Fluid Inclusion and Oxygen Isotope Characteristics of Vein Quartz Associated with the Nabeba Iron Deposit, Republic of Congo: Implications for the Enrichment of Hypogene Ores
Abstract
1. Introduction
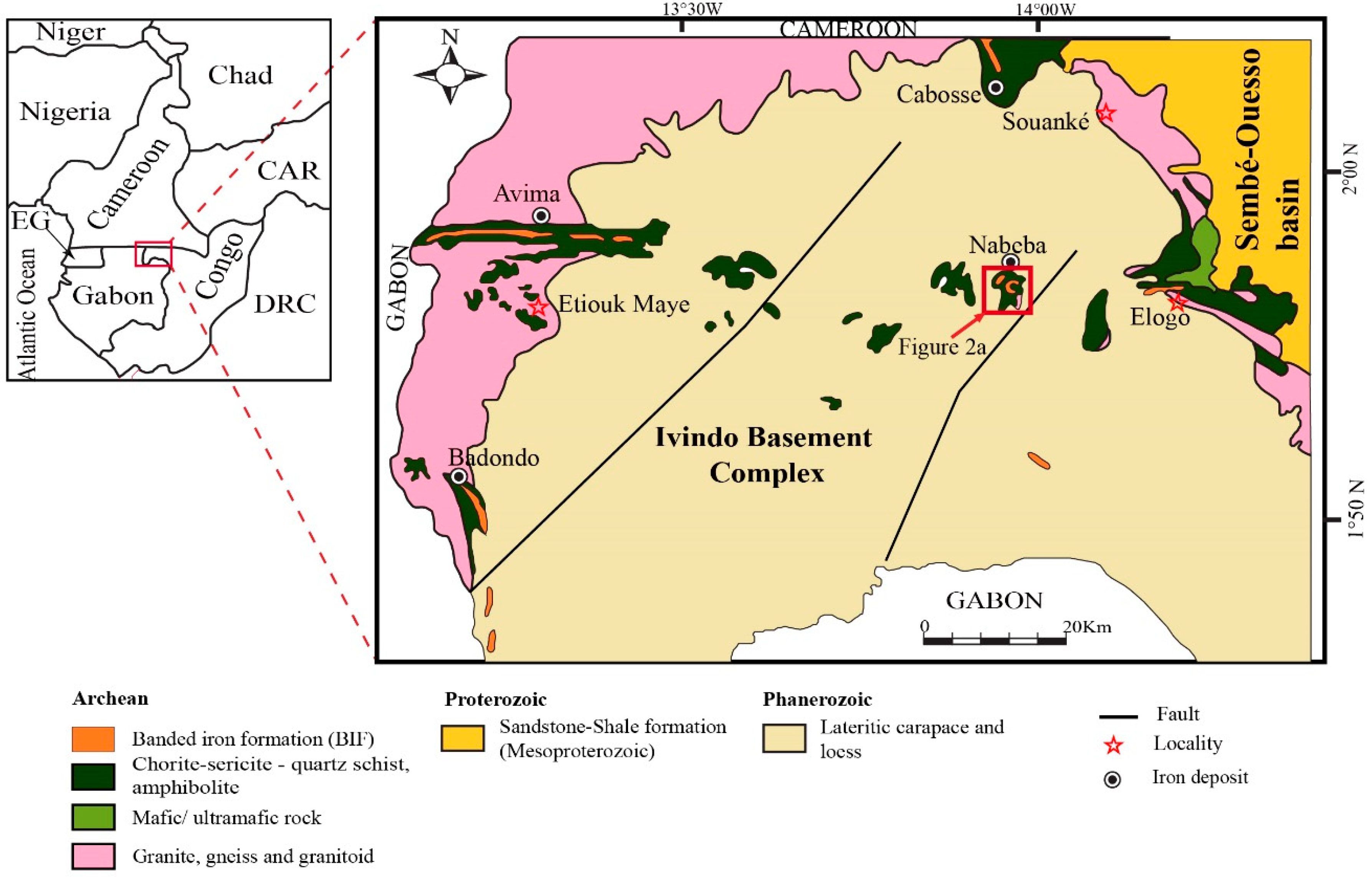
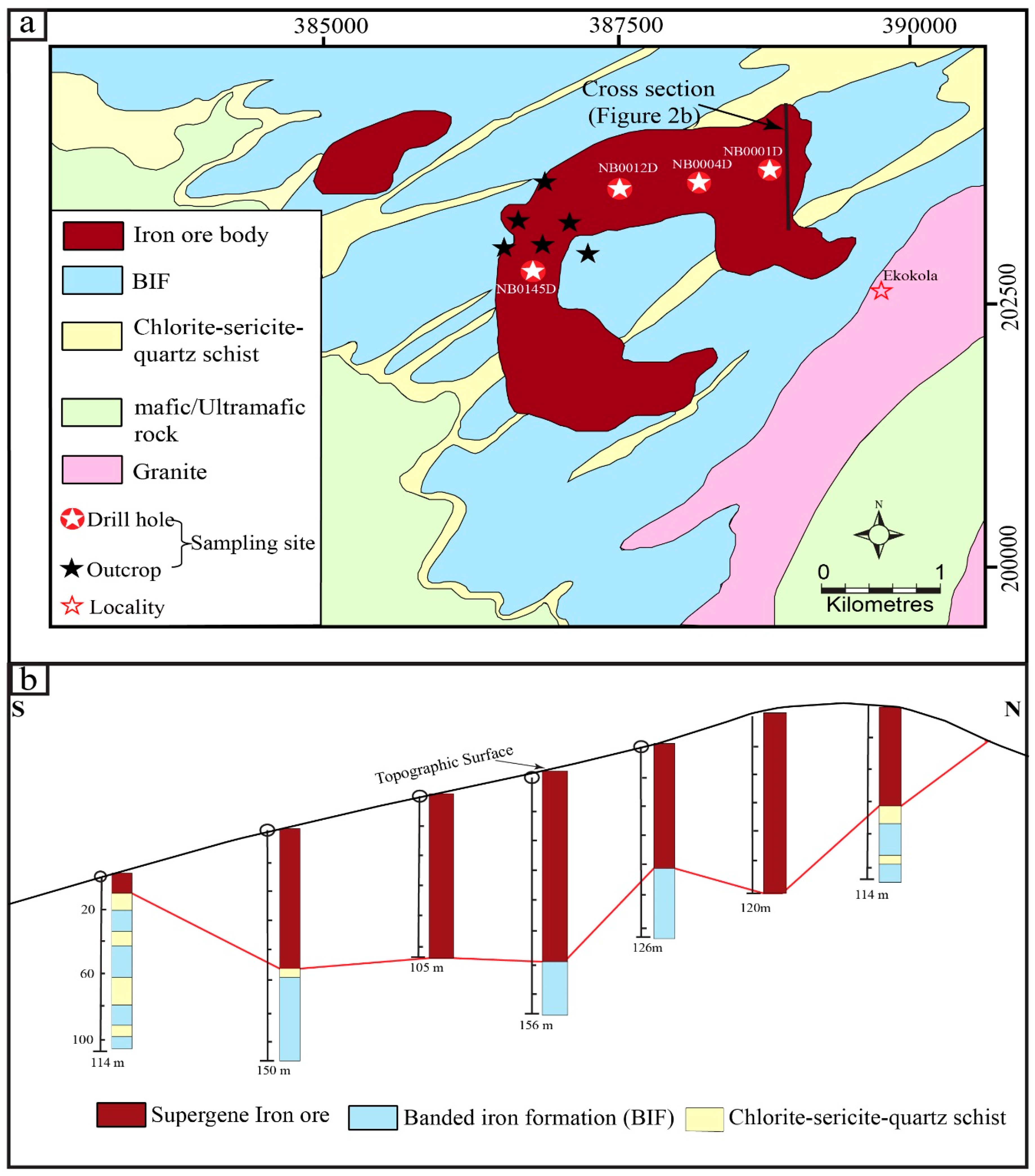
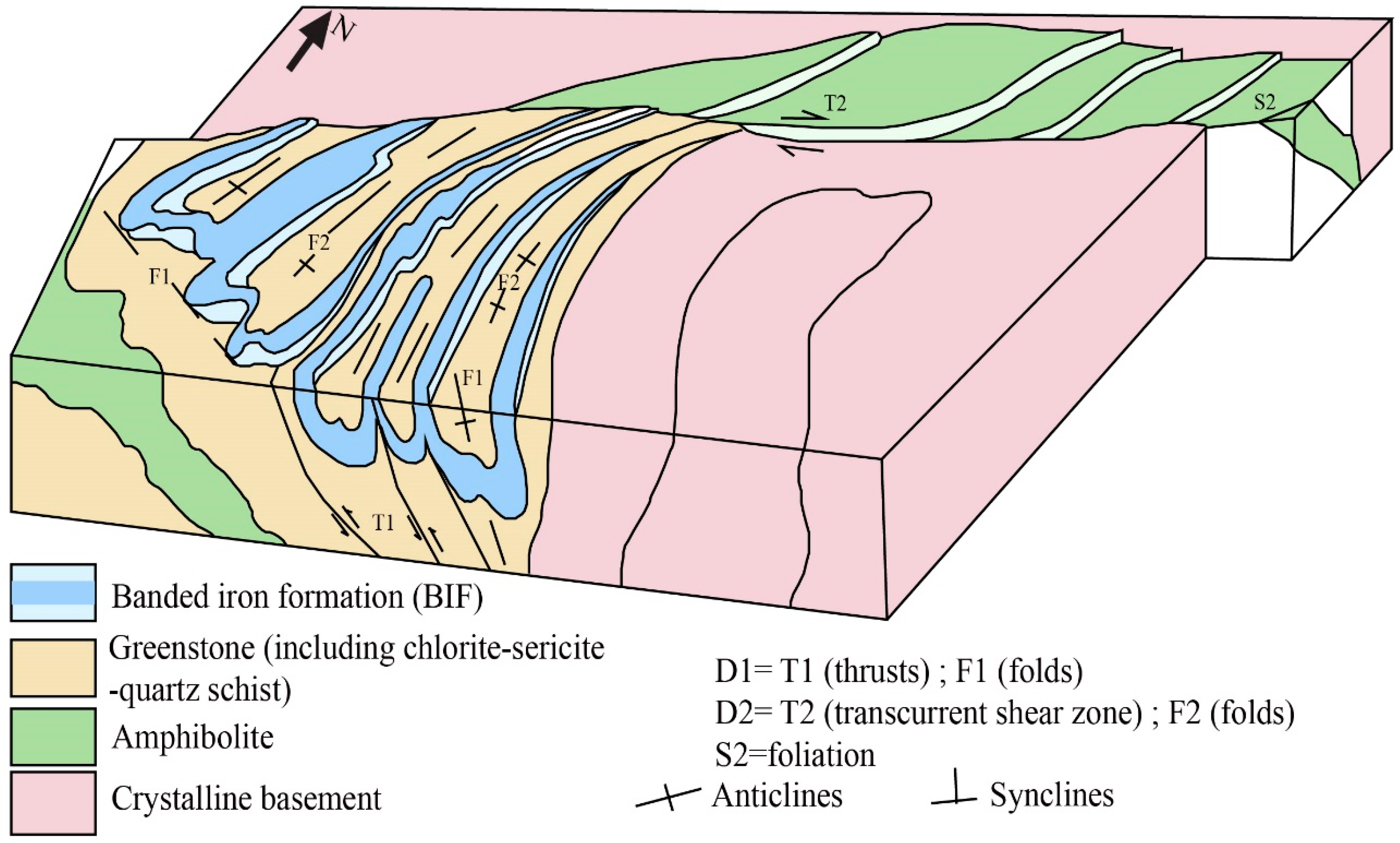
2. Geological Setting
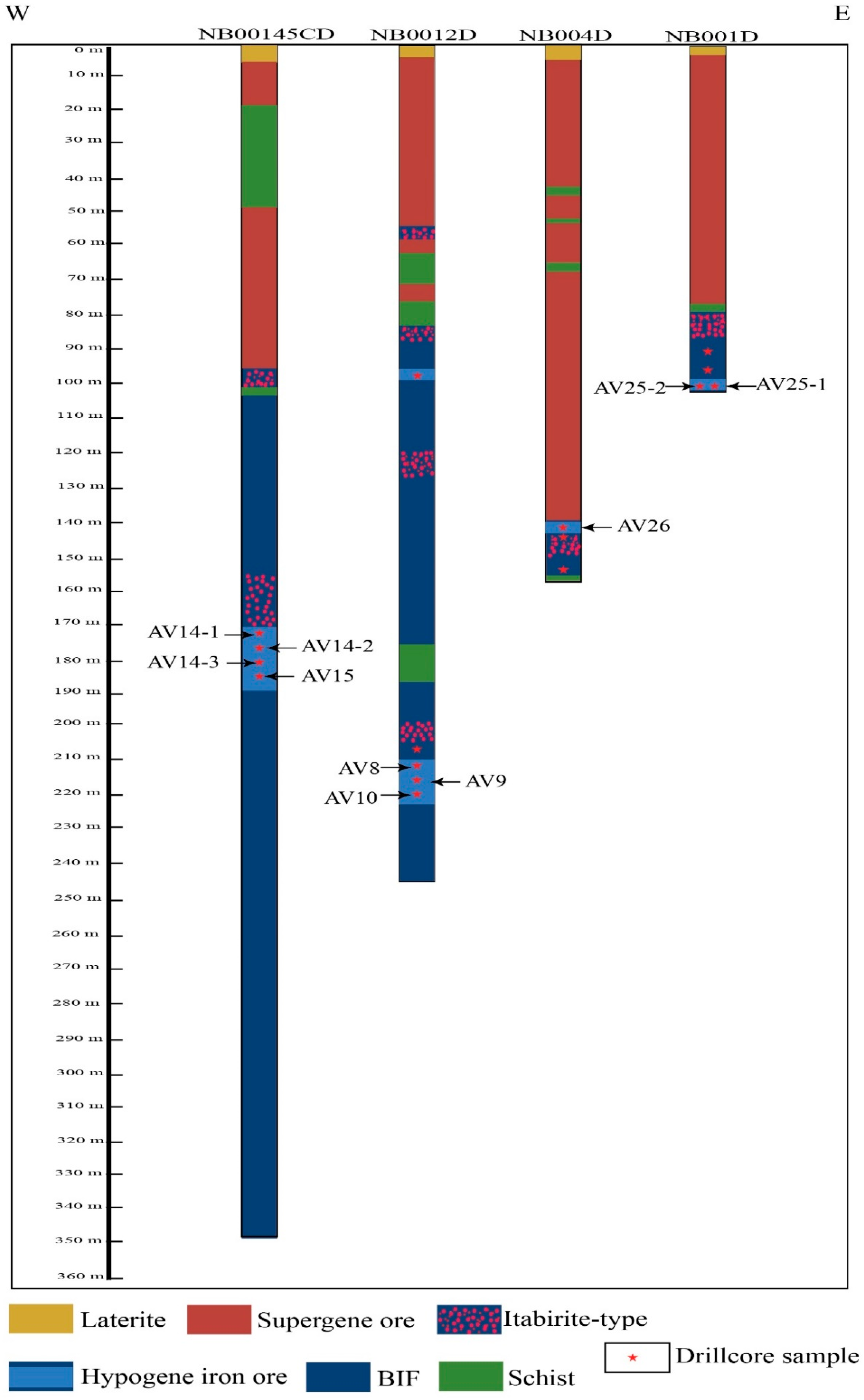
3. Sampling and Analytical Methods
4. Results
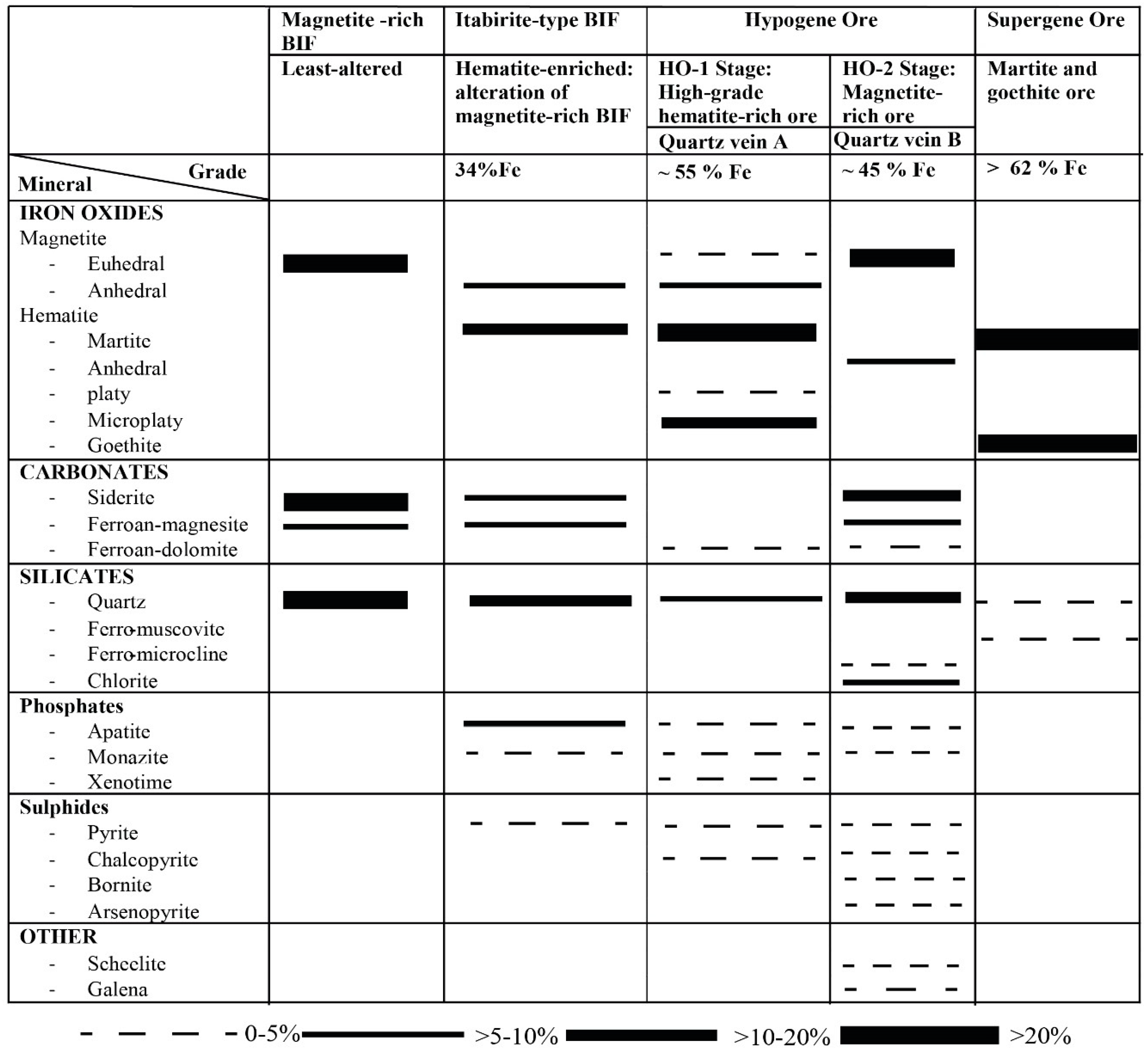
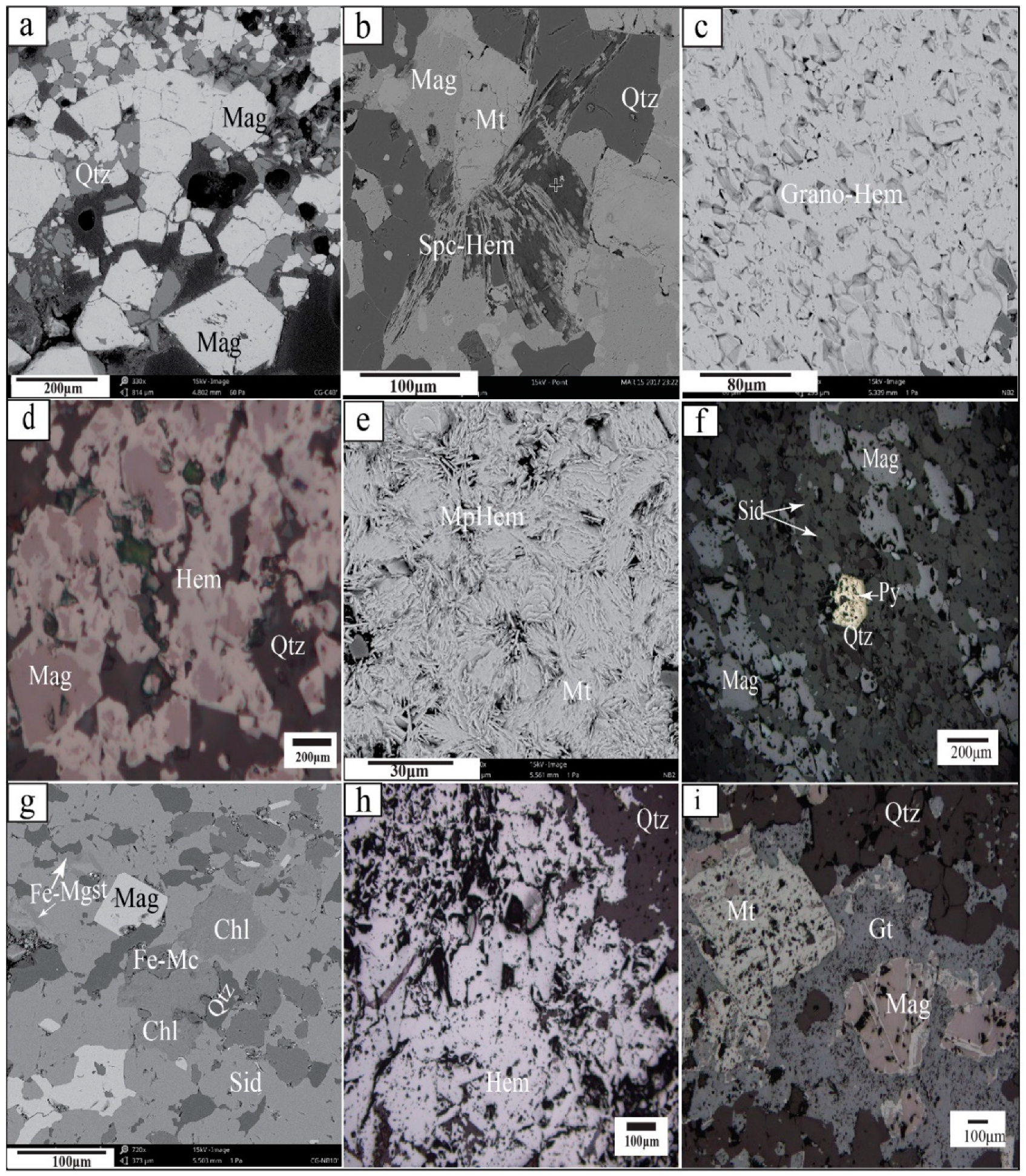
4.1. Ore Mineral Parageneses
4.2. Quartz Veins Associated with Nabeba Hypogene Iron Ore
4.3. Fluid Inclusion Study
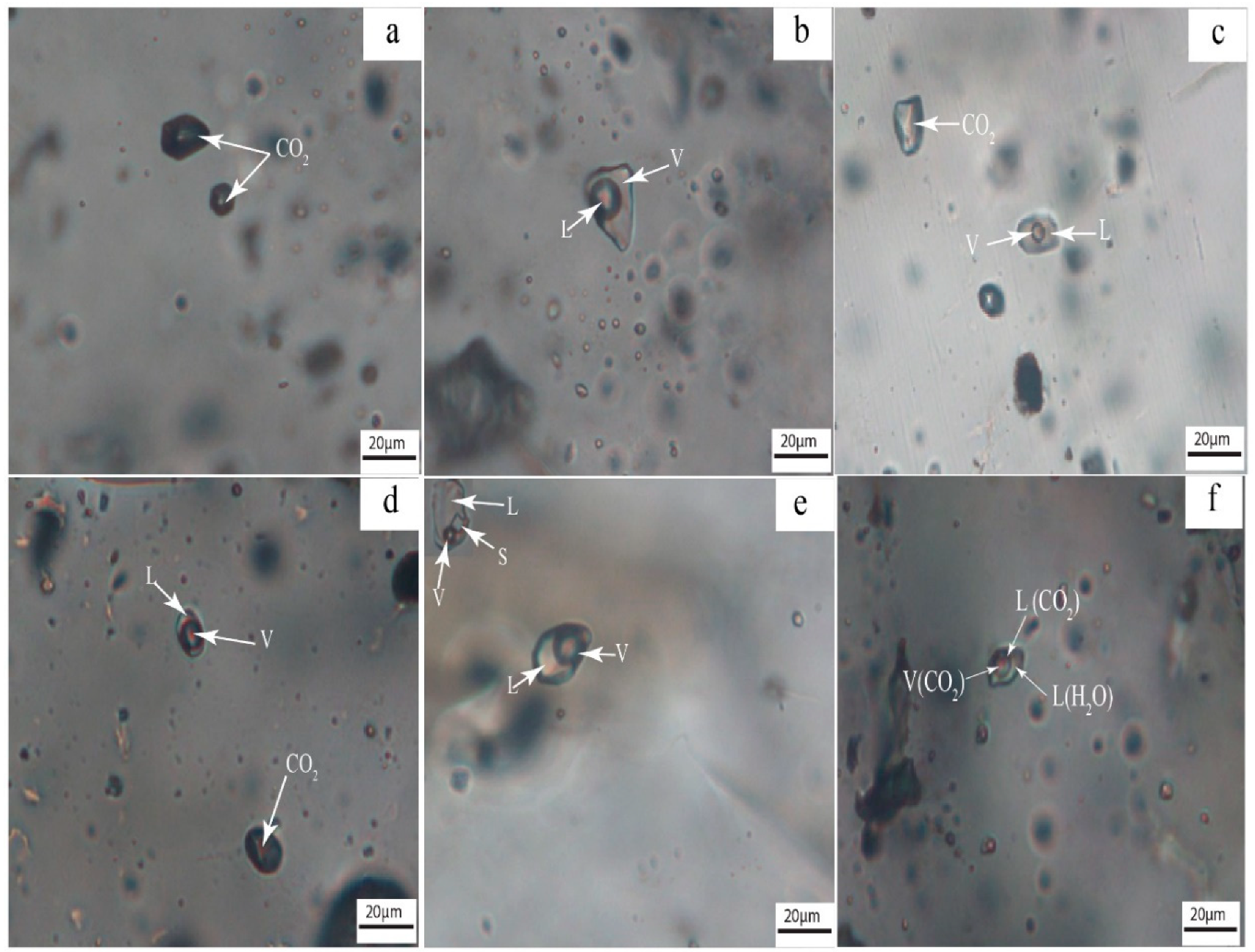
4.3.1. Fluid Inclusion Petrography
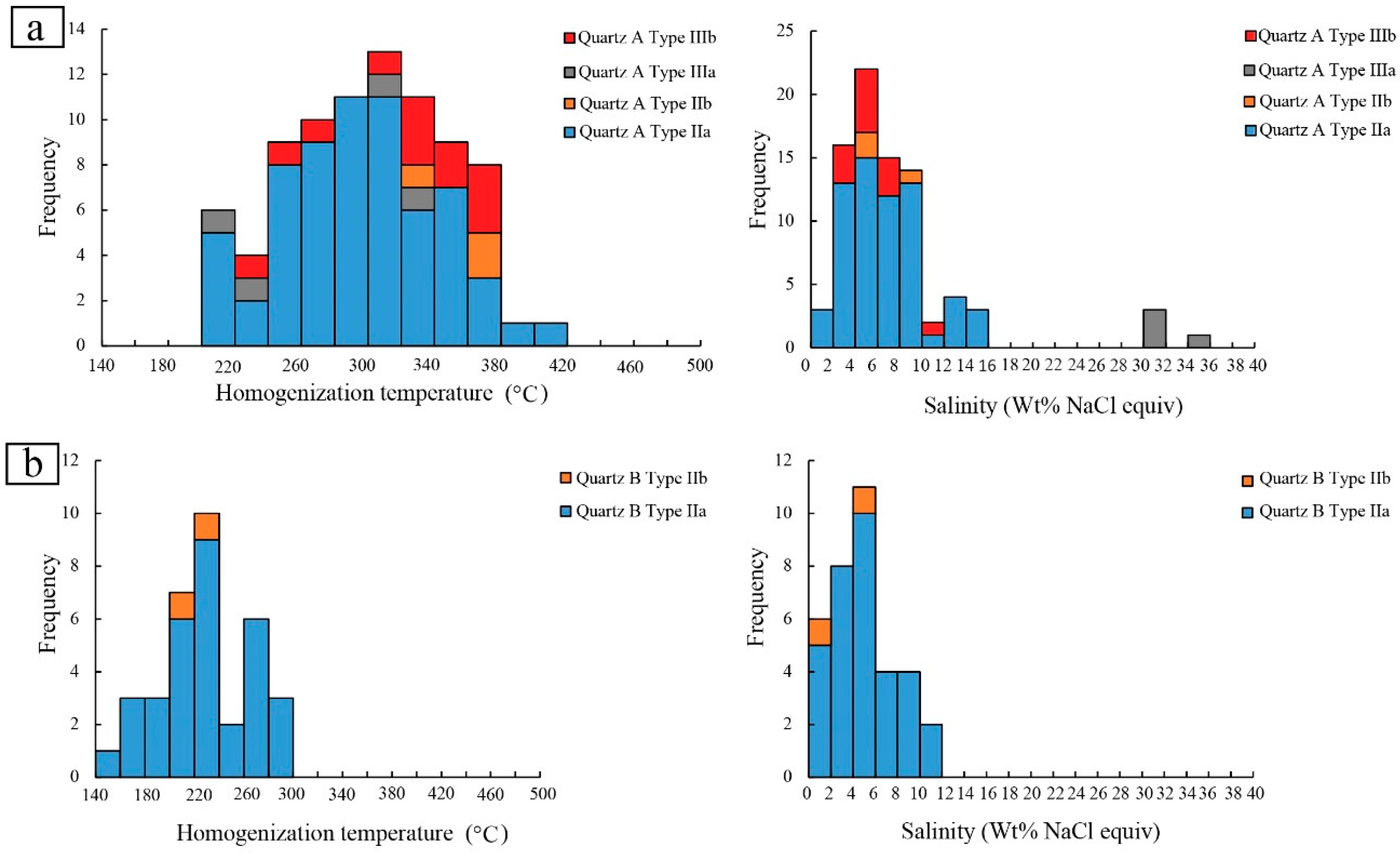
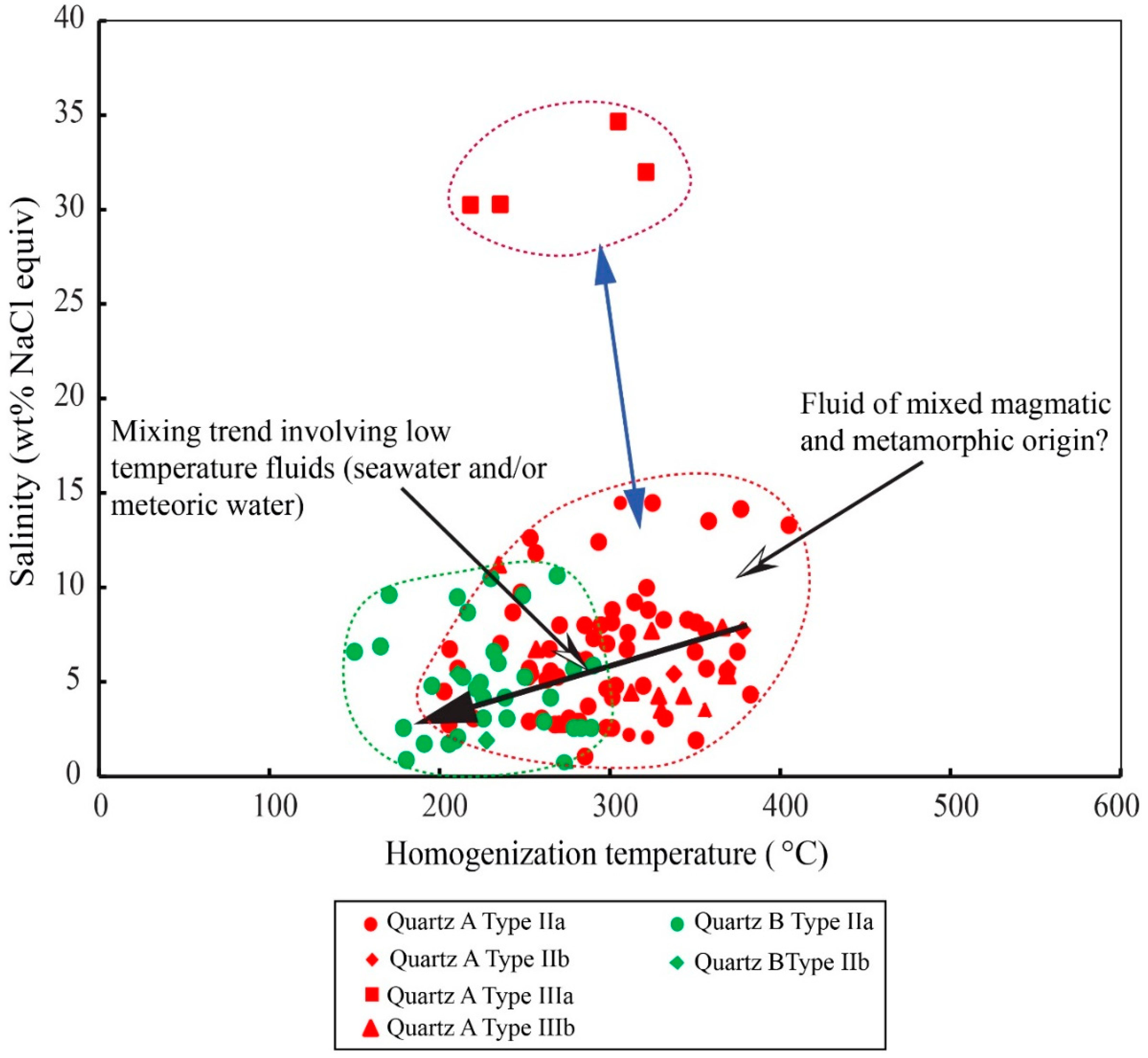
4.3.2. Microthermometry and Raman Spectrometry
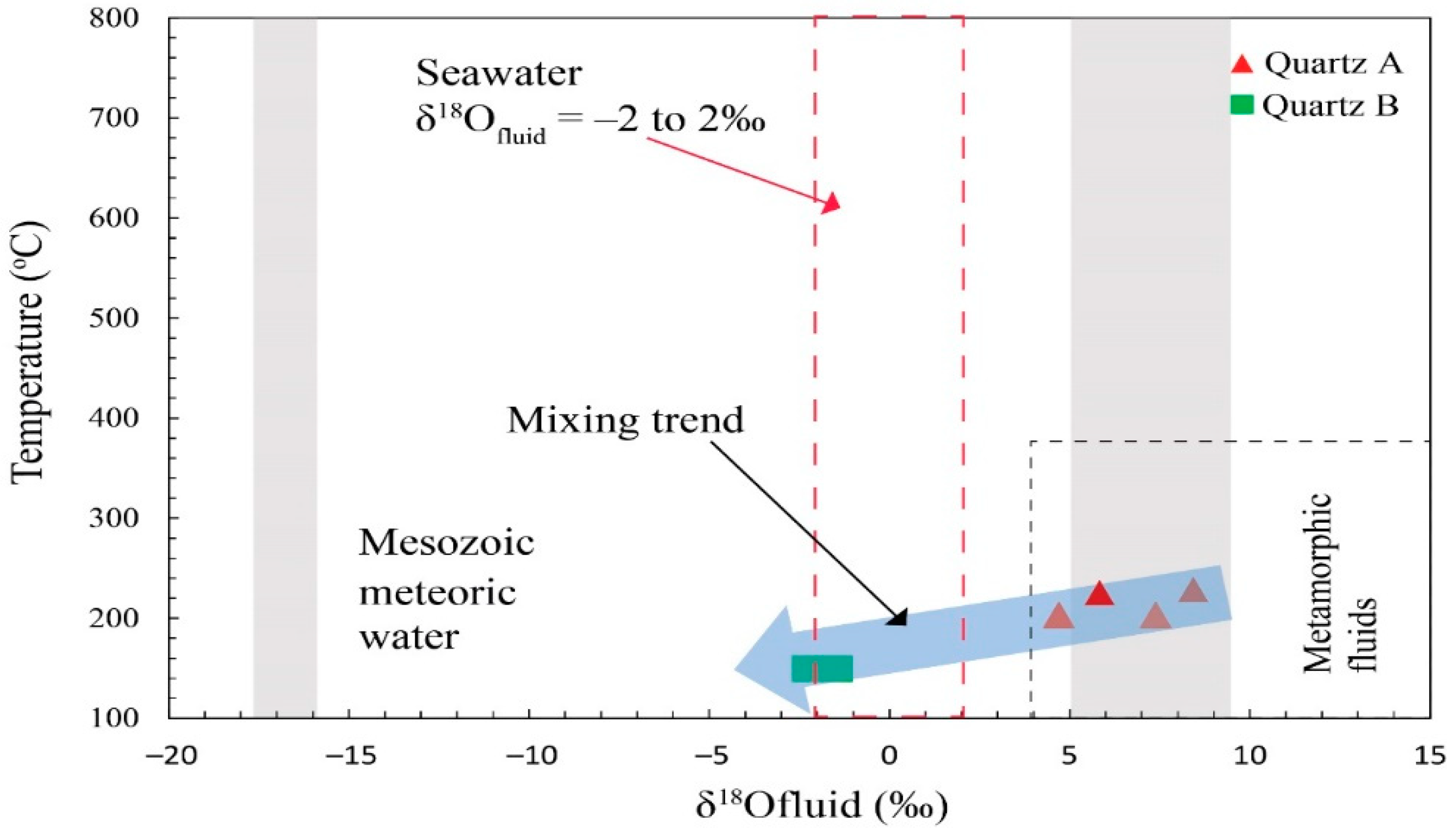
4.4. Oxygen Isotopes
5. Discussion
5.1. Trapping Mechanism and Implications for Temperature, Pressure, and depth Conditions of Hypogene Iron Ore Mineralization
5.2. Nature and Origin of the Hypogene Ore-Forming Fluids
5.3. Comparison with Other BIF-Hosted Iron Ore Deposits.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duuring, P.; Hagemann, S.G.; Novikova, Y.; Cudahy, T.; Laukamp, C. Targeting iron ore in banded iron formations using ASTER data: Weld range Greenstone Belt, Yilgarn Craton, Western Australia. Econ. Geol. 2012, 107, 585–597. [Google Scholar] [CrossRef]
