Theoretical Modeling of Defects, Dopants, and Diffusion in the Mineral Ilmenite
Abstract
1. Introduction
2. Computational Methods
3. Results
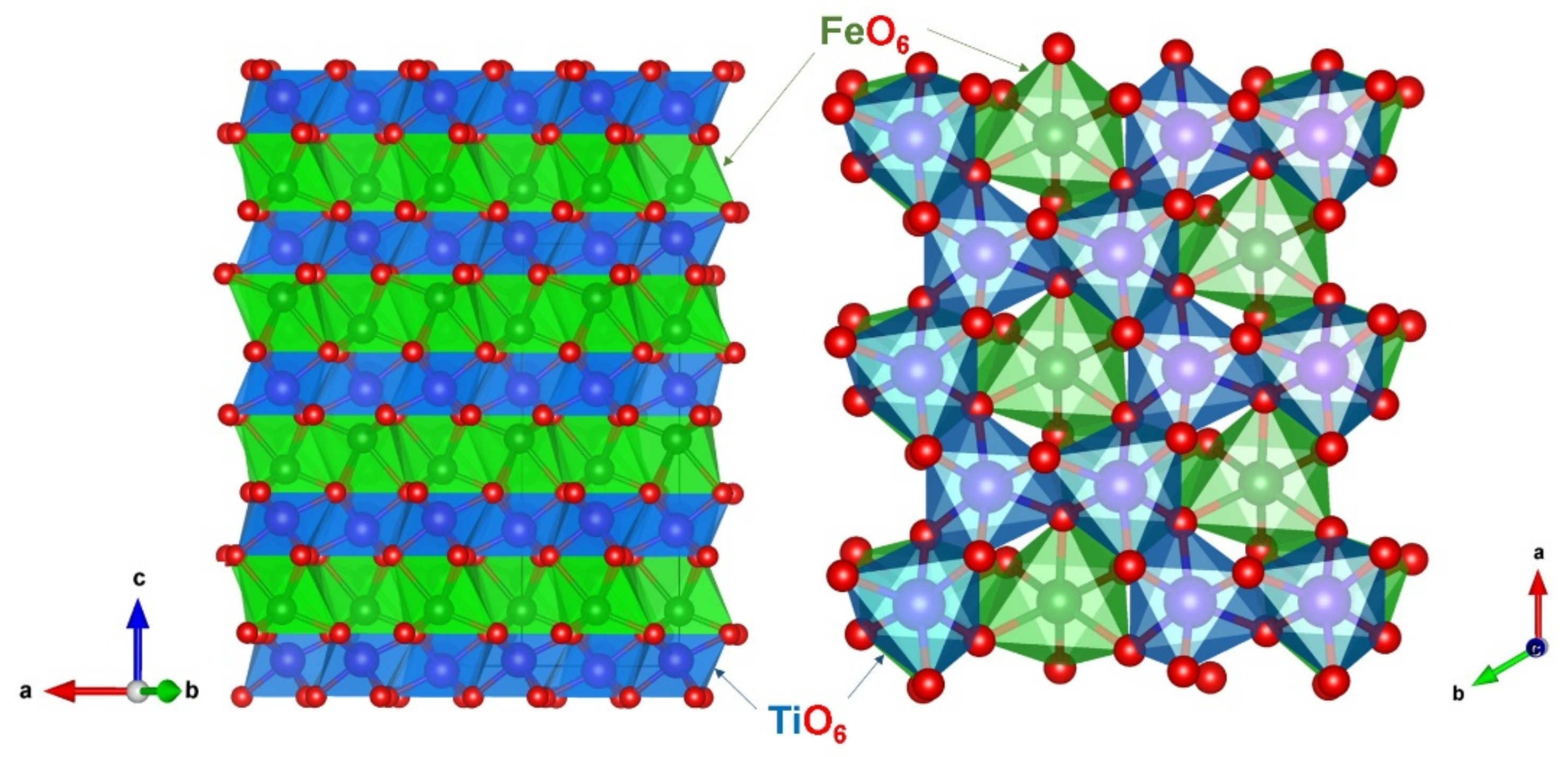
3.1. Crystal Structure of FeTiO3
3.2. Energetics of Intrinsic Defects
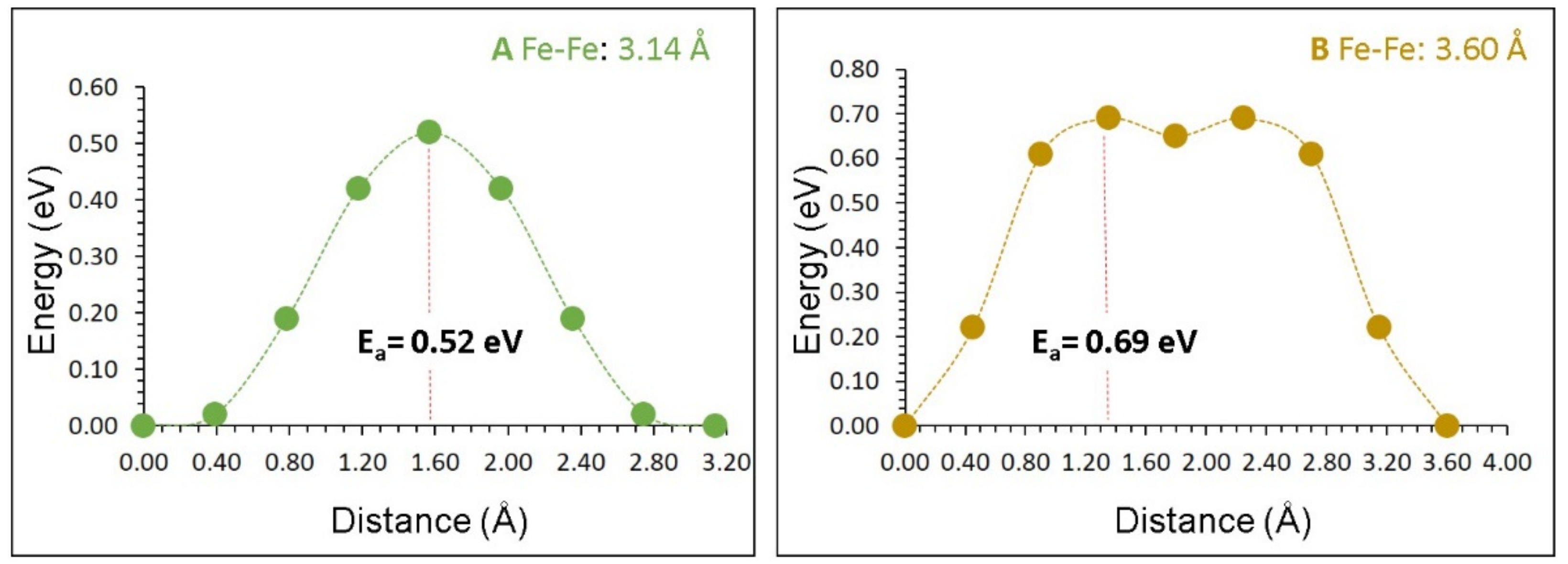
3.3. Self-Diffusion of Iron
3.4. Dopant Substitution
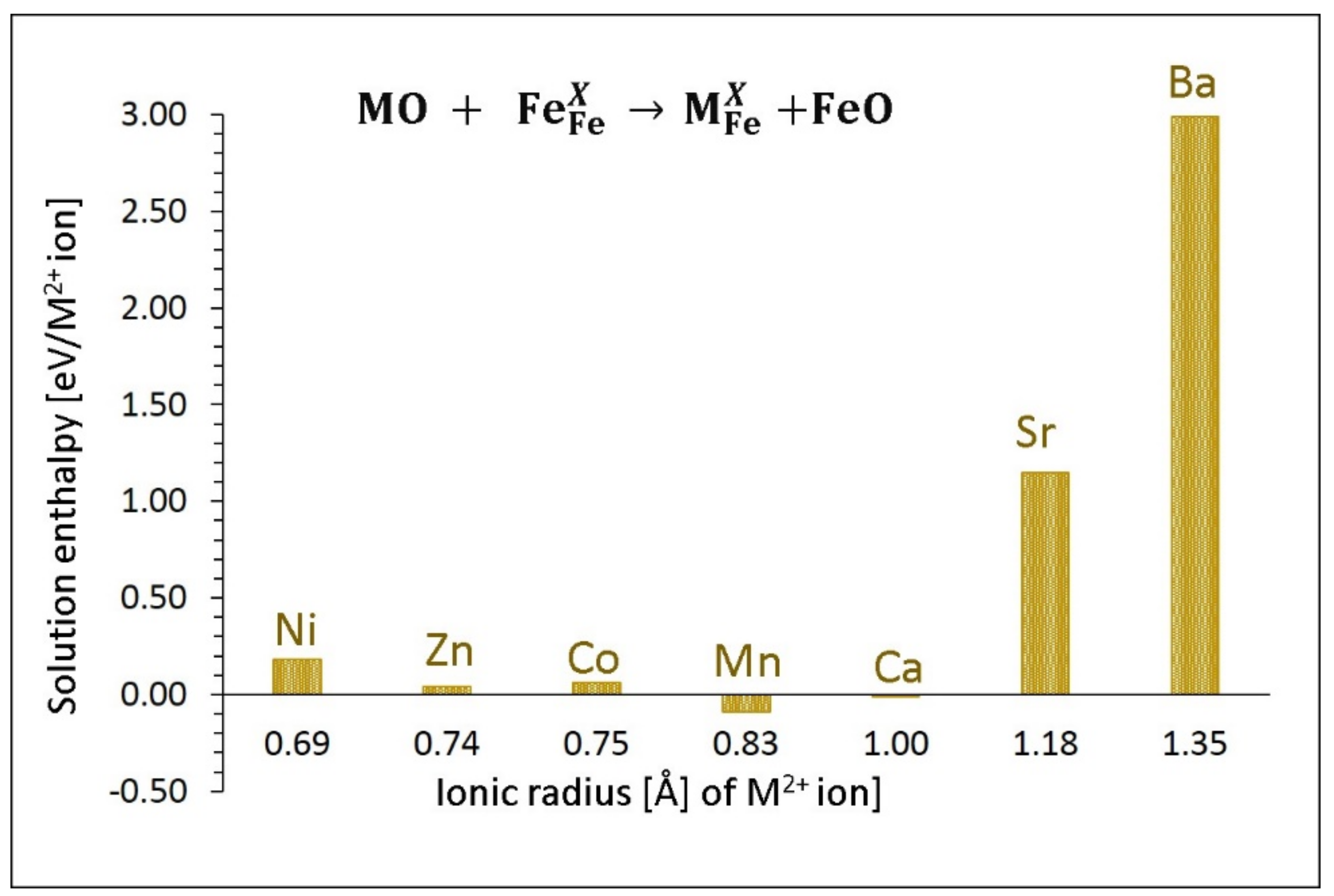
3.4.1. Divalent Dopants
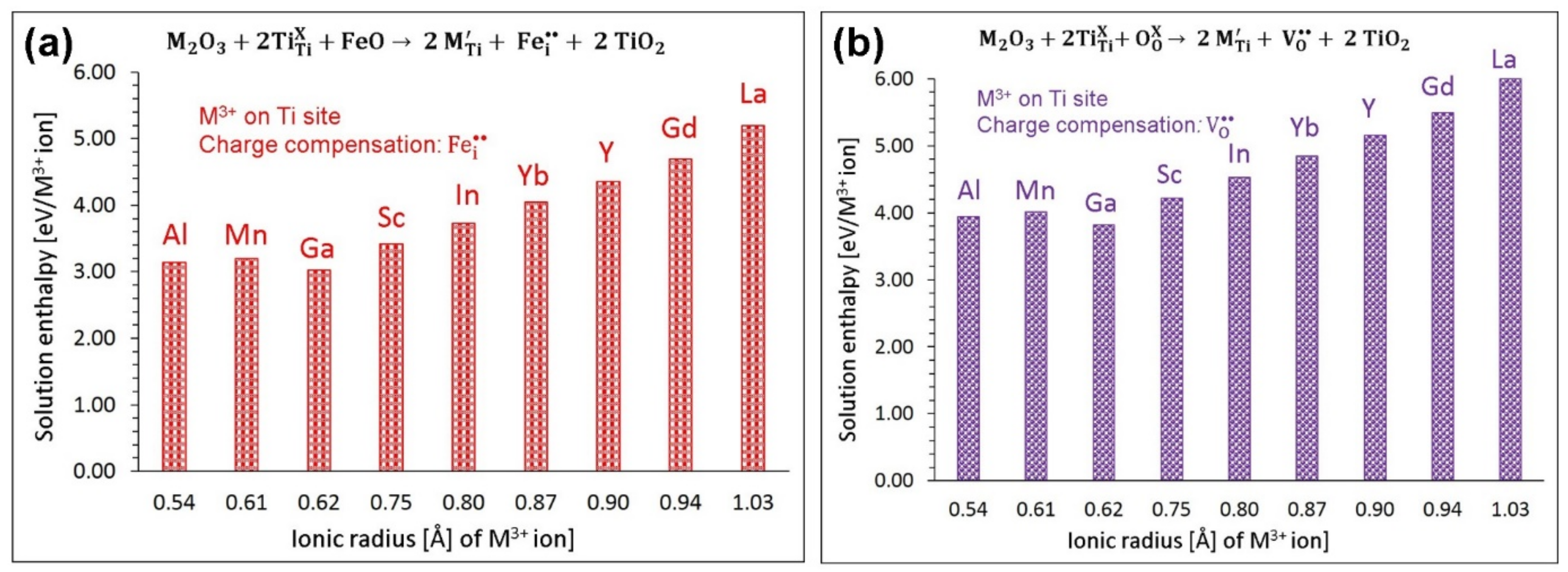
3.4.2. Trivalent Dopants
3.4.3. Tetravalent Dopants
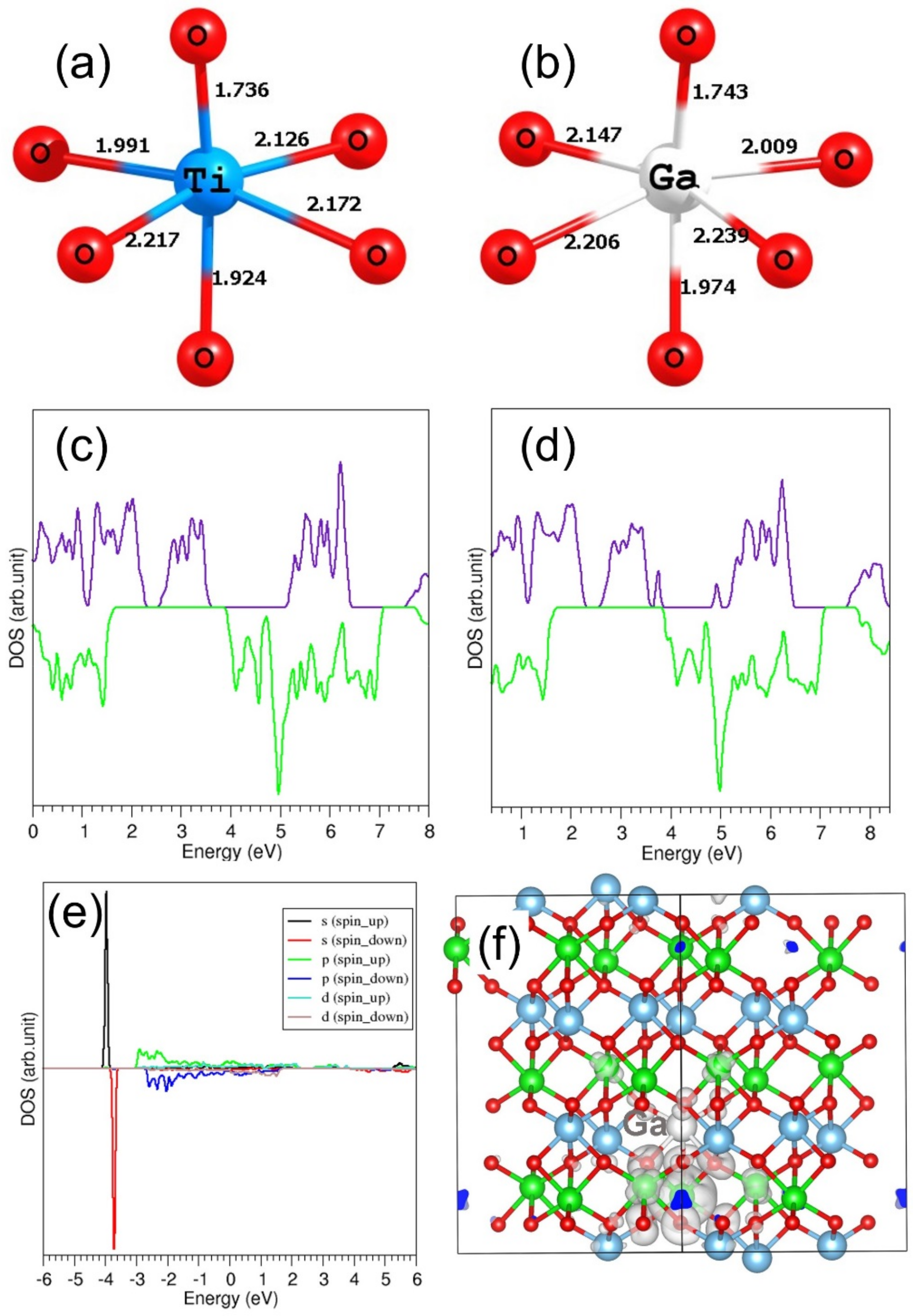
3.5. Electronic Structures of Doped FeTiO3 Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Premaratne, W.A.P.J.; Rowson, N.A. The Processing of Beach Sand from Sri Lanka for the Recovery of Titanium Using Magnetic Separation. Phys. Sep. Sci. Eng. 2003, 12, 13–22. [Google Scholar] [CrossRef]
- Ismail, M.G.M.U.; Amarasekera, J.; Kumarasinghe, J.S.N. The upgrading of ilmenite from SRI lanka by the oxidation-reduction-leach process. Int. J. Miner. Process. 1983, 10, 161–164. [Google Scholar] [CrossRef]
- Premaratne, W.A.P.J.; Rowson, N.A. Microwave Assisted Dissolution of Sri Lankan Ilmenite: Extraction and Leaching Kinetics of Titanium and Iron Metals. J. Sci. Univ. Kelaniya Sri Lanka 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Edwards, R.; Atkinson, K. Magmatic deposits. In Ore Deposit Geology and Its Influence on Mineral Exploration; Edwards, R., Atkinson, K., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1986; pp. 18–68. [Google Scholar]
