1. Introduction
Talc (Mg
3Si
4O
10(OH)
2) is a phyllosilicate mineral with a T‒O‒T layer composed of tetrahedral silicon and octahedral magnesium, which share oxygen and are strongly bonded with each other. The weak van der Waals bonds between the T‒O‒T layers is the origin of softness of talc. The silicon in the siloxane sheet renders talc hydrophobic, since it cannot easily be substituted with aluminum [
1]. Talc is physically easy to handle due to low hardness, does not react with acid due to its chemical stability, and has high adsorptivity, low plasticity, and low thermal/electrical conductivity. Because of these characteristics, talc is used as a coating, refractory, and additive in various industrial fields such as paper, paint, rubber, ceramic, refractory material, and polymer manufacturing. Chemical and pharmaceutical industries also require high-grade talc powders with high purity and uniform particle sizes. The grade and usage of talc is classified considering the purity, whiteness, particle size, and more. For example, talc with a particle size of 44 μm is used in ceramics and paints, 8–12 μm in paper, 7 μm in cosmetics, and 2 μm or smaller in rubber [
2]. In addition, the nanoscale talc recently received attention for its application in areas such as improving the heat resistance of nanocomposites, and for use in waste water filter materials [
3,
4,
5].
Given the importance of the above, various attempts have been made to produce ultrafine talc powders and better understand their physicochemical properties [
6,
7,
8,
9,
10,
11]. Various processes, such as planetary ball milling, tumbling milling, stirred ball milling, and disk milling, have been used to grind talc [
10,
12,
13,
14,
15,
16,
17]. A reduction in particle size and the collapse of the crystalline structure of talc have been observed in mechanical grinding studies [
8,
10,
12,
13,
15,
18,
19,
20].
Table 1 summarize the particle size and/or surface area of ground talc with processed various milling methods. Delamination of the layered structure in the direction of the (00
l) surface is the predominant mechanism of grinding, which, consequently, accompanies the breaking of individual plates and disordering in the talc crystal [
8,
10,
15,
21]. Further excessive grinding generally causes the collapse of the crystalline structure into the amorphous phase and the aggregation of ultrafine particles [
13,
14]. The degree of particle size reduction varies depending on the type of milling machine and milling conditions such as duration and rotating speed [
11,
21,
22,
23,
24,
25,
26]. Besides the collapse of the crystalline structure, the physicochemical properties of talc such as thermal and dispersion behaviors, cation exchange capacity, wettability, and whiteness are also changed by mechanical grinding [
6,
10,
12,
14,
27,
28,
29]. In accordance with the increasing demand for high-quality and ultrafine talc powder, the importance of comminution technology, capable of controlling crystallinity, particle shape, and particle size, is increasing.
Planetary ball milling, which is one of the most frequently used lab-scale milling tools, has been applied to increase the grinding efficiency of talc. The collision impact and friction between powder samples and balls in a rotating jar grind the microscale talc into sub-microscale particles. The grinding efficiency of ball milling is greatly affected by the jar rotation speed, ball size, and ratio of balls and sample talc [
22,
24,
25]. A faster rotation speed generally produces talc with a smaller particle size and lower crystallinity [
11]. Recently developed high-energy ball mills reach rotation speeds of up to 2000 rpm and provide an opportunity to reduce the size of talc powder to the nanoscale level. The high-energy ball mill system consists of turn discs and jars. The oppositely rotating jar and turning disc produce highly energetic impact energy (
Figure 1) [
30]. However, while the ball size is an important factor in ball milling, the effect of the ball size on the comminution of talc has not yet been fully understood [
31,
32]. While the particle size is known to generally increase with an increasing ball size, in 2013, Shin et al. showed that particle size decreased and then increased as the ball size increased from 1 to 10 mm during the comminution of Al
2O
3 powder at low rotation speeds (50–153 rpm) [
32]. The optimum ball size is dependent on the milling conditions and the properties of the mineral. Thus, to obtain ultrafine talc powder while minimizing the loss of crystallinity, a systematic approach is necessary to understand the effect of ball size in high-energy ball milling on the comminution of talc.
