Sintering Behavior and Technological Properties of Low-Temperature Porcelain Tiles Prepared Using a Lithium Ore and Silica Crucible Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Base Materials
2.2. Preparation of Porcelain Tiles
2.3. Sample Characterization
3. Results and Discussion
3.1. Phase Composition of the Raw Materials
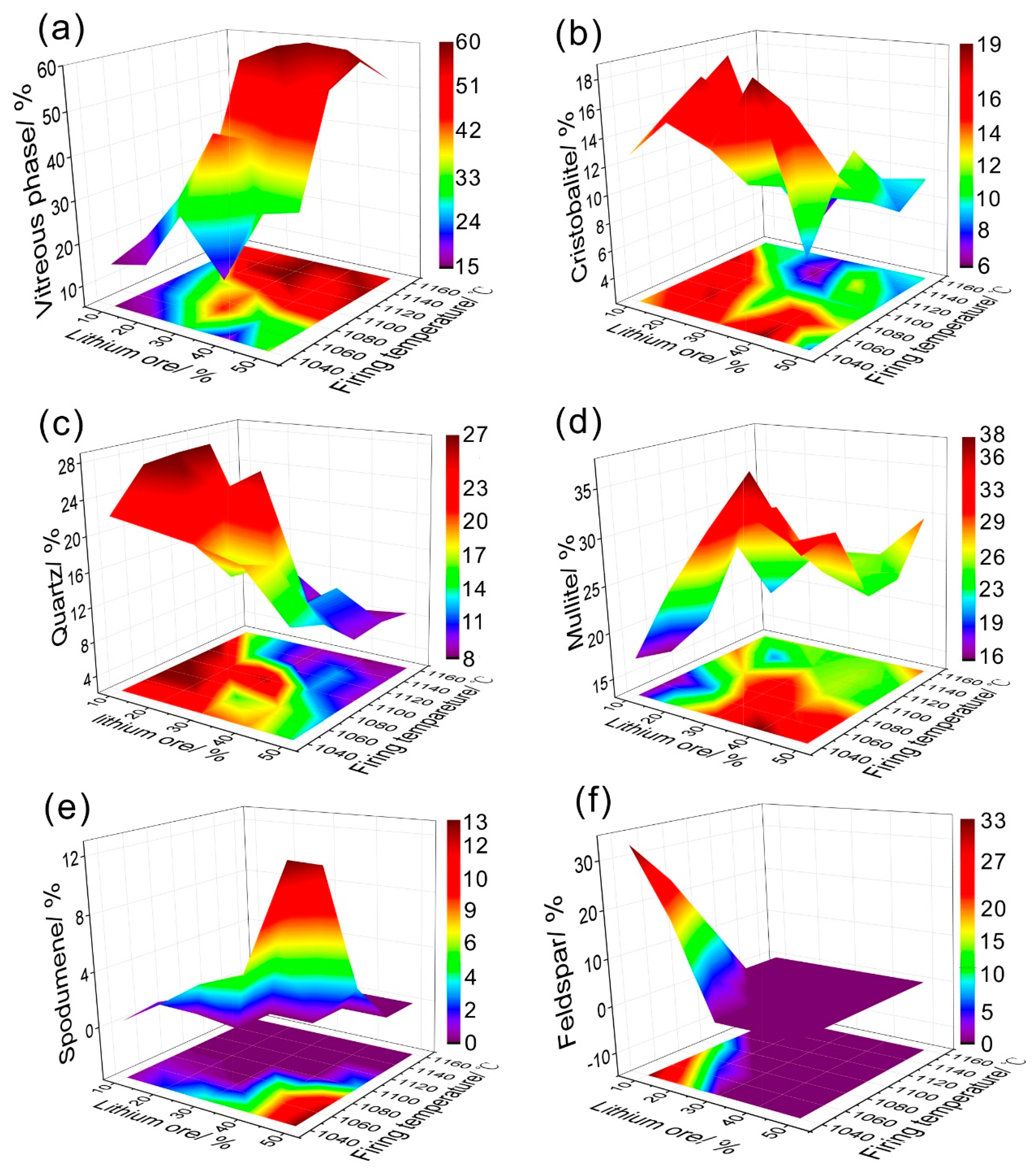
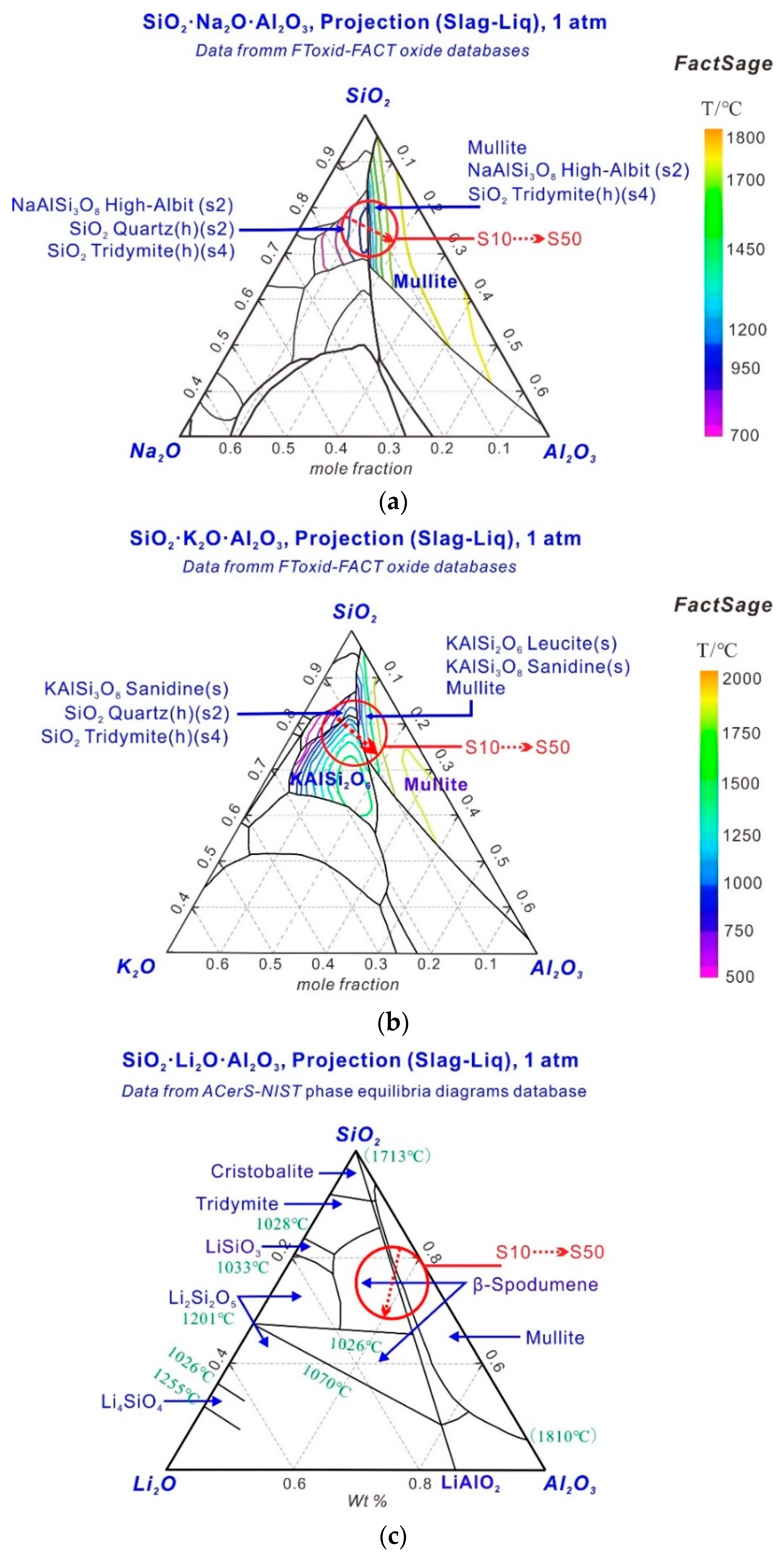
