Novel Selective Depressant of Titanaugite and Implication for Ilmenite Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Samples
2.2. Flotation
2.2.1. Microflotation
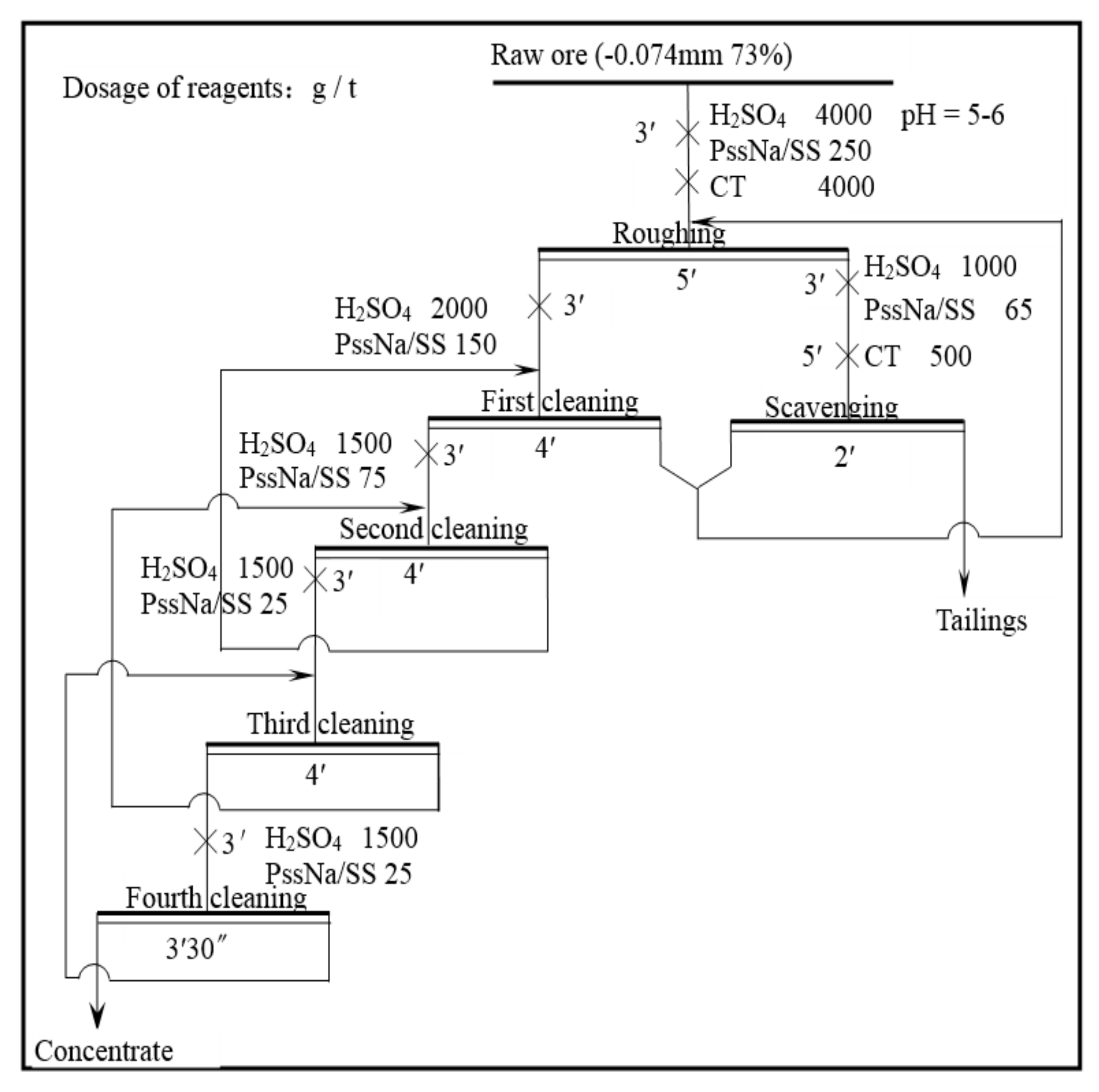
2.2.2. Bench Scale Flotation
2.3. Electrokinetic Potential Tests
2.4. Adsorption Measurements
2.5. FTIR Measurement
2.6. XPS Measurements
3. Results and Discussion
3.1. Flotation
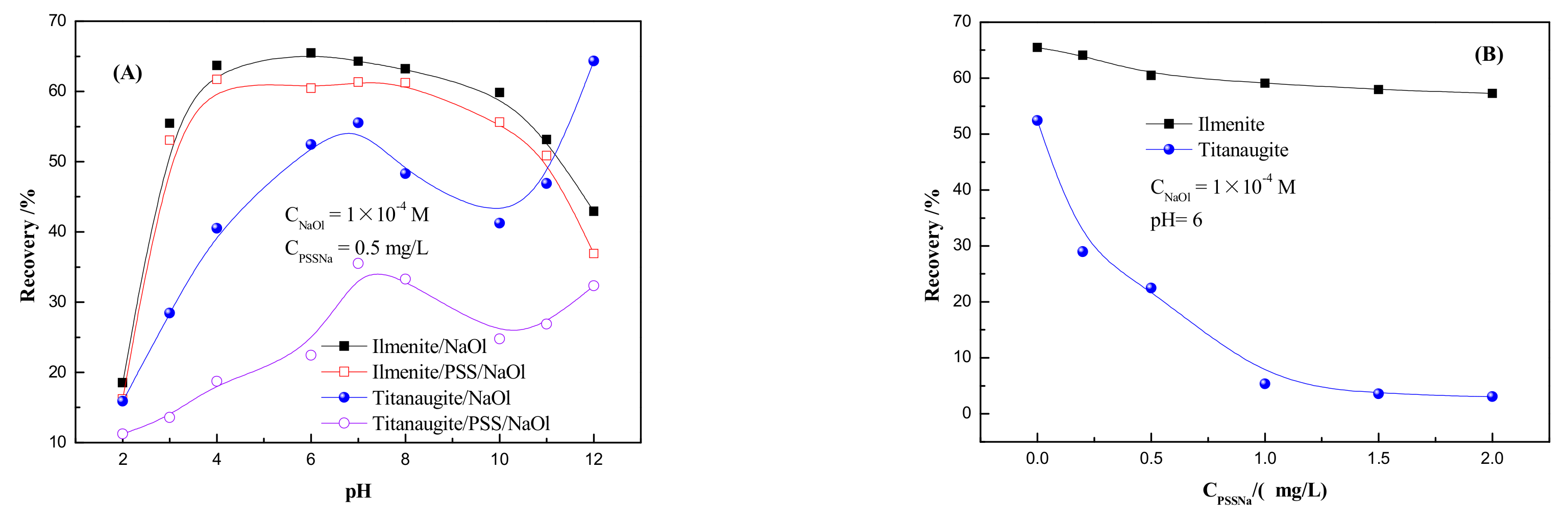
3.1.1. Microflotation
3.1.2. Bench Scale Flotation
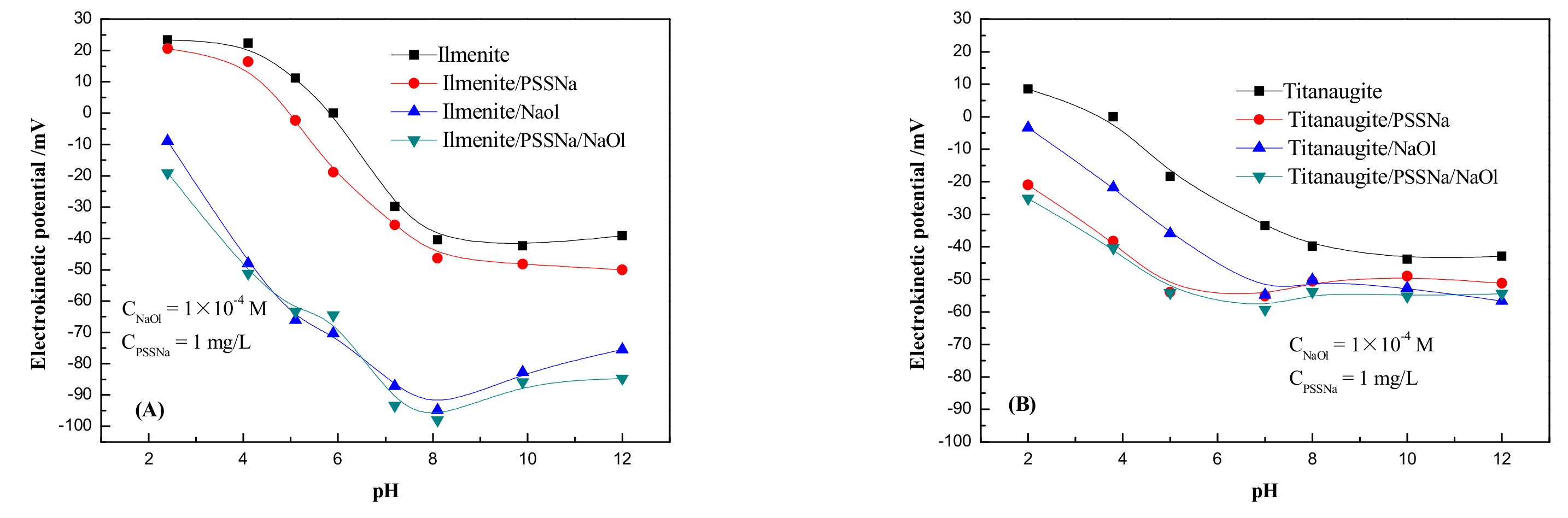
3.2. Electrokinetic Potential
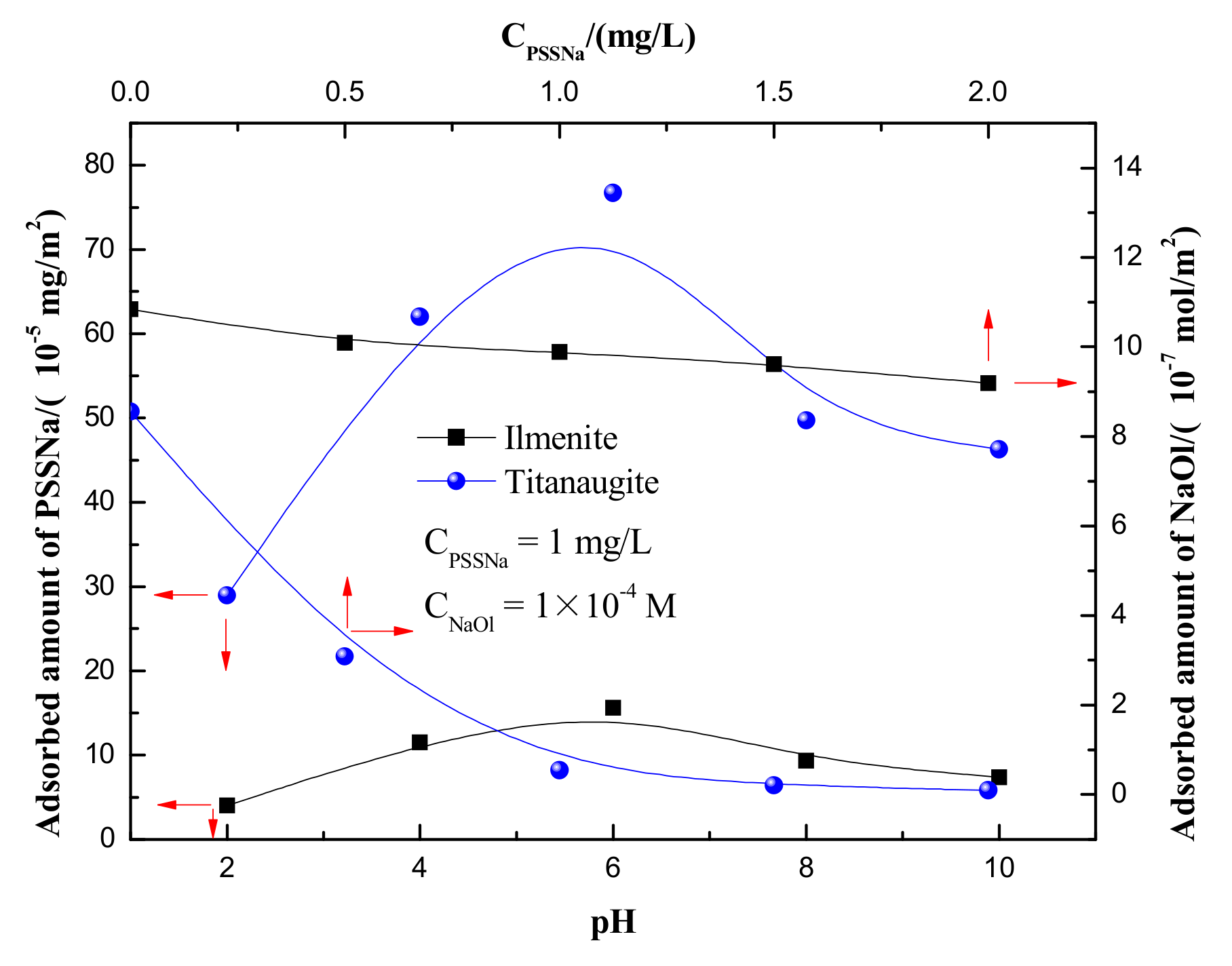
3.3. Adsorption Amounts
3.4. FTIR
3.5. XPS Detection
3.6. Suggested Adsorption Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, D.S.; Zhao, L.S.; Tao, Q.I.; Hu, G.; Zhao, H.X.; Jie, L.I.; Wang, L.N. Desilication from titanium–vanadium slag by alkaline leaching. Trans. Nonferr. Metal. Soc. 2013, 23, 3076–3082. [Google Scholar] [CrossRef]
- Samal, S.; Mohapatra, B.K.; Mukherjee, P.S.; Chatterjee, S.K. Integrated XRD, EPMA and XRF study of ilmenite and titania slag used in pigment production. J. Alloy. Compd. 2009, 474, 484–489. [Google Scholar] [CrossRef]
- Drzymala, J.; Luszczkiewicz, A.; Simiczyjew, P. Flotation study on high-hercynite ilmenite ores. Int. J. Miner. Process. 1983, 10, 289–296. [Google Scholar] [CrossRef]