In this study, high-energy ball milling with high rotation speed of 2000 rpm is applied to obtain the nanoscale talc powder. The effect of the ball size for planetary ball milling on the grinding rate and behavior of talc is investigated with a varying ball size (2, 1, and 0.1 mm). Changes in the agglomeration characteristics of mechanochemical talc and the relationship with the ball size is also investigated.
3. Results
3.1. X-Ray Diffraction Analysis
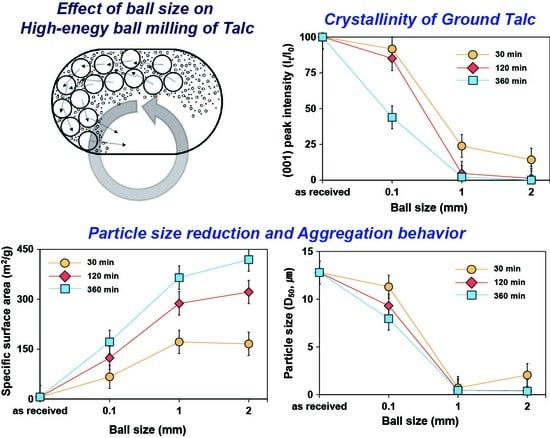
Figure 2 shows the XRD patterns of talc ground for up to 360 min using different ball sizes, which shows the effect of ball size on the crystallinity of ground talc.
In the as-received talc, (001), (002), and (003) peaks of talc with a narrow width were observed at 2θ angles of 9.4°, 18.9°, and 28.6°, respectively [
12]. The small peaks for chlorite and quartz were also observed, which were often found with talc as a result of carbonate alteration [
33]. Upon grinding, the X-ray diffraction peak intensity gradually decreased while the peak width increased, which implied the decrease in crystallinity of ground talc. The degree and rate of reduction in peak intensity varies with the size of the balls used in grinding. When 2 mm or 1 mm balls were used for grinding, the peak intensity of the talc markedly decreased until 60 min of grinding. After 120 min of grinding, most diffraction peaks disappeared when the 2 mm balls were used. When 1 mm balls were used, small diffraction peaks were still observed, even though their intensities are very small compared with prior to grinding. A further change in the diffraction pattern was not observed for grinding by more than 120 min. These results indicated that ground talc for 120 min and more has a disordered or amorphous structure when 2 mm or 1 mm balls were used for grinding. When 0.1 mm balls were used, the decrease in peak intensity was relatively sluggish. The peak intensity consistently decreased even after 120 min of grinding, which was in contrast with the cases using 2 mm or 1 mm balls. The unique diffraction pattern of talc was still apparent even after 360 min of grinding, which indicated a crystalline structure of talc left after grinding.
To quantitatively compare the effects of the ball size on the crystallinity of ground talc, the change in the relative height of the talc (001) peak, according to grinding, was analyzed (
Figure 3). When 2 mm balls were used, the peak height after 60 min of grinding decreased to approximately 9% compared with the height prior to grinding.
Almost no peak was observed after 120 min of grinding. Similar results were obtained when 1 mm balls were used, but, after 120 min, the peak height decreased slightly to less than that obtained using 2 mm balls. When 0.1 mm balls were used, the peak height showed a slight decrease, and approximately 44% remained even after 360 min of grinding. These results indicate that the crystallinity of talc decreases more when 2 mm and 1 mm balls are used than when 0.1 mm balls are used for milling. As the ball size increases, greater size reduction of the talc particle and loss of crystallinity occur during grinding. This is due to the reduced kinetic energy of collision between rotating balls in the mill with 0.1 mm balls when compared with 2 mm and 1 mm balls [
31,
32].
Figure 4 shows the variation in relative peak intensities (I
t/I
0) of talc’s main diffraction peaks such as (001), (132), (003), and (020) upon grinding, which shows the effect of the ball size on the direction in the fracture of talc. For 2 mm balls, the (001) and (003) lattice planes, which are perpendicular to the c-axis of talc, decreased faster than the (132) and (020) peaks. In particular, the decrease rate of (001) and (003) peaks was very high during the first 10 min and gradually slows at 60 min of grinding. The (001) and (003) peaks mostly disappeared after 120 min of grinding. In contrast, the decrease rate of the (132) and (020) peaks is relatively low and its peak intensities are approximately 20% after 360 min of grinding compared with that prior to grinding. The use of 1 mm balls yielded similar results to those obtained using 2 mm balls. The decrease rate of (001) and (003) peaks was very high in the early stage of grinding, and most of the peaks subsequently disappear. The (132) and (020) peak intensities are approximately 50% after 360 min of grinding when compared with that prior to grinding, which indicates less amorphization when using 1 mm balls than when using 2 mm balls.