- Spitz, K.; Trudinger, J. Mining and the Environment; CRC Press: London, UK, 2019. [Google Scholar]
- Filippou, D.; Hudon, G. Iron removal and recovery in the titanium dioxide feedstock and pigment industries. JOM 2009, 61, 36. [Google Scholar] [CrossRef]
- Herath, J.W. Mineral resources of Sri Lanka. Eco. Bull. 1975, 2, 1–75. [Google Scholar]
- Deep, A.; Malik, P.; Gupta, B. Extraction and Separation of Ti(IV) Using Thiophosphinic Acids and Its Recovery from Ilmenite and Red Mud. Sep. Sci. Technol. 2001, 36, 671–685. [Google Scholar] [CrossRef]
- Das, G.; Pranolo, Y.; Zhu, Z.; Cheng, C. Leaching of ilmenite ores by acidic chloride solutions. Hydrometallurgy 2013, 133, 94–99. [Google Scholar] [CrossRef]
- Rao, D.S.; Sengupta, D. Electron Microscopic Studies of Ilmenite from the Chhatrapur Coast, Odisha, India, and Their Implications in Processing. J. Geochem. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Palliyaguru, L.; Arachchi, N.D.H.; Jayaweera, C.D.; Jayaweera, P.M. Production of synthetic rutile from ilmenite via anion-exchange. Miner. Process. Extr. Metall. 2018, 127, 169–175. [Google Scholar] [CrossRef]
- Parapari, P.S.; Irannajad, M.; Mehdilo, A. Modification of ilmenite surface properties by superficial dissolution method. Miner. Eng. 2016, 92, 160–167. [Google Scholar] [CrossRef]