- Fan, X.; Rowson, N.A. The effect of Pb (NO3)2 on ilmenite flotation. Miner. Eng. 2000, 13, 205–215. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Mahmud, M.A.P.; Lang, C. Towards sustainable TiO2 production: An investigation of environmental impacts of ilmenite and rutile processing routes in Australia. J. Clean. Prod. 2018, 196, 1016–1025. [Google Scholar] [CrossRef]
- Zvyagin, B.B.; Drits, V.A. An introduction to rock forming minerals. Vols. IV by WA Deer, RA Howie and J. Zussman. J. Appl. Crystallogr. 1970, 3, 426–428. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Effect of crystal chemistry and surface properties on ilmenite flotation behavior. Int. J. Miner. Process. 2015, 137, 71–81. [Google Scholar] [CrossRef]
- Bulatovic, S.; Wyslouzil, D.M. Process development for treatment of complex perovskite, ilmenite and rutile ores. Miner. Eng. 1999, 12, 1407–1417. [Google Scholar] [CrossRef]
- Song, Q.; Tsai, S.C. Flotation of ilmenite using benzyl arsonic acid and acidified sodium silicate. Int. J. Miner. Process. 1989, 26, 111–121. [Google Scholar] [CrossRef]
- Wang, D.Z.; Hu, Y.H. Solution Chemistry of Flotation; Hunan Scientific Press: Changsha, China, 1988; Volume 2, p. 35. [Google Scholar]
- Zhu, Y.G.; Zhang, G.F.; Feng, Q.M.; Yan, D.C.; Wang, W.Q. Effect of surface dissolution on flotation separation of fine ilmenite from titanaugite. Trans. Nonferr. Metal. Soc. 2011, 21, 1149–1154. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Jia, T.; Liu, Y.; Han, Y. Selective flotation of ilmenite from olivine using the acidified water glass as depressant. Int. J. Miner. Process. 2016, 157, 73–79. [Google Scholar] [CrossRef]