In contrast, when 0.1 mm balls were used, the decrease rate of (132) and (020) peaks was higher than that of the (001) and (003) peaks, especially during the first 60 min of grinding. After 360 min of grinding, all peaks except the (020) peak reached approximately 40% compared with that prior to grinding. As the ball size increases, the reduction of peak intensity prior to grinding increases for most peaks. The delamination of (00l) planes, in particular, are significantly affected by the ball size. When 2 mm or 1 mm balls were used, the peak intensity of (00l) planes reduced to less than 5% of its state prior to grinding, while it only reduced to 40% when using 0.1 mm balls. The relative intensities of (020) plane after 360 min of grinding were approximately 20%, 55%, and 60% when using 2 mm, 1 mm, and 0.1 mm balls, respectively. These results indicate that the size reduction mechanism of talc changes with varying ball size. The contribution of delamination toward the decrease in particle size is high when 2 mm or 1 mm balls are used but low when 0.1 mm balls are used. The particle size reduction of talc occurred in all directions when using 0.1 mm balls.
3.2. Analysis of Specific Surface Area
Figure 5 shows the variation in the specific surface area of talc according to the ball size used in grinding.
As the grinding progresses, the increase in the specific surface area of talc is initially abrupt and then becomes gradual. The overall tendency of the increase in the specific surface area is similar for all ball sizes. However, the degree of increase varied according to the ball size. When 2 mm or 1 mm balls were used, the tendency was very similar until 60 min of grinding. The specific surface area was slightly higher when 1 mm balls were used than when 2 mm balls were used. However, since the grinding time is increased above 60 min, the specific surface area of the particles ground using 2 mm balls increased more toward the end of the grinding period. The specific surface area was approximately 6.1 m
2/g before grinding and increased to approximately 419.1 m
2/g for 2 mm balls and approximately 365.1 m
2/g for 1 mm balls after 360 min of grinding. The grinding efficiency when using 2 mm balls was slightly lower than when using 1 mm balls for the first 60 min of grinding, but prolonged grinding yielded a higher specific surface area when 2 mm balls were used. The 0.1 mm ball showed the lowest grinding efficiency, with a specific surface area of only 171.7 m
2/g even after 360 min of grinding. These results indicate that the use of 1 mm and 2 mm balls in grinding talc could yield a larger specific surface area than the use of 0.1 mm balls could. In planetary ball milling, the proper ball size could maximize the grinding efficiency with a balance of aggregation and particle size reduction [
32]. Our results indicate that the specific surface area eventually increases when the 2 mm balls are used for grinding, even though it is not clearly observed during the early stage of grinding.
The estimated spherical diameters (ESDs) were calculated using the equation D = 6/ρS, where ρ is the density of talc (2.8 g/m
3),
S is the specific surface area (m
2·g
−1), and the constant 6 is the shape factor assuming that the talc particles are spherical. The results are listed in
Table 2.
The powder had ESD of approximately 350 nm prior to grinding, which decreased to 5.1, 5.9, and 12.5 nm after 360 min of grinding using 2 mm, 1 mm, and 0.1 mm balls, respectively. These are much lower ESDs of talc than previously reported, as shown in
Table 1 [
10,
14]. Data from previous reports listed in
Table 1 were generally acquired under dry conditions, unlike the wet milling performed in this study.
We also obtained ground talc series with a similar specific surface area, even though the ball size varied. Similar specific surface areas of 81.1, 81.5, and 87.7 m2/g (approximately 85 m2/g) were obtained after 10 min of grinding with 2 mm and 1 mm balls and after 60 min of grinding with 0.1 mm balls, respectively. Furthermore, similar specific surface areas of 165.8, 171.6, and 171.7 m2/g (approximately 170 m2/g) were obtained after 30 min of grinding with 2 mm and 1 mm balls and after 360 min of grinding with 0.1 mm balls, respectively. These two series with a similar specific surface area will be discussed below.
