The Study of Crystal Structure on Grossular–Andradite Solid Solution
Abstract
:1. Introduction
2. Experimental Methods
3. Results
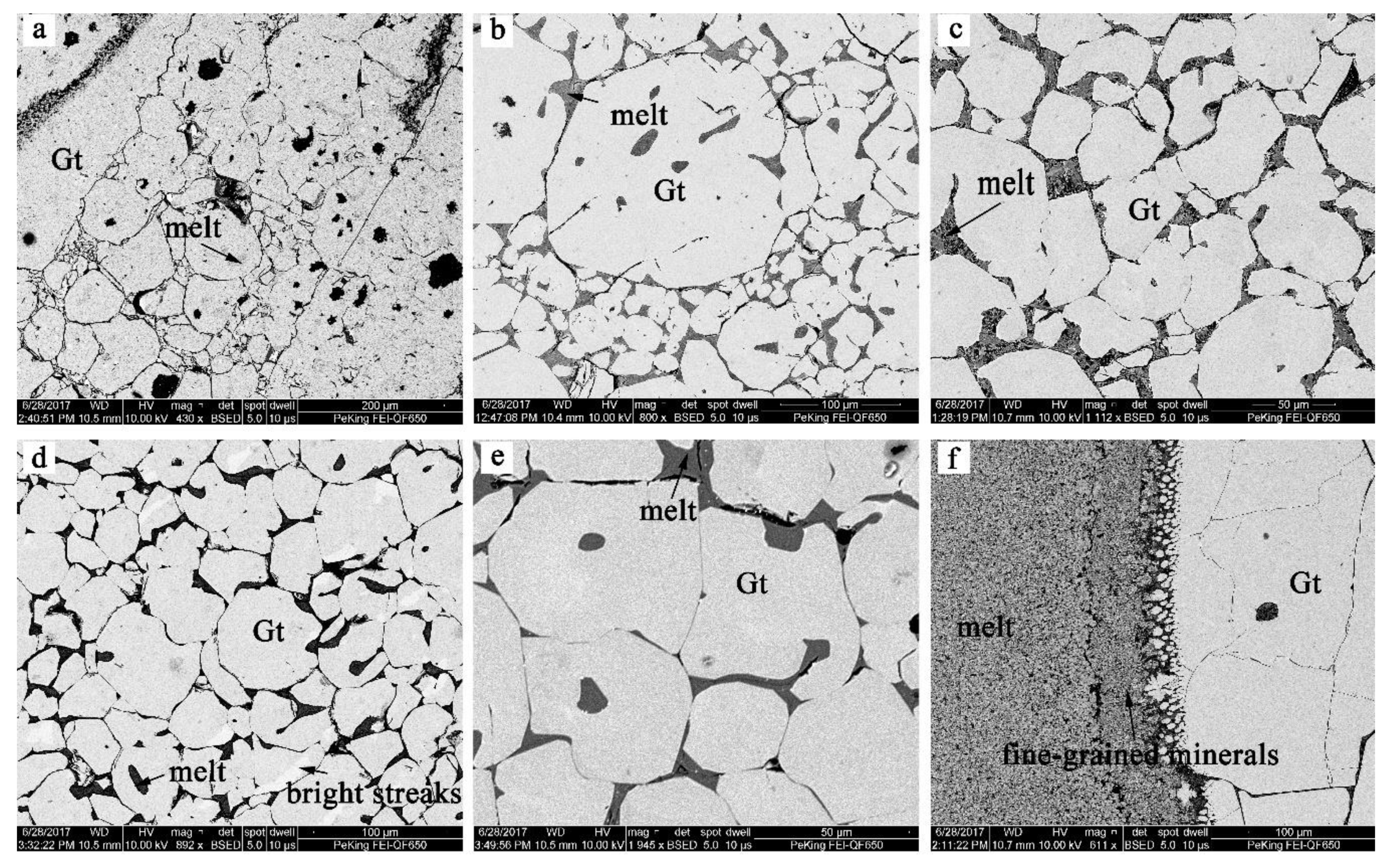
3.1. Phase Compositions
3.2. Unit-Cell Parameters
3.3. Excess Volume
3.4. Bond Length and Polyhedral Volume
3.5. XRD Peak Broadening
4. Discussion
4.1. Excess Volume and Distortion
4.2. Microstrain
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Irifune, T. An experimental investigation of the pyroxene-garnet transformation in a pyrolite composition and its bearing on the constitution of the mantle. Phys. Earth Planet. Inter. 1987, 45, 324–336. [Google Scholar] [CrossRef]
- Irifune, T.; Sekine, T.; Ringwood, A.E.; Hibberson, W.O. The eclogite–garnetite transformation at high-pressure and some geophysical implications. Earth Planet. Sci. Lett. 1986, 77, 245–256. [Google Scholar] [CrossRef]
- Conrad, P.G.; Zha, C.S.; Mao, H.K.; Hemley, R.J. The high-pressure, single-crystal elasticity of pyrope, grossular, and andradite. Am. Mineral. 1999, 84, 374–383. [Google Scholar] [CrossRef]
- Dachs, E.; Geiger, C.A. Thermodynamic behaviour of grossular-andradite, Ca3(Alx)2Si3O12, garnets: A calorimetric study. Eur. J. Mineral. 2019, 31, 443–451. [Google Scholar] [CrossRef]
- Du, W.; Clark, S.M.; Walker, D. Thermo-compression of pyrope–grossular garnet solid solutions: Non-linear compositional dependence. Am. Mineral. 2015, 100, 215–222. [Google Scholar] [CrossRef]
- Novak, G.A.; Gibbs, G.V. The crystal chemistry of the silicate garnets. Am. Mineral. 1971, 56, 791–825. [Google Scholar]
- Sun, C.G.; Liang, Y. The importance of crystal chemistry on REE partitioning between mantle minerals (garnet, clinopyroxene, orthopyroxene, and olivine) and basaltic melts. Chem. Geol. 2013, 358, 23–36. [Google Scholar] [CrossRef]
- Ganguly, J.; Cheng, W.J.; Oneill, H.S. Syntheses, volume, and structural changes of garnets in the pyrope–grossular join: Implications for stability and mixing properties. Am. Mineral. 1993, 78, 583–593. [Google Scholar]
- Geiger, C.A. A powder infrared spectroscopic investigation of garnet binaries in the system Mg3Al2Si3O12–Fe3Al2Si3O12–Mn3Al2Si3O12–Ca3Al2Si3O12. Eur. J. Mineral. 1998, 10, 407–422. [Google Scholar] [CrossRef]
- Geiger, C.A. Spectroscopic investigations relating to the structural, crystal-chemical and lattice-dynamic properties of (Fe2+, Mn2+, Mg, Ca)3Al2Si3O12 garnet: A review and analysis. In Spectroscopic Methods in Mineralogy; Beran, E.l.A., Libowitzky, E., Eds.; Eötvös University Press: Budapest, Hungary, 2004; Volume 6, pp. 589–645. [Google Scholar]