- Hagemann, S.G. The role of hydrothermal fluids in the formation of high grade BIF-hosted iron ore deposits. In Proceedings of the ECROFI XXIII, Leeds, UK, 27–29 June 2015; p. 20. [Google Scholar]
- Hagemann, S.G.; Barley, M.E.; Folkert, S.L.; Yardley, B.W.D. A hydrothermal origin for the Giant BIF-Hosted Tom Price Iron Ore Deposit. In Mineral Deposits, Processes to Processing; Stanley, C.J., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 1999; pp. 41–44. [Google Scholar]
- Taylor, D.; Dalstra, H.J.; Harding, A.E.; Broadbent, G.C.; Barley, M.E.; Barley, M. Genesis of high-grade hematite orebodies of the Hamersley Province, Western Australia. Econ. Geol. 2001, 96, 837–873. [Google Scholar] [CrossRef]
- Thorne, W.S.; Hagemann, S.G.; Barley, M.E. Petrographic and geochemical evidence for hydrothermal evolution of the North Deposit, Mt Tom Price, Western Australia. Miner. Deposita 2004, 39, 766–783. [Google Scholar] [CrossRef]
- Silva, R.F.E.; Lobato, L.M.; Rosiere, C.; Hagemann, S.; Zucchetti, M.; Baars, F.; Morais, R.; Andrade, I. A hydrothermal origin for the Jaspilite-hosted, giant serra norte iron ore deposits in the Carajas mineral province, Para state, Brazil. In Banded Iron Formation-Related High-Grade Iron Ore; Society of Economic Geologists: Littleton, CO, USA, 2008; pp. 255–289. [Google Scholar]