3.3. Analysis of Particle Size by the Laser Diffraction Method
Figure 6 shows the evolution of the talc’s particle size upon grinding, as measured by the laser diffraction particle size analysis.
The as-received talc shows a single distribution with a mean particle size of approximately 12 μm. With an increase in grinding time, the particle size generally decreased. However, the minimum particle size and reduction rate varied according to the ball size. When 2 mm and 1 mm balls were used, the particle size rapidly decreased to less than 1 μm for the first 60 min of grinding and did not change much after 120 min. However, when 0.1 mm balls were used, the particle size gradually decreases until 360 min of grinding, but the changes are relatively insignificant compared with the changes when using 1 mm and 2 mm balls.
The distribution pattern of particle sizes also varies upon grinding. The particle size of as-received talc shows a monomodal distribution ranging from ~3 to ~50 μm with a center at ~10 μm. As the grinding proceeds, an increase in submicron particles appeared in addition to the existing predominately micron-sized particles of approximately ~10 μm, which forms a bimodal distribution. In this case, we refer to the two distribution groups as a microscale group and a sub-microscale group. When using 1 mm and 2 mm balls, the sub-microscale group appears even after 10 min of grinding and its population gradually increases until 120 min of grinding at the expense of the microscale group. The mean particle size of the microscale group gradually decreases from ~10 to ~2 μm as the grinding proceeds for up to 60 min. The microscale group is not observed after 120 min of grinding, which indicates that talc powders are fully pulverized into sub-microscale after 120 min of grinding. However, re-aggregation occurred in some samples, and, thus, small amounts of ~4 and ~20 μm particles were observed after 120 min of grinding. When using 0.1 mm balls, the population of the microscale group does not show a significant reduction even after 360 min of grinding and the population of the sub-microscale group is relatively insignificant when compared to the results when using 1 mm and 2 mm balls. We note that dispersion of the ground talc may be incomplete in aqueous solution and the sizes of the aggregated particles can be measured by the laser diffraction method.
Figure 7 shows the change in the D
50, which corresponds to 50% of the cumulative volume of the particle size distribution, upon grinding, according to the ball size.
When 2 mm balls were used, D50 decreased sharply to approximately 0.5 μm after 60 min of grinding, but did not show significant changes until 360 min. Similarly, when 1 mm balls were used, D50 decreased to approximately 0.7 μm in the first 30 min of grinding, but it did not change significantly after that. These results show that the particle size rarely decrease after approximately 60 min of grinding, which indicates that it reached the grinding limit under the grinding condition employed in this study. Until 60 min of grinding, the grinding efficiency of the 2 mm balls was slightly lower than that of the 1 mm balls. However, beyond this time frame, the D50 was smaller and the particle size distribution was narrower. In contrast, when 0.1 mm balls were used, the particle size steadily decreased until 360 min from the beginning of grinding. The final D50 was approximately 7.9 μm, which is approximately 7 μm larger than that obtained using 2 mm or 1 mm balls. These results indicate that, when 0.1 mm balls are used, the pulverization rate is slow and showed a lower grinding efficiency compared with the 2 mm and 1 mm balls.
3.4. Aggregation of Ground Talc
Laser diffraction particle size analysis and BET-specific surface area analysis are general methods to analyze the changes in particle size upon grinding. Though the calculated ESD based on the BET-specific surface area is much smaller than the results of particle size analysis because the shape of phyllosilicate talc and the increase in specific surface area generally indicates the decrease in particle size. According to the laser diffraction particle size analysis, the particle size of talc ground using 2 mm and 1 mm balls significantly decreased until 60 min of grinding, and further size reduction was not subsequently observed. On the contrary, BET-specific surface area continuously increases after 60 min of grinding. In addition, the significant difference in a specific surface area between talc powders ground using 2 mm and 1 mm balls is observed while the result of the particle size analysis shows only a trivial difference. These differences between the laser diffraction particle size and surface area analysis can appear when the ultrafine talc particles are aggregated by grinding [
12]. In the BET-specific surface area analysis, there is a rare effect of aggregation because the N
2 gas is adsorbed to the particle surface. However, in the particle size analysis using laser diffraction, the size of aggregates can be measured rather than the individual particles. These results indicate that aggregation of ultrafine talc powder occurred as a result of grinding.