- Liu, X.; Huang, G.Y.; Li, C.X.; Cheng, R.J. Depressive effect of oxalic acid on titanaugite during ilmenite flotation. Miner. Eng. 2015, 79, 62–67. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Liu, R.; Sun, W.; Xiao, J. Comparative studies of flotation and adsorption with cetyl pyridinium chloride on molybdite and fluorapatite. Int. J. Miner. Process. 2015, 143, 112–116. [Google Scholar] [CrossRef]
- Xiong, F.; Chen, C.; Liu, S. Preparation of chitosan/polystyrene sulfonate multilayered composite metal nanoparticles and its application. J. Nanosci. Nanotechnol. 2016, 16, 6027–6031. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, l.; Wang, J.; Wang, L.; Xiao, J. A comparison study of adsorption of benzohydroxamic acid and amyl xanthate on smithsonite with dodecylamine as co-collector. Appl. Surf. Sci. 2017, 426, 1141–1147. [Google Scholar] [CrossRef]
- Lyu, F.; Gao, J.; Sun, N.; Liu, R.; Sun, X.; Cao, X.; Wang, L.; Sun, W. Utilisation of propyl gallate as a novel selective collector for diaspore flotation. Miner. Eng. 2019, 131, 66–72. [Google Scholar] [CrossRef]