- Figueiredo e Silva, R.C.; Hagemann, S.; Lobato, L.M.; Rosière, C.A.; Banks, D.A.; Davidson, G.J.; Vennemann, T.; Hergt, J. Hydrothermal fluid processes and evolution of the giant Serra Norte jaspilite-hosted iron ore deposits, Carajás Mineral Province, Brazil. Econ. Geol. 2013, 108, 739–779. [Google Scholar] [CrossRef]
- Longley, R.; Kitto, P.; Thornett, S. Iron Ore, Exploration History of the Mbalam Iron Ore Project; Sundance Resources Ltd.: Perth, Australia, 2013; Volume 80. [Google Scholar]
- Thornett, S.; Longley, R.; Robertson, I.; Meehan, D. Mbalam-Nabeba Iron Ore Project; 100-RG-GO-0009; Sundance Resources Ltd.: Perth, Australia, 2013; Volume 479, Unpublished. [Google Scholar]
- Gatsé Ebotehouna, C.; Issambo Dom, S. Geological Knowledge of the Iron Formation of the Nabeba Iron Deposit; Northwestern Republic of Congo: Brazzaville, Republic of Congo, 2016; Unpublished. [Google Scholar]
- Ministère des mines et de l’énergie; Direction Générale des mines. Carte géologique de la Republique du Congo au 1: 1 000 000; Ministère des mines et de l’energie: Brazzaville, Republic of Congo, 1995.
- Desthieux, F. Notice Explicative de la Carte Géologique de la République du Congo au 1/1.000. 000; Ministère des mines et de l’énergie and Direction Générale des Mines: Brazzaville, Congo, 1993; 27p. [Google Scholar]