- Fan, X.; Rowson, N.A. Fundamental Investigation of Microwave Pretreatment on the Flotation of Massive Ilmenite Ores. Dev. Chem. Eng. Miner. Process. 2000, 8, 167–182. [Google Scholar] [CrossRef]
- Fan, X.; Rowson, N.A. The effect of Pb(NO3)2 on ilmenite flotation. Miner. Eng. 2000, 13, 205–215. [Google Scholar] [CrossRef]
- Wilson, N.C.; Muscat, J.; Mkhonto, D.; Ngoepe, P.E.; Harrison, N.M. Structure and properties of ilmenite from first principles. Phys. Rev. B 2005, 71, 075202. [Google Scholar] [CrossRef]
- Ribeiro, R.A.P.; Camilo, A.; de Lazaro, S.R. Electronic structure and magnetism of new ilmenite compounds for spintronic devices: FeBO3 (B = Ti, Hf, Zr, Si, Ge, Sn). J. Magn. Magn. Mater. 2015, 394, 463–469. [Google Scholar] [CrossRef]
- Kishore, N.; Nagarajan, V.; Chandiramouli, R. High-pressure studies on electronic and mechanical properties of FeBO3 (B = Ti, Mn, Cr) ceramics—A first-principles study. Phase Transit. 2018, 91, 382–397. [Google Scholar] [CrossRef]
- Carmichael, C.M.; Blackett, P.M.S. The magnetic properties of ilmenite-haematite crystals. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1961, 263, 508–530. [Google Scholar]
- Ribeiro, R.A.P.; de Lázaro, S.R. Structural, electronic and elastic properties of FeBO3 (B = Ti, Sn, Si, Zr) ilmenite: a density functional theory study. RSC Adv. 2014, 4, 59839–59846. [Google Scholar] [CrossRef]