- Liu, J.; Ejtemaei, M.; Nguyen, A.V.; Wen, S.; Zeng, Y. Surface chemistry of Pb-activated sphalerite. Miner. Eng. 2020, 145, 10658. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, Y.; Feng, Q.; Lu, Y.; Ou, L. Flotation mechanism of fine ilmenite by sodium oleate. Chin. J. Nonferr. Metal. 2009, 2, 372–377. (In Chinese) [Google Scholar]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z. The activation mechanism of lead ions in the flotation of ilmenite using sodium oleate as a collector. Miner. Eng. 2017, 111, 100–107. [Google Scholar] [CrossRef]
- Da Silva, L.; Paula, M.M.S.; Oenning, L.W.; Benavides, R. Properties of polystyrene/acrylic acid membranes after sulphonation reactions. J. New Mater. Electrochem. Sys. 2014, 17, 085–090. [Google Scholar]
- Kong, H.; Luo, P.; Gao, C.; Yan, D. Polyelectrolyte-functionalized multiwalled carbon nanotubes: Preparation, characterization and layer-by-layer self-assembly. Polymer 2005, 46, 2472–2485. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H.; Dahlan, K.Z.M. Kinetic investigations of graft copolymerization of sodium styrene sulfonate onto electron beam irradiated poly (vinylidene fluoride) films. Radiat. Phys. Chem. 2011, 80, 66–75. [Google Scholar] [CrossRef]
- Cepeda-Jiménez, C.M.; Pastor-Blas, M.M.; Ferrándiz-Gómez, T.P.; MartíN-MartíNez, J.M. Influence of the styrene content of thermoplastic styrene–butadiene rubbers in the effectiveness of the treatment with sulfuric acid. Int. J. Adhes. Adhes. 2001, 21, 161–172. [Google Scholar] [CrossRef]