- Meloux, J.; Bigot, M.; Viland, J. Plan Minéral de la République Populaire du Congo; BRGM: Orléans, France, 1986. [Google Scholar]
- Goldstein, H. Systematics of Fluid Inclusions in Diagenetic Minerals. SEPM Short Course 1994, 31, 199. [Google Scholar]
- Steele-MacInnis, M.; Lecumberri-Sanchez, P.; Bodnar, R.J. Short note: HokieFlincs_H2O-NaCl: A Microsoft Excel spreadsheet for interpreting microthermometric data from fluid inclusions based on the PVTX properties of H2O-NaCl. Comput. Geosci. 2012, 49, 334–337. [Google Scholar] [CrossRef]
- Steele-MacInnis, M. Fluid inclusions in the system H2O-NaCl-CO2: An algorithm to determine composition, density and isochore. Chem. Geol. 2018, 498, 31–44. [Google Scholar] [CrossRef]
- Hansteen, T.H.; Klugel, A. Fluid inclusion thermobarometry as a tracer for magmatic processes. Rev. Mineral. Geochem. 2008, 69, 143–177. [Google Scholar] [CrossRef]
- Cadusch, P.; Hlaing, M.; Wade, S.; McArthur, S.; Stoddart, P. Improved methods for fluorescence background subtraction from Raman spectra. J. Raman Spectrosc. 2013, 44, 1587–1595. [Google Scholar] [CrossRef]
- Clayton, R.N.; O’Neil, J.R.; Mayeda, T.K. Oxygen isotope exchange between quartz and water. J. Geophys. Res. 1972, 77, 3057–3067. [Google Scholar] [CrossRef]
- Roedder, E. Fluid Inclusions. Rev. Mineral. 1984, 12, 12–45. [Google Scholar]