- Geiger, C.A.; Feenstra, A. Molar volumes of mixing of almandine–pyrope and almandine–spessartine garnets and the crystal chemistry and thermodynamic-mixing properties of the aluminosilicate garnets. Am. Mineral. 1997, 82, 571–581. [Google Scholar] [CrossRef]
- Merli, M.; Callegari, A.; Cannillo, E.; Caucia, F.; Leona, M.; Oberti, R.; Ungaretti, L. Crystal-chemical complexity in natural garnets: Structural constraints on chemical variability. Eur. J. Mineral. 1995, 7, 1239–1249. [Google Scholar] [CrossRef]
- Ungaretti, L.; Leona, M.; Merli, M.; Oberti, R. Non-ideal solid-solution in garnet: Crystal-structure evidence and modelling. Eur. J. Mineral. 1995, 7, 1299–1312. [Google Scholar] [CrossRef]
- Dachs, E.; Geiger, C.A. Heat capacities and entropies of mixing of pyrope–grossular (Mg3Al2Si3O12–Ca3Al2Si3O12) garnet solid solutions: A low-temperature calorimetric and a thermodynamic investigation. Am. Mineral. 2006, 91, 894–906. [Google Scholar] [CrossRef]
- Du, W.; Clark, S.M.; Walker, D. Excess mixing volume, microstrain, and stability of pyrope-grossular garnets. Am. Mineral. 2016, 101, 193–204. [Google Scholar] [CrossRef]
- Newton, R.C.; Charlu, T.V.; Kleppa, O.J. Thermochemistry of high pressure garnets and clinopyroxenes in the system CaO–MgO–Al2O3–SiO2. Geochim. Cosmochim. Acta 1977, 41, 369–377. [Google Scholar] [CrossRef]
- Boffa-Ballaran, T.; Woodland, A.B. Local structure of ferric iron-bearing garnets deduced by IR-spectroscopy. Chem. Geol. 2006, 225, 360–372. [Google Scholar] [CrossRef]
- McAloon, B.P.; Hofmeister, A.M. Single-crystal IR spectroscopy of grossular-andradite garnets. Am. Mineral. 1995, 80, 1145–1156. [Google Scholar] [CrossRef]
- Heuss-Aßbichler, S.; Fehr, K.T. Intercrystalline exchange of Al and Fe3+ between grossular–andradite and clinozoisite–epidote solid solutions. Neues Jahrb. für Mineral.–Abh. 1997, 172, 69–100. [Google Scholar]
- Liu, X.; Chen, J.L.; Tang, J.J.; He, Q.; Li, S.C.; Peng, F.; He, D.W.; Zhang, L.F.; Fei, Y.W. A large volume cubic press with a pressure-generating capability up to about 10 GPa. High Press. Res. 2012, 32, 239–254. [Google Scholar] [CrossRef]
- He, Q.; Tang, J.J.; Wang, F.; Liu, X. High Temperature Stable Assembly Designed for Cubic Press. Chin. J. High Press. Phys. 2014, 28, 145–151. [Google Scholar]