- Li, H.M.; Liu, J.C.; Zhu, F.M.; Lin, S.A. Synthesis and physical properties of sulfonated syndiotactic polystyrene ionomers. Polym. Int. 2001, 50, 421–428. [Google Scholar] [CrossRef]
- Brijmohan, S.B.; Swier, S.; Weiss, R.A.; Shaw, M.T. Synthesis and characterization of cross-linked sulfonated polystyrene nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 8039–8045. [Google Scholar] [CrossRef]
- Xing, W.; Ma, Q.; Xu, J.; Peng, X. Baeyer–Villiger oxidation of cyclic ketones with hydrogen peroxide catalyzed by silica–VTMO–OSO3H. J. Porous Mater. 2015, 22, 487–492. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Wang, Z.; Han, H.; Yang, Y.; Hu, Y.; Tang, H.; Liu, R. Self-assembly of mixed dodecylamine–dodecanol molecules at the air/water interface based on large-scale molecular dynamics. J. Mol. Liq. 2019, 276, 867–874. [Google Scholar] [CrossRef]
- Bekri-Abbes, I.; Bayoudh, S.; Baklouti, M. Converting waste polystyrene into adsorbent: Potential use in the removal of lead and cadmium ions from aqueous solution. J. Polym. Environ. 2006, 14, 249–256. [Google Scholar] [CrossRef]
- Cruz-Cruz, I.; Reyes-Reyes, M.; López-Sandoval, R. Formation of polystyrene sulfonic acid surface structures on poly (3, 4-ethylenedioxythiophene): Poly (styrenesulfonate) thin films and the enhancement of its conductivity by using sulfuric acid. Thin Solid Films 2013, 531, 385–390. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Wang, J.; Xiao, J.; Liu, J.; Xu, L.; Fu, K. Strengthened floatation of molybdite using oleate with suitable co-collector. Miner. Eng. 2018, 122, 99–105. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Zheng, Y.; Xiao, J. Role of calcium dioleate in the flotation of powellite particles using oleate. Miner. Eng. 2019, 138, 95–100. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y. Recovering Cobalt and Sulfur in Low Grade Cobalt-Bearing V–Ti Magnetite Tailings Using Flotation Process. Processes 2019, 7, 536. [Google Scholar] [CrossRef] [Green Version]
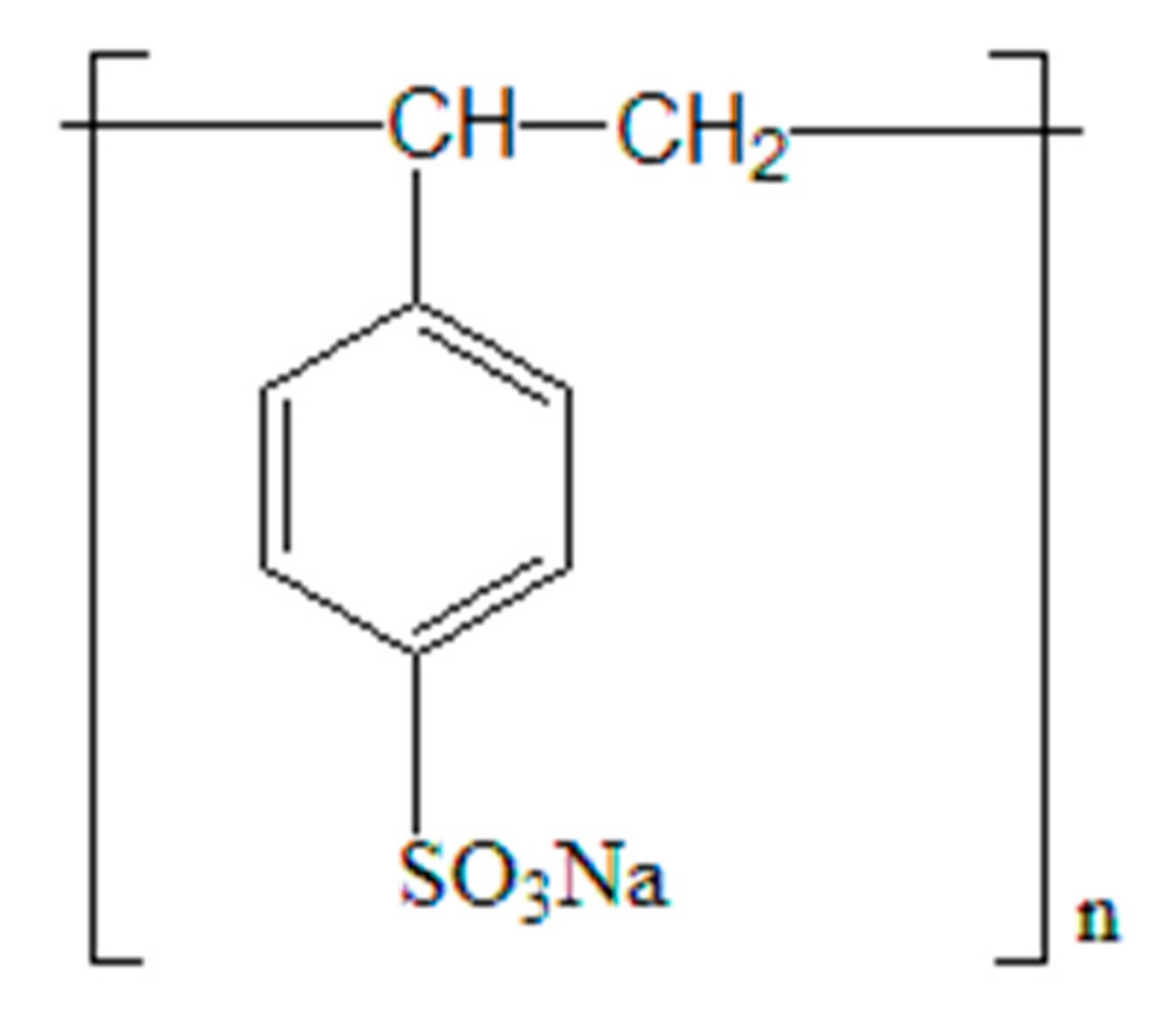





| Samples | TiO2 | Fe2O3 | MgO | Al2O3 | SiO2 | CaO | MnO | Specific Surface Area/m2·g−1 |
|---|---|---|---|---|---|---|---|---|
| Ilmenite | 51.14 | 39.21 | 4.46 | 1.85 | 1.47 | 0.37 | 0.61 | 0.89 |
| Titanaugite | 1.91 | 14.58 | 11.36 | 5.88 | 41.09 | 18.16 | 0.17 | 0.74 |
| Component | Fe | TiO2 | S | P2O5 | Na2O | K2O | CaO | Al2O3 | MgO | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 16.628 | 15.617 | 0.08 | 0.122 | 0.747 | 0.307 | 11.251 | 6.101 | 8.915 | 30.843 |
| Product Name | Yield (%) | TiO2 Grade (%) | TiO2 Recovery (%) | |||
|---|---|---|---|---|---|---|
| PSSNa | Sodium Silicate | PSSNa | Sodium Silicate | PSSNa | Sodium Silicate | |
| Ilmenite concentrate | 25.93 | 25.19 | 45.97 | 43.54 | 76.32 | 70.18 |
| Tailings | 74.07 | 74.81 | 5.01 | 6.23 | 23.68 | 29.82 |
| Crudes | 100.00 | 100.00 | 15.63 | 15.63 | 100.00 | 100.00 |