3.2. Phase Transitions in the Sintering Process
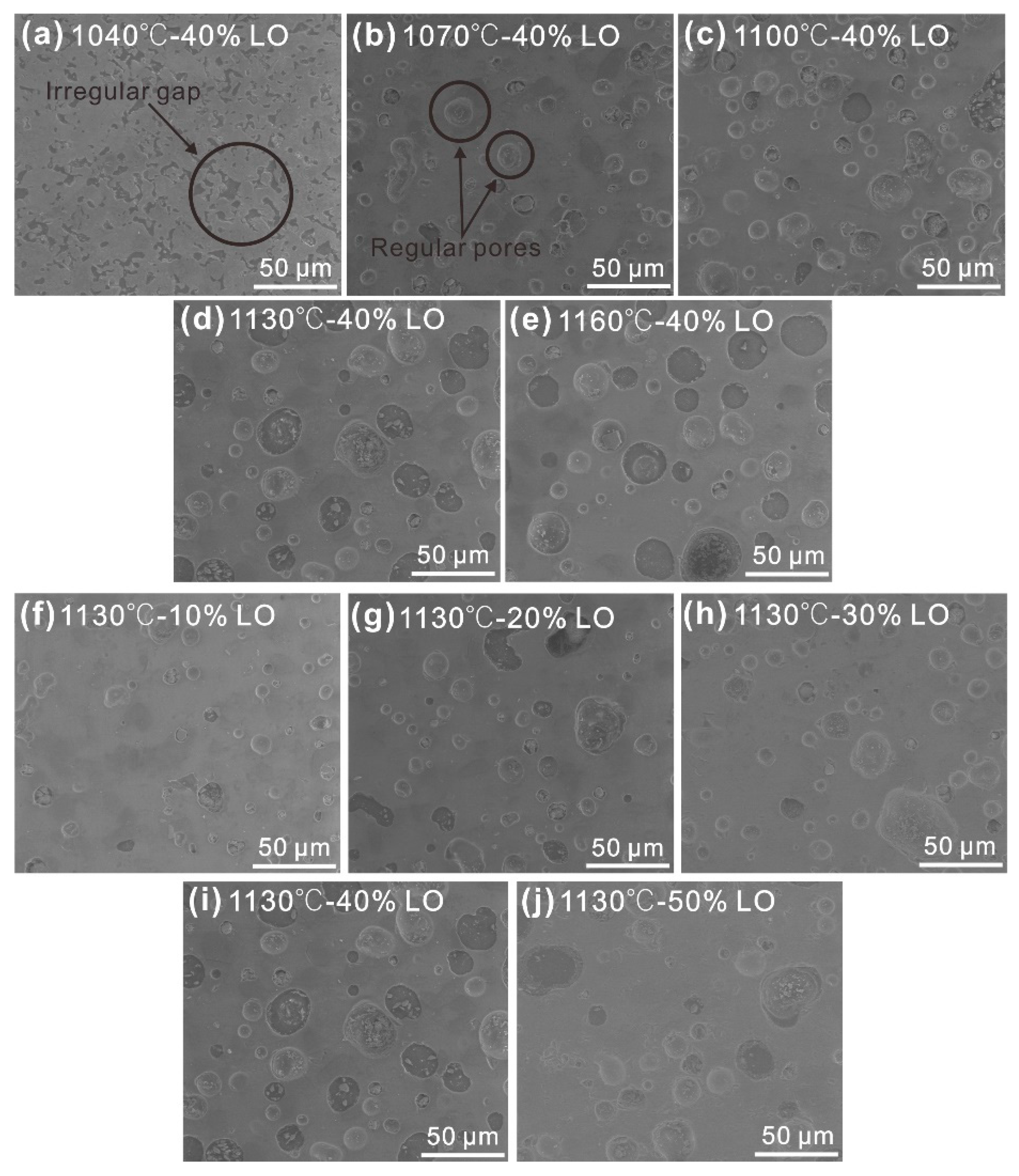
3.3. Microstructure Analysis
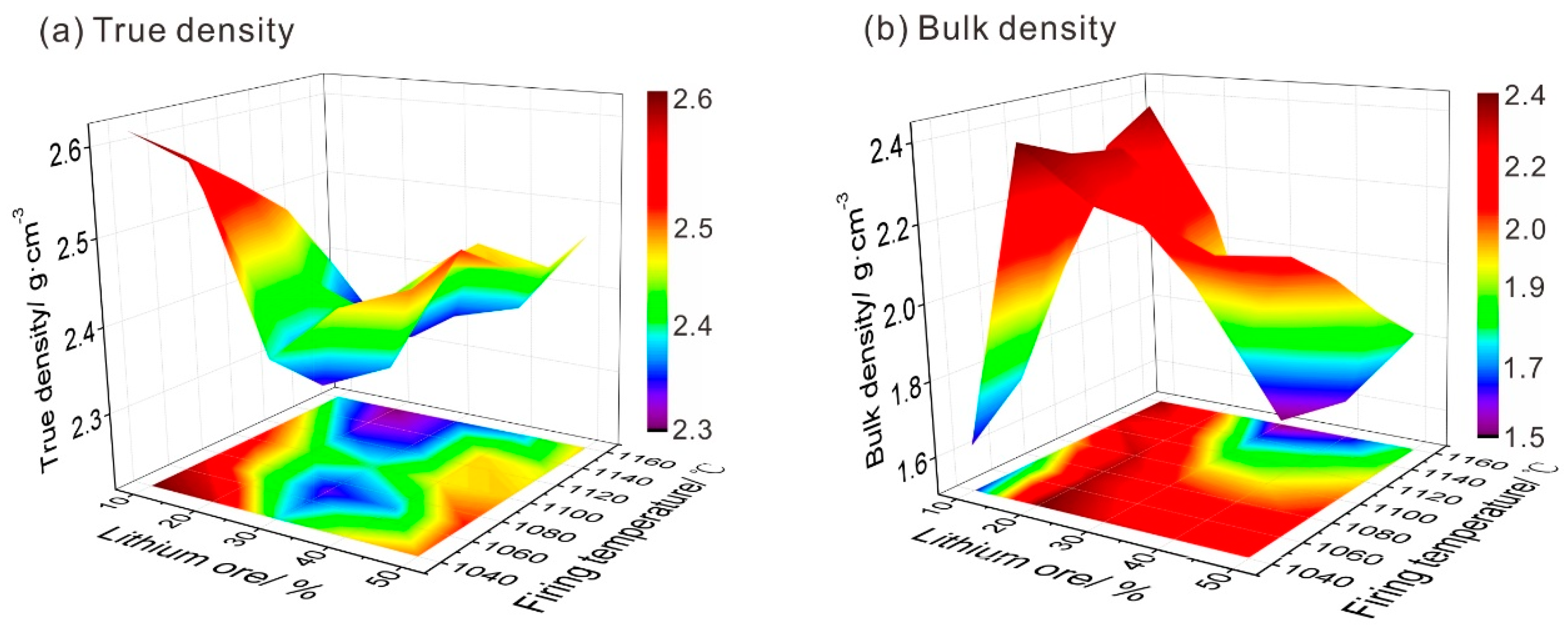
3.4. True Density and Bulk Density
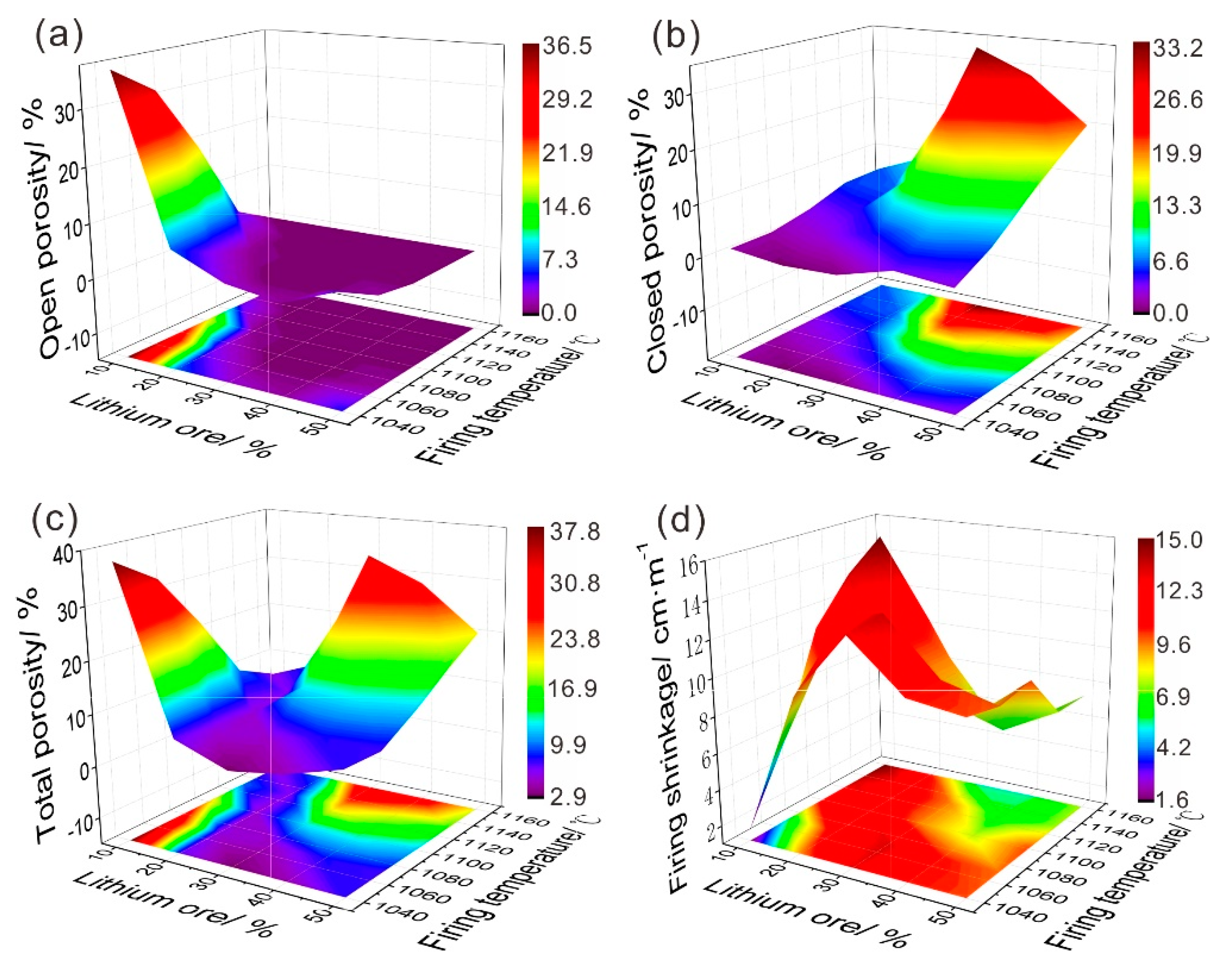
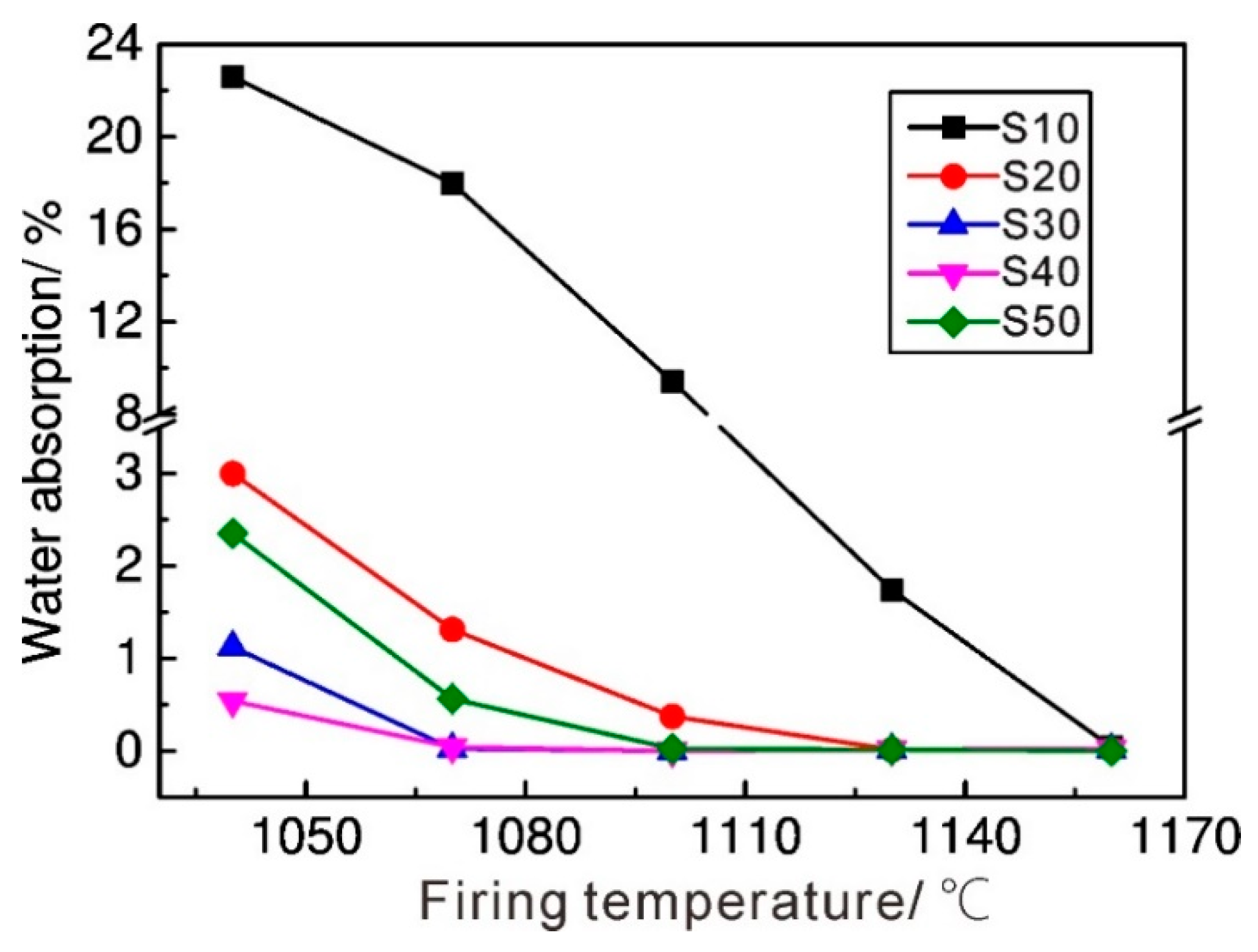
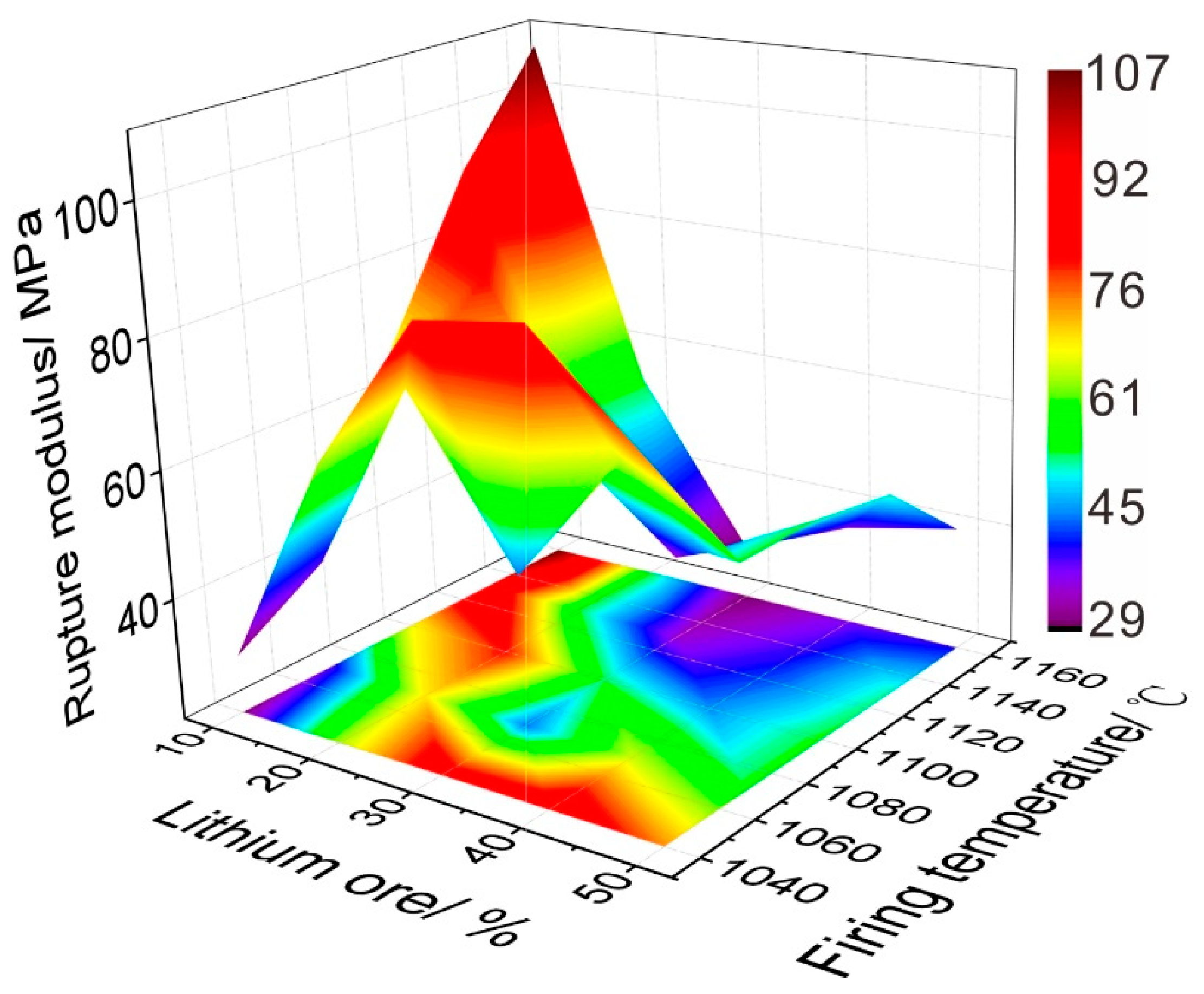
3.5. Water Absorption and Rupture Modulus
3.6. Environmental and