- Shepherd, T.J.; Rankin, A.H.; Alderton, D.H.M. A Practical Guide to Fluid Inclusion Studies; Blackie & Son: Glasgow/London, UK, 1985; p. 240. [Google Scholar]
- Roedder, E. Natural occurrence and significance of fluids indicating high pressure and temperature. Phys. Chem. Earth 1981, 13, 9–39. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, F.-F.; Li, B.-C. Genesis of the Yandong porphyry Cu deposit in eastern Tianshan, NW China: Evidence from geology, fluid inclusions and isotope systematics. Ore Geol. Rev. 2017, 86, 280–296. [Google Scholar] [CrossRef]
- Taylor, H. The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposition. Econ. Geol. 1974, 69, 843–883. [Google Scholar] [CrossRef]
- Duuring, P.; Hagemann, S.G.; Banks, D.A.; Schindler, C. A synvolcanic origin for magnetite-rich orebodies hosted by BIF in the Weld Range District, Western Australia. Ore Geol. Rev. 2018, 93, 211–254. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Zhang, D.; Fu, X.; Bao, X.; Li, H.; Ai, Y. Ore Controlling Factors and Ore-Prospecting Models for Copper-Polymetallic Deposits in Southeast Inner Mongolia; Seismological Press: Beijing, China, 1994. [Google Scholar]
- Pope, E.C.; Bird, D.K.; Rosing, M.T. Isotope composition and volume of Earth’s early oceans. Proc. Natl. Acad. Sci. USA 2012, 109, 4371–4376. [Google Scholar] [CrossRef]