The aggregation of ground talc particles can be observed in SEM images of the talc particles grounded using 1 mm balls (
Figure 8).
Before grinding, a unique laminar structure of talc is clearly observed with a size of tens of micrometers. With increasing grinding time, the particle size decreases to approximately 5 μm after 30 min, less than approximately 1 μm after 240 min, and approximately 200–300 nm after 360 min of grinding. The aggregation of ultrafine particles is clearly observed after 240 min of grinding, which is consistent with results of the laser diffraction particle size and BET-specific surface area analysis.
In order to understand the effect of ball size on the aggregation of ground talc,
Figure 9 compares the particle size analysis results of talc powders with similar specific surface areas. As described above, we obtain the ground talc series that have a similar specific surface area of talc particles that are approximately 85 m
2/g and 170 m
2/g. The laser diffraction particle size of the ground talc using 2 mm and 1 mm balls showed similar particle size distribution when compared with the case of using 0.1 mm balls. In both series, the intensity of the sub-microscale group was larger when using 2 mm and 1 mm balls than when using 0.1 mm balls, which indicates a larger particle size when using 0.1 mm balls.
The different particle size distributions with similar surface area could be observed if the aggregation of ultrafine particles occurred as a result of grinding. The larger particle size distribution indicates the more aggregated status of talc powder. In this study, the ground talc powder using 0.1 mm balls shows a larger particle size than other talc powders with similar surface areas, which indicates that it is easily aggregated due to grinding.
The increased aggregation of ground talc with 0.1 mm balls than with 1 mm or 2 mm balls could be related to the degree of dispersion in the aqueous solution used in the particle size analysis. The aggregation of ultrafine particles generally occurs in order to reduce the high surface energy [
34,
35]. The talc powders with a similar surface area and different aggregation behavior indicate that these ground talc powders have a different surface energy and/or property. The results of XRD analysis showed that different crystallinity of ground talc powder depends on ball size. The crystallinity of ground talc is higher when using 0.1 mm balls than when using 1 mm and 2 mm balls. Crystalline talc is hydrophobic due to the siloxane sheet of the T‒O‒T layer. Hence, it aggregates well in aqueous solution [
1,
36]. On the other hand, disordered talc with lower crystallinity due to grinding is delaminated. Breakage of the layers exposes the hydroxyl groups in the octahedral layer. As a result, the talc is less hydrophobic due to the loss of crystallinity [
8,
37,
38]. In an aqueous solution, the more hydrophilic talc is better dispersed.
As shown in
Figure 3, the crystallinity of ground talc prepared using 0.1 mm balls during grinding is higher than that using 1 mm and 2 mm balls even though these talc powders have similar surface areas. The ground talc with relatively higher crystallinity (i.e., when using 0.1 mm balls for grinding) has higher surface energy than other ground talc powders (i.e., when using 1 mm and 2 mm balls for grinding). Thus, increased aggregation could occur in ground talc using 0.1 mm balls due to the loss of crystallinity and hydrophobicity.
5. Conclusions
In this study, talc was ground at a fast rotation speed of 2000 rpm using a high-energy ball mill, and ultrafine talc particles were obtained. Furthermore, the effects of ball size on the grinding efficiency and crystallinity of talc were investigated. When a larger ball size was used in ball milling, the crystallinity decreased faster, and the grinding efficiency and the specific surface area of the talc increased. In the case of crystallinity, the smaller the ball size was, the smaller the decrease in crystallinity was. Therefore, using a small ball size can produce talc nanoparticles with higher crystallinity. Upon the progress of milling, the aggregation of talc particles occurs and the degree of aggregation shows the relations with crystallinity of talc. Comparing the ground talc with similar specific surface areas, the more disordered the structure of the ground talc is, the fewer aggregation and more hydrophilic properties are observed.