- Matko, V.; Jezernik, K. Greatly Improved Small Inductance Measurement Using Quartz Crystal Parasitic Capacitance Compensation. Sensors 2010, 10, 3954–3960. [Google Scholar] [CrossRef]
- Matko, V. Next generation AT-cut quartz crystal sensing devices. Sensors 2011, 11, 4474–4482. [Google Scholar] [CrossRef]
- Jay, E.E.; Rushton, M.J.D.; Chroneos, A.; Grimes, R.W.; Kilner, J.A. Genetics of superionic conductivity in lithium lanthanum titanates. Phys. Chem. Chem. Phys. 2015, 17, 178–183. [Google Scholar] [CrossRef]
- Fisher, C.A.J.; Hart Prieto, V.M.; Islam, M.S. Lithium Battery Materials LiMPO4 (M = Mn, Fe, Co, and Ni): Insights into Defect Association, Transport Mechanisms, and Doping Behavior. Chem. Mater. 2008, 20, 5907–5915. [Google Scholar] [CrossRef]
- Islam, M.S.; Driscoll, D.J.; Fisher, C.A.J.; Slater, P.R. Atomic-Scale Investigation of Defects, Dopants, and Lithium Transport in the LiFePO4 Olivine-Type Battery Material. Chem. Mater. 2005, 17, 5085–5092. [Google Scholar] [CrossRef]
- Kuganathan, N.; Kordatos, A.; Chroneos, A. Li2SnO3 as a Cathode Material for Lithium-ion Batteries: Defects, Lithium Ion Diffusion and Dopants. Sci. Rep. 2018, 8, 12621. [Google Scholar] [CrossRef] [PubMed]