- Chen, T.; Wang, Y.C.; Bao, X.J.; Ma, Y.L.; Liu, L.P.; Liu, X. Heating Technique, Temperature Measurement, and Temperature Distribution in High Pressure Experiments on Large Volume Press. J. Earth Sci. Environ. 2018, 40, 428–448. [Google Scholar]
- Holland, T.J.B.; Redfern, S.A.T. Unit cell refinement from powder diffraction data: The use of regression diagnostics. Mineral. Mag. 1997, 61, 65–77. [Google Scholar] [CrossRef]
- Liu, X.; O’Neill, H.S.C. The effect of Cr2O3 on the partial melting of spinel lherzolite in the system CaO–MgO–Al2O3–SiO2–Cr2O3 at 1.1 GPa. J. Petrol. 2004, 45, 2261–2286. [Google Scholar] [CrossRef]
- Xu, C.; Kynicky, J.; Tao, R.B.; Liu, X.; Zhang, L.F.; Pohanka, M.; Song, W.L.; Fei, Y.W. Recovery of an oxidized majorite inclusion from Earth’s deep asthenosphere. Sci. Adv. 2017, 3, e1601589. [Google Scholar] [CrossRef]
- Geiger, C.A.; Newton, R.C.; Kleppa, O.J. Enthalpy of mixing of synthetic almandine–grossular and almandine–pyrope garnets from high temperature solution calorimetry. Geochim Cosmochim Acta 1987, 51, 1755–1763. [Google Scholar] [CrossRef]
- Armbruster, T.; Geiger, C.A. Andradite crystal chemistry, dynamic X-site disorder, and structural strain in silicate garnets. Eur. J. Mineral. 1993, 5, 59–71. [Google Scholar] [CrossRef]
- Woodland, A.B.; Ross II, C.R. A crystallographic and Mossbauer Spectroscopy Study of Fe32+Al2Si3O12–Fe32+Fe23+Si3O12 (Almandine–”Skiagite”) and Ca3Fe23+Si3O12–Fe32+Fe23+Si3O12 (Andradite–”Skiagite”) Garnet Solid Solutions. Phys. Chem. Miner. 1994, 21, 117–132. [Google Scholar] [CrossRef]
- Antao, S.M.; Klincker, A.M. Crystal structure of a birefringent andradite–grossular from Crowsnest Pass, Alberta, Canada. Powder Diffr. 2014, 29, 20–27. [Google Scholar] [CrossRef]
- Antao, S.M.; Salvador, J.J. Crystal chemistry of Birefringent Uvarovite Solid Solutions. Minerals 2019, 9, 395. [Google Scholar] [CrossRef]
- Dapiaggi, M.; Geiger, C.A.; Artioli, G. Microscopic strain in synthetic pyrope–grossular solid solutions determined by synchrotron X-ray powder diffraction at 5 K: The relationship to enthalpy of mixing behavior. Am. Mineral. 2005, 90, 506–509. [Google Scholar] [CrossRef]
- Cressey, G.; Schmid, R.; Wood, B.J. Thermodynamic properties of almandine–grossular garnet solid solutions. Contrib. Mineral. Petrol. 1978, 67, 397–404. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Euler, F.; Bruce, J.A. Oxygen coordinates of compounds with garnet structure. Acta Crystallogr. 1965, 19, 971–978. [Google Scholar] [CrossRef]