- Roedder, E.; Bodnar, R. Geologic pressure determinations from fluid inclusion studies. Annu. Rev. Earth Planet. Sci. 1980, 8, 263–301. [Google Scholar] [CrossRef]
- Driesner, T.; Heinrich, C.A. The system H2O–NaCl. Part I: Correlation formulae for phase relations in temperature–pressure–composition space from 0 to 1000 C, 0 to 5000 bar, and 0 to 1 XNaCl. Geochim. Cosmochim. Acta 2007, 71, 4880–4901. [Google Scholar] [CrossRef]
- Wilkinson, J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Baker, T.; Lang, J.R. Reconciling fluid inclusion types, fluid processes, and fluid sources in skarns: An example from the Bismark Deposit, Mexico. Miner. Deposita 2003, 38, 474–495. [Google Scholar] [CrossRef]
- Sośnicka, M.; Bakker, R.J.; Broman, C.; Pitcairn, I.; Paranko, I.; Burlinson, K. Fluid types and their geneticmeaning for the BIF-hosted iron ores, Krivoy Rog, Ukraine. Ore Geol. Rev. 2015, 68, 171–194. [Google Scholar] [CrossRef]
- Thorne, W.S.; Hagemann, S.G.; Sepe, D.; Dalstra, H.J.; Banks, D.A. Structural control, hydrothermal alteration zonation, and fluid chemistry of the concealed, high-grade 4EE iron orebody at the Paraburdoo 4E Deposit, Hamersley Province, Western Australia. Econ. Geol. 2014, 109, 1529–1562. [Google Scholar] [CrossRef]