- Kuganathan, N.; Kordatos, A.; Fitzpatrick, M.E.; Vovk, R.V.; Chroneos, A. Defect process and lithium diffusion in Li2TiO3. Solid State Ion. 2018, 327, 93–98. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Defects, Dopants and Sodium Mobility in Na2MnSiO4. Sci. Rep. 2018, 8, 14669. [Google Scholar] [CrossRef] [PubMed]
- Treacher, J.C.; Wood, S.M.; Islam, M.S.; Kendrick, E. Na2CoSiO4 as a cathode material for sodium-ion batteries: structure, electrochemistry and diffusion pathways. Phys. Chem. Chem. Phys. 2016, 18, 32744–32752. [Google Scholar] [CrossRef]
- Kuganathan, N.; Iyngaran, P.; Vovk, R.; Chroneos, A. Defects, dopants and Mg diffusion in MgTiO3. Sci. Rep. 2019, 9, 4394. [Google Scholar] [CrossRef]
- Gale, J.D.; Rohl, A.L. The General Utility Lattice Program (GULP). Mol. Simul. 2003, 29, 291–341. [Google Scholar] [CrossRef]
- Gale, J.D. GULP: A computer program for the symmetry-adapted simulation of solids. J. Chem. Soc. Faraday Trans. 1997, 93, 629–637. [Google Scholar] [CrossRef]
- Mott, N.F.; Littleton, M.J. Conduction in polar crystals. I. Electrolytic conduction in solid salts. Trans. Faraday Soc. 1938, 34, 485–499. [Google Scholar] [CrossRef]
- Varotsos, P. Point defect parameters in β-PbF2 revisited. Solid State Ion. 2008, 179, 438–441. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes. The Art of Scientific Computing; Cambridge University Press: Cambridge, UK, 1986; p. 818. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Morosin, B.; Baughman, R.J.; Ginley, D.S.; Butler, M.A. The influence of crystal structure on the photoresponse of iron–titanium oxide electrodes. J. Appl. Crystallogr. 