- Born, L.; Zemann, J. Abstandsberechnung und gitterenergetische Berechnungen an Granaten. Beitr Miner. Petrogr 1964, 10, 2–23. [Google Scholar]
- Armbruster, T.; Geiger, C.A.; Lager, G.A. Single-crystal X-ray structure study of synthetic pyrope almandine garnets at 100 and 293 K. Am. Mineral. 1992, 77, 512–521. [Google Scholar]
- Meagher, E.P. The crystal structures of pyrope and grossularite at elevated temperatures. Am. Mineral. 1975, 60, 218–228. [Google Scholar]
- Williamson, G.K.; Hall, W.H. X-ray broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Boffa-Ballaran, T.; Carpenter, M.A.; Geiger, C.A.; Koziol, A.M. Local structural heterogeneity in garnet solid solutions. Phys. Chem. Miner. 1999, 26, 554–569. [Google Scholar] [CrossRef]
- Becker, U.; Pollok, K. Molecular simulations of interfacial and thermodynamic mixing properties of grossular–andradite garnets. Phys. Chem. Miner. 2002, 29, 52–64. [Google Scholar] [CrossRef]









| Target x a | Composition | SiO2 b | Al2O3 b | Fe2O3 b | CaO b |
|---|---|---|---|---|---|
| 0.0 | Gro c100And d0 | 40.02 | 22.64 | 0.00 | 37.35 |
| 0.1 | Gro90And10 | 39.51 | 20.11 | 3.50 | 36.88 |
| 0.2 | Gro80And20 | 39.02 | 17.66 | 6.91 | 36.42 |
| 0.3 | Gro70And30 | 38.53 | 15.26 | 10.24 | 35.97 |
| 0.4 | Gro60And40 | 38.06 | 12.92 | 13.49 | 35.53 |
| 0.5 | Gro50And50 | 37.61 | 10.64 | 16.66 | 35.10 |
| 0.6 | Gro40And60 | 37.16 | 8.41 | 19.75 | 34.68 |
| 0.7 | Gro30And70 | 36.72 | 6.23 | 22.77 | 34.27 |
| 0.8 | Gro20And80 | 36.29 | 4.11 | 25.72 | 33.88 |
| 0.9 | Gro10And90 | 35.88 | 2.03 | 28.61 | 33.49 |
| 1.0 | Gro0And100 | 35.47 | 0.00 | 31.42 | 33.11 |
| Run # | Xa | T (°C) | t (h) b | Phase Assemblage | Notes |
|---|---|---|---|---|---|
| LMD611 | 0.0 | 1200 | 24 | Gt + melt | Gt size ~20–100 μm; vol % of melt ~5% c |
| LMD634 | 0.1 | 1200 | 28 | Gt + melt + gas d | Gt size ~20–500 μm; vol % of melt ~20%; almost all Fe lost to the Pt capsule |
| LMD584 | 0.2 | 1200 | 24 | Gt + melt | Gt size ~30–50 μm; vol % of melt ~5% |
| LMD618 | 0.3 | 1200 | 24 | Gt + melt + gas | Gt size ~20–200 μm; vol % of melt ~5% |
| LMD589 | 0.4 | 1200 | 24 | Gt + melt | Gt size ~30–80 μm; vol % of melt ~5% |
| LMD622 | 0.5 | 1200 | 28 | Gt + melt + gas | Gt size ~20–50 μm; vol % of melt ~5% |
| LMD631 | 0.6 | 1200 | 28 | Gt + melt + gas | Gt size ~40–60 μm; vol % of melt ~5% |
| LMD613 | 0.7 | 1200 | 24 | Gt + melt + gas | Gt size ~ 50 μm; vol % of melt ~5% |
| LMD581 | 0.8 | 1200 | 24 | Gt + melt | Gt size ~50–100 μm; vol % of melt < 5% |