| Minerals | Element | Binding Energy/eV | Chemical Shift ΔE/eV | |
|---|---|---|---|---|
| Without PSSNa | Treated with Pssna | |||
| Ilmenite | Fe2p3/2 | 710.7 | 711.0 | 0.3 |
| Fe 2p1/2 | 724.1 | 724.2 | 0.1 | |
| Ti2p3/2 | 463.5 | 463.7 | 0.2 | |
| Ti2p1/2 | 457.9 | 458.1 | 0.2 | |
| Titanaugite | Ca2p3/2 | 347.1 | 347.8 | 0.7 |
| Ca2p1/2 | 350.7 | 351.4 | 0.7 | |
| Mg2p | 49.9 | 50.4 | 0.5 | |
| Al2p | 74.4 | 74.7 | 0.3 | |
| Fe2p3/2 | 710.6 | 710.9 | 0.3 | |
| Fe2p1/2 | 723.6 | 723.8 | 0.2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Wang, Z.; Xiao, J.; Wang, H.; Deng, B.; Zhang, Y.; Chen, C. Novel Selective Depressant of Titanaugite and Implication for Ilmenite Flotation. Minerals 2019, 9, 703. https://doi.org/10.3390/min9110703
Liu N, Wang Z, Xiao J, Wang H, Deng B, Zhang Y, Chen C. Novel Selective Depressant of Titanaugite and Implication for Ilmenite Flotation. Minerals. 2019; 9(11):703. https://doi.org/10.3390/min9110703
Chicago/Turabian StyleLiu, Nengyun, Zhen Wang, Junhui Xiao, Hongbin Wang, Bing Deng, Yushu Zhang, and Chao Chen. 2019. "Novel Selective Depressant of Titanaugite and Implication for Ilmenite Flotation" Minerals 9, no. 11: 703. https://doi.org/10.3390/min9110703
APA StyleLiu, N., Wang, Z., Xiao, J., Wang, H., Deng, B., Zhang, Y., & Chen, C. (2019). Novel Selective Depressant of Titanaugite and Implication for Ilmenite Flotation. Minerals, 9(11), 703. https://doi.org/10.3390/min9110703







