dy2O3 | Total | Eu | Tb | Ho | Er | Tm | Yb | Lu | |
| Average | 3.60 | 1.65 | 0.28 | 99.12 | 33 | 972 | 254 | 407 | 49 | 306 | 27 |
| 2SD | 0.35 | 0.30 | 0.05 | 1.38 | 2 | 58 | 18 | 34 | 5 | 42 | 5 |
| 2RSD (%) | 9.85 | 18.09 | 19.14 | 1.39 | 5 | 6 | 7 | 8 | 10 | 14 | 18 |
| Monazite | Date | Weight (mg) | δ18OVSMOW (‰) | Yield (%) |
|---|---|---|---|---|
| RW-1 | 2017/11/8 | 2.04 | 6.34 | 90 |
| 2017/11/8 | 2.03 | 6.20 | 92 | |
| 2018/1/25 | 1.75 | 6.37 | 97 | |
| 2018/1/25 | 1.87 | 6.28 | 93 | |
| Average | 6.30 | |||
| 2SD | 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.-G.; Li, X.-H.; Ling, X.-X.; Yang, Y.-H.; Li, C.-F.; Li, Y.-L.; Mao, Q.; Li, Q.-L.; Putlitz, B. Further Characterization of the RW-1 Monazite: A New Working Reference Material for Oxygen and Neodymium Isotopic Microanalysis. Minerals 2019, 9, 583. https://doi.org/10.3390/min9100583
Wu L-G, Li X-H, Ling X-X, Yang Y-H, Li C-F, Li Y-L, Mao Q, Li Q-L, Putlitz B. Further Characterization of the RW-1 Monazite: A New Working Reference Material for Oxygen and Neodymium Isotopic Microanalysis. Minerals. 2019; 9(10):583. https://doi.org/10.3390/min9100583
Chicago/Turabian StyleWu, Li-Guang, Xian-Hua Li, Xiao-Xiao Ling, Yue-Heng Yang, Chao-Feng Li, You-Lian Li, Qian Mao, Qiu-Li Li, and Benita Putlitz. 2019. "Further Characterization of the RW-1 Monazite: A New Working Reference Material for Oxygen and Neodymium Isotopic Microanalysis" Minerals 9, no. 10: 583. https://doi.org/10.3390/min9100583
APA StyleWu, L.-G., Li, X.-H., Ling, X.-X., Yang, Y.-H., Li, C.-F., Li, Y.-L., Mao, Q., Li, Q.-L., & Putlitz, B. (2019). Further Characterization of the RW-1 Monazite: A New Working Reference Material for Oxygen and Neodymium Isotopic Microanalysis. Minerals, 9(10), 583. https://doi.org/10.3390/min9100583