| LMD632 | 0.9 | 1100 | 28 | Gt + melt + gas | Gt size ~20–100 μm; vol % of melt ~5% |
| LMD625 | 1.0 | 1100 | 28 | Gt + melt | Gt size ~100–500 μm; vol % of melt ~30% |
| Comp. b | Oxygen Atom Coordinate | Refinement Results | ||||
|---|---|---|---|---|---|---|
| x | y | z | R1 | wR2 | GooF | |
| 0.00 (0) c | 0.0384 (1) | 0.5453 (1) | 0.3487 (1) | 0.0267 | 0.0769 | 1.012 |
| 0.15 (2) | 0.0387 (2) | 0.5456 (2) | 0.3480 (1) | 0.0269 | 0.0780 | 1.001 |
| 0.26 (1) | 0.0383 (5) | 0.5465 (6) | 0.3475 (5) | 0.0682 | 0.1517 | 1.077 |
| 0.33 (2) | 0.0379 (4) | 0.5464 (4) | 0.3471 (4) | 0.0521 | 0.0937 | 1.059 |
| 0.45 (2) | 0.0390 (3) | 0.5467 (3) | 0.3466 (3) | 0.0377 | 0.0938 | 1.015 |
| 0.54 (2) | 0.0389 (3) | 0.5471 (3) | 0.3464 (3) | 0.0377 | 0.0657 | 1.014 |
| 0.70 (2) | 0.0394 (4) | 0.5475 (3) | 0.3456 (3) | 0.0423 | 0.1048 | 1.010 |
| 0.75 (1) | 0.0392 (1) | 0.5479 (1) | 0.3455 (1) | 0.0189 | 0.0541 | 1.045 |
| 0.90 (1) | 0.0393 (2) | 0.5485 (2) | 0.3449 (2) | 0.0274 | 0.0746 | 1.091 |
| 0.99 (0) | 0.0395 (3) | 0.5488 (2) | 0.3446 (2) | 0.0371 | 0.0940 | 1.006 |
| Run # | LMD611 | LMD634 | LMD584 | LMD618 | LMD589 | LMD622 | LMD631 | LMD613 | LMD581 | LMD632 | LMD625 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Target x | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
| EMPA # | 11 | 18 | 19 | 23 | 12 | 20 | 28 | 24 | 10 | 28 | 12 |
| Wt % | |||||||||||
| SiO2 | 39.92(15) a | 40.27(21) | 39.27(16) | 38.49(17) | 38.23(28) | 37.35(23) | 37.06(29) | 35.86(17) | 36.05(36) | 35.54(29) | 34.73(23) |
| Al2O3 | 22.49(20) | 22.23(22) | 18.88(51) | 16.49(28) | 14.77(48) | 12.11(52) | 9.69(52) | 6.48(36) | 5.12(34) | 2.01(20) | 0.15(2) |
| Fe2O3 b | 0.00(0) | 0.40(34) | 5.30(55) | 8.87(44) | 11.27(81) | 15.67(67) | 18.14(71) | 22.89(58) | 24.38(58) | 28.68(50) | 31.85(31) |
| FeO b | 0.00(0) | 0.32(27) | 0.65(40) | 0.46(36) | 0.90(45) | 0.24(25) | 0.70(36) | 0.05(9) | 1.02(44) | 0.66(47) | 0.00(0) |
| CaO | 37.76(22) | 37.30(36) | 36.07(35) | 35.53(26) | 34.92(33) | 34.67(26) | 33.98(23) | 33.63(25) | 32.80(39) | 32.61(28) | 32.62(14) |
| Total | 100.23(37) | 100.61(32) | 100.26(41) | 99.93(45) | 100.08(54) | 100.11(50) | 99.64(42) | 99.01(47) | 99.47(79) | 99.59(55) | 99.44(51) |
| Cations on the basis of 12 oxygen | |||||||||||
| Si | 2.99(1) | 3.01(1) | 3.00(2) | 2.99(1) | 2.99(1) | 2.96(1) | 2.99(2) | 2.96(2) | 2.99(2) | 2.99(2) | 2.96(1) |
| Al | 1.98(2) | 1.96(1) | 1.70(4) | 1.51(2) | 1.36(4) | 1.13(4) | 0.92(5) | 0.63(3) | 0.50(3) | 0.20(2) | 0.01(0) |