1978, 11, 121–124. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the Concentrations of Imperfections in Crystalline Solids. In Solid State Physics; Seitz, F., Turnbull, D., Eds.; Academic Press: Cambridge, MA, USA, 1956; Volume 3, pp. 307–435. [Google Scholar]
- Varotsos, P.; Miliotis, D. New aspects on the dielectric properties of the alkali halides with divalent impurities. J. Phys. Chem. Solids 1974, 35, 927–930. [Google Scholar] [CrossRef]
- Gambhire, A.B.; Lande, M.K.; Rathod, S.B.; Arbad, B.R.; Vidhate, K.N.; Gholap, R.S.; Patil, K.R. Synthesis and characterization of FeTiO3 ceramics. Arab. J. Chem. 2016, 9, S429–S432. [Google Scholar] [CrossRef]
- Ribeiro, R.A.P.; de Lazaro, S.R.; Gatti, C. The role of exchange–correlation functional on the description of multiferroic properties using density functional theory: the ATiO3 (A = Mn, Fe, Ni) case study. RSC Adv. 2016, 6, 101216–101225. [Google Scholar] [CrossRef]





| Parameter | Calculated | Experiment [40] | |∆| (%) | ||
|---|---|---|---|---|---|
| Force Field | DFT | Force Field | DFT | ||
| a = b (Å) | 5.1465 | 5.0724 | 5.0870 | 1.17 | 0.29 |
| c (Å) | 13.8593 | 14.0066 | 14.0420 | 1.30 | 0.25 |
| α = β (°) | 90.00 | 90.00 | 90.00 | 0.00 | 0.00 |
| γ (°) | 120.0 | 120.0 | 120.0 | 0.00 | 0.00 |