| Stage | Samples | F.I Type (*n) | Size (μm) | Tm_ice (°C) | Tm_CO2 | Th_CO2 | Th _Total (°C) | Tm_Clathrate | Ts_Halite (°C) | Salinity (wt. % NaCl Equiv.) | Bulk Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HO-1 stage | Quartz vein A associated with hypogene hematite-rich ore (Samples AV8, AV9, AV10, AV14-1, AV14-2, AV14-3, AV15) | Type I (9) | 5–10 | −60.4 to −56.7 | 18.3 to 30.7 | 0.55 to 0.79 | |||||
| Type IIa Aqueous (64) | 6–22 | −10.6 to −0.6 | 203 to 405 | 1 to 15 | 0.59 to 0.98 | ||||||
| Type IIb Aqueous (3) | 7.5–15.8 | −4.9 to −3.3 | 338 to 378 | 5.4 to 7.7 | 0.64 to 0.70 | ||||||
| Type IIIa Aqueous (4) | 9–12 | 210 to 321 | 165 to 250 | 30 to 35 | 1.01 to 1.10 | ||||||
| Type IIIb Aqueous-carbonic (12) | 7–13.5 | −59.9 to −56.7 | 14.2 to 29.9 | 235 to 369 | 3.5 to 8.6 | 2.8 to 11.2 | 0.89 to 1.04 | ||||
| HO-2 stage | Quartz vein B associated with hypogene magnetite-rich ore (Samples AV25-1, AV25-2, AV26) | Type IIa Aqueous (33) | 5–13.9 | −7.1 to −0.4 | 150 to 290 | 0.7 to 10.6 | 0.76 to 0.97 | ||||
| Type IIb Aqueous (2) | 6–8 | −3.3 to −1.1 | 211 to 228 | 1.9 to 5.4 | 0.85 to 0.90 |
| Sample Number | Sample Description | δ18OV-SMOW (‰) | Ttmin | δ18Ofluid (‰) |
|---|---|---|---|---|
| CG-NB_AV25 | Quartz vein B, HO-2 stage | 13.2 | 150 | −2.3 |
| CG-NB_AV26 | Quartz vein B, HO-2 stage | 14 | 150 | −1.5 |
| CG-NB_AV8 | Quartz vein A, HO-1 stage | 18.9 | 203–215 | 7.4–8.1 |