| Fe3+ | 0.00(0) | 0.03(2) | 0.31(3) | 0.52(3) | 0.66(5) | 0.94(5) | 1.10(5) | 1.45(4) | 1.52(4) | 1.82(3) | 2.07(2) |
| Fe2+ | 0.00(0) | 0.02(2) | 0.04(3) | 0.03(2) | 0.06(3) | 0.02(2) | 0.05(2) | 0.00(1) | 0.07(3) | 0.05(3) | 0.00(0) |
| Ca | 3.03(1) | 2.99(2) | 2.95(2) | 2.95(1) | 2.93(3) | 2.95(2) | 2.94(2) | 2.98(2) | 2.91(2) | 2.94(2) | 2.98(1) |
| Total | 8.00(0) | 8.00(1) | 8.00(1) | 8.00(1) | 8.00(0) | 8.00(1) | 8.00(0) | 8.02(2) | 8.00(0) | 8.00(0) | 8.02(1) |
| Fe3+/∑Fe | – | – | 0.88(7) | 0.95(4) | 0.92(4) | 0.98(2) | 0.96(2) | 1.00(0) | 0.96(2) | 0.98(2) | 1.00(0) |
| Observed x | 0.00(0) | 0.01(1) | 0.15(2) | 0.26(1) | 0.33(2) | 0.45(2) | 0.54(2) | 0.70(2) | 0.75(1) | 0.90(1) | 0.99(0) |
| X2+ | 3.03(1) | 3.01(1) | 2.99(1) | 2.98(1) | 2.99(1) | 2.96(1) | 2.98(2) | 2.98(1) | 2.98(2) | 2.99(2) | 2.98(1) |
| Y3+ | 1.98(2) | 1.98(2) | 2.00(3) | 2.03(3) | 2.02(2) | 2.07(3) | 2.02(3) | 2.08(4) | 2.02(3) | 2.02(3) | 2.08(2) |
| Z4+ | 2.99(1) | 3.01(1) | 3.00(2) | 2.99(1) | 2.99(1) | 2.96(1) | 2.99(2) | 2.96(2) | 2.99(2) | 2.99(2) | 2.96(1) |
| Si | 2.99(1) | 3.01(1) | 3.00(2) | 2.99(1) | 2.99(1) | 2.96(1) | 2.99(2) | 2.96(2) | 2.99(2) | 2.99(2) | 2.96(1) |
| Al | 1.98(2) | 1.96(1) | 1.70(4) | 1.51(2) | 1.36(4) | 1.13(4) | 0.92(5) | 0.63(3) | 0.50(3) | 0.20(2) | 0.01(0) |
| Fe3+ | 0.00(0) | 0.03(2) | 0.31(3) | 0.52(3) | 0.66(5) | 0.94(5) | 1.10(5) | 1.45(4) | 1.52(4) | 1.82(3) | 2.07(2) |
| Observed x | 0.00(0) | 0.01(1) | 0.15(2) | 0.26(1) | 0.33(2) | 0.45(2) | 0.54(2) | 0.70(2) | 0.75(1) | 0.90(1) | 0.99(0) |
| X2+ | 3.03(1) | 3.01(1) | 2.99(1) | 2.98(1) | 2.99(1) | 2.96(1) | 2.98(2) | 2.98(1) | 2.98(2) | 2.99(2) | 2.98(1) |
| Y3+ | 1.98(2) | 1.98(2) | 2.00(3) | 2.03(3) | 2.02(2) | 2.07(3) | 2.02(3) | 2.08(4) | 2.02(3) | 2.02(3) | 2.08(2) |
| Z4+ | 2.99(1) | 3.01(1) | 3.00(2) | 2.99(1) | 2.99(1) | 2.96(1) | 2.99(2) | 2.96(2) | 2.99(2) | 2.99(2) | 2.96(1) |
| Comp.X a | Powder XRD | Single-Crystal XRD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a (Å) | V (Å3) | Mol V | Excess V | Microstr | a (Å) | V (Å3) | Mol V | Excess V | |
| (cm3/mol) | (cm3/mol) | ain(10−2) | (cm3/mol) | (cm3/mol) | |||||
| 0.00(0)b | 11.8484(3) | 1663.31(11) | 125.16(1) | 0 | 0 | 11.8418(3) | 1660.55(11) | 124.96(1) | 0 |
| 0.15(2) | 11.8736(3) | 1673.97(11) | 125.97(1) | −0.209(1) | 1.06(28) | 11.8677(4) | 1671.47(11) | 125.78(1) | −0.213(1) |
| 0.26(1) | 11.8909(3) | 1681.30(11) | 126.52(1) | −0.348(1) | 1.39(25) | 11.8869(9) | 1681.30(11) | 126.52(1) | −0.308(1) |