| Defect Process | Defect Energy (eV) | Defect Energy (eV)/Defect |
|---|---|---|
| Fe Frenkel/1 | 8.40 | 4.20 |
| Ti Frenkel/2 | 19.58 | 9.79 |
| O Frenkel/3 | 9.02 | 4.51 |
| Schottky/4 | 30.64 | 6.13 |
| FeO Schottky/5 | 9.98 | 4.99 |
| TiO2 Schottky-like/6 | 21.06 | 7.02 |
| Fe/Ti anti-site (isolated)/7 | 4.52 | 2.26 |
| Fe/Ti anti-site (cluster)/8 | 1.10 | 0.55 |
| Migration Path | Fe‒Fe Separation (Å) | Activation Energy (eV) |
|---|---|---|
| A | 3.14 | 0.52 |
| B | 3.60 | 0.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuganathan, N.; Srikaran, R.; Fossati, P.C.M.; Chroneos, A. Theoretical Modeling of Defects, Dopants, and Diffusion in the Mineral Ilmenite. Minerals 2019, 9, 610. https://doi.org/10.3390/min9100610
Kuganathan N, Srikaran R, Fossati PCM, Chroneos A. Theoretical Modeling of Defects, Dopants, and Diffusion in the Mineral Ilmenite. Minerals. 2019; 9(10):610. https://doi.org/10.3390/min9100610
Chicago/Turabian StyleKuganathan, Navaratnarajah, Ratnasothy Srikaran, Paul C. M. Fossati, and Alexander Chroneos. 2019. "Theoretical Modeling of Defects, Dopants, and Diffusion in the Mineral Ilmenite" Minerals 9, no. 10: 610. https://doi.org/10.3390/min9100610
APA StyleKuganathan, N., Srikaran, R., Fossati, P. C. M., & Chroneos, A. (2019). Theoretical Modeling of Defects, Dopants, and Diffusion in the Mineral Ilmenite. Minerals, 9(10), 610. https://doi.org/10.3390/min9100610