| CG- NB_AV15 | Quartz vein A, HO-1 stage | 16.2 | 203–215 | 4.7–5.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatsé Ebotehouna, C.; Xie, Y.; Adomako-Ansah, K.; Pei, L. Fluid Inclusion and Oxygen Isotope Characteristics of Vein Quartz Associated with the Nabeba Iron Deposit, Republic of Congo: Implications for the Enrichment of Hypogene Ores. Minerals 2019, 9, 677. https://doi.org/10.3390/min9110677
Gatsé Ebotehouna C, Xie Y, Adomako-Ansah K, Pei L. Fluid Inclusion and Oxygen Isotope Characteristics of Vein Quartz Associated with the Nabeba Iron Deposit, Republic of Congo: Implications for the Enrichment of Hypogene Ores. Minerals. 2019; 9(11):677. https://doi.org/10.3390/min9110677
Chicago/Turabian StyleGatsé Ebotehouna, Chesther, Yuling Xie, Kofi Adomako-Ansah, and Liang Pei. 2019. "Fluid Inclusion and Oxygen Isotope Characteristics of Vein Quartz Associated with the Nabeba Iron Deposit, Republic of Congo: Implications for the Enrichment of Hypogene Ores" Minerals 9, no. 11: 677. https://doi.org/10.3390/min9110677
APA StyleGatsé Ebotehouna, C., Xie, Y., Adomako-Ansah, K., & Pei, L. (2019). Fluid Inclusion and Oxygen Isotope Characteristics of Vein Quartz Associated with the Nabeba Iron Deposit, Republic of Congo: Implications for the Enrichment of Hypogene Ores. Minerals, 9(11), 677. https://doi.org/10.3390/min9110677