| 0.33(2) | 11.8977(3) | 1684.17(11) | 126.73(1) | −0.620(1) | 3.33(56) | 11.8950(4) | 1683.04(11) | 126.65(1) | −0.549(1) |
| 0.45(2) | 11.9338(3) | 1699.55(11) | 128.08(1) | −0.283(1) | 3.02(29) | 11.9253(8) | 1695.93(11) | 127.62(1) | −0.417(1) |
| 0.54(2) | 11.9610(3) | 1711.19(12) | 129.04(1) | −0.053(1) | 2.83(34) | 11.9524(15) | 1702.52(12) | 128.49(1) | −0.206(1) |
| 0.70(2) | 11.9899(3) | 1723.64(12) | 129.70(1) | −0.080(1) | 1.57(24) | 11.9878(14) | 1722.73(12) | 129.64(1) | −0.047(1) |
| 0.75(1) | 12.0070(3) | 1731.02(12) | 130.26(1) | 0.100(1) | 3.18(44) | 12.0018(3) | 1728.78(12) | 130.09(1) | 0.024(1) |
| 0.90(1) | 12.0390(3) | 1744.89(12) | 131.30(1) | 0.153(1) | 0.60(38) | 12.0332(3) | 1742.38(12) | 131.11(1) | 0.034(1) |
| 0.99(0) | 12.0542(3) | 1751.50(12) | 131.80(1) | 0 | 0.31(32) | 12.0525(7) | 1750.78(12) | 131.75(1) | 0 |
| Comp a | T (°C) | P (GPa) | t (h) b | a (Å) | Reference |
|---|---|---|---|---|---|
| Gro c | 1200 | 3.0 | 24 | 11.8484(3) | This study, powder XRD |
| Gro | 1200 | 3.0 | 28 | 11.8418(3) | This study, single-crystal XRD |
| Gro | 1150 | 2.7 | 48 | 11.851(1) | Geiger et al., (1987) [26], powder XRD |
| Gro | 1350–1400 | 4.0–4.2 | 48 | 11.8515(2) | Ganguly et al., (1993) [8], powder XRD |
| Gro | 1400 | 6.0 | 0.5 | 11.850(2) | Du et al., (2016) [15], powder XRD |
| Andd | 1100 | 3.0 | 24 | 12.0542(3) | This study, powder XRD |
| And | 1100 | 3.0 | 28 | 12.0525(7) | This study, single-crystal XRD |
| And | 1200 | 2.0 | 12.063(1) | Armbruster and Geiger (1993) [27], single-crystal XRD | |
| And | 1100 | 1.2 | 91 | 12.0596(2) | Woodland and Ross (1994) [28], powder XRD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sun, Q.; Duan, D.; Bao, X.; Liu, X. The Study of Crystal Structure on Grossular–Andradite Solid Solution. Minerals 2019, 9, 691. https://doi.org/10.3390/min9110691
Wang Y, Sun Q, Duan D, Bao X, Liu X. The Study of Crystal Structure on Grossular–Andradite Solid Solution. Minerals. 2019; 9(11):691. https://doi.org/10.3390/min9110691
Chicago/Turabian StyleWang, Yichuan, Qiang Sun, Dengfei Duan, Xinjian Bao, and Xi Liu. 2019. "The Study of Crystal Structure on Grossular–Andradite Solid Solution" Minerals 9, no. 11: 691. https://doi.org/10.3390/min9110691
APA StyleWang, Y., Sun, Q., Duan, D., Bao, X., & Liu, X. (2019). The Study of Crystal Structure on Grossular–Andradite Solid Solution. Minerals, 9(11), 691. https://doi.org/10.3390/min9110691