Economic Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raimondo, M.; Zanelli, C.; Matteucci, F.; Guarini, G.; Dondi, M.; Labrincha, J.A. Effect of waste glass (TV/PC cathodic tube and screen) on technological properties and sintering behaviour of porcelain stoneware tiles. Ceram. Int. 2007, 33, 615–623. [Google Scholar] [CrossRef]
- Tucci, A.; Esposito, L.; Rastelli, E.; Palmonari, C.; Rambaldi, E. Use of soda-lime scrap-glass as a fluxing agent in a porcelain stoneware tile mix. J. Eur. Ceram. Soc. 2004, 24, 83–92. [Google Scholar] [CrossRef]
- Matteucci, F.; Dondi, M.; Guarini, G. Effect of soda-lime glass on sintering and technological properties of porcelain stoneware tiles. Ceram. Int. 2002, 28, 873–880. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Liu, H.; Han, C. Recycling of waste red mud for production of ceramic floor tile with high strength and lightweight. J. Alloys Compd. 2018, 748, 876–881. [Google Scholar] [CrossRef]
- Huang, T. Latest Data of American Ceramic Brick Market. Available online: http://www.xbcd.cn/hyxw/jjgc/201905/499691822.html (accessed on 16 May 2019).
- Matthew, G.O.; Fatile, B.O. Characterization of vitrified porcelain tiles using feldspar from three selected deposits in Nigeria. Res. J. Recent Sci. 2014, 3, 2277–2502. [Google Scholar]
- Yue, L. Experimental study on preparation of vitrified brick from coal gangue (in Chinese). J. Liaoning Technol. Univ. (Nat. Sci.) 2010, 29, 201–204. [Google Scholar]
- Dana, K.; Das, S.K. Enhanced resistance to thermal cycling of slag-containing vitrified porcelain tiles. Ceram. Int. 2008, 28, 121–124. [Google Scholar]
- Pinatti, D.G.; Conte, R.A.; Borlini, M.C.; Santos, B.C.; Oliveira, I.; Vieira, C.M.F.; Monteiro, S.N. Incorporation of the ash from cellulignin into vitrified ceramic tiles. J. Eur. Ceram. Soc. 2006, 26, 305–310. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Agathopoulos, S.; Fernandes, H.R.; Ferreira, J.M.F. Influence of lithium oxide as auxiliary flux on the properties of triaxial porcelain bodies. J. Eur. Ceram. Soc. 2006, 26, 1131–1139. [Google Scholar] [CrossRef]
- Ismatova, R. Properties and structure of spodumene-porcelain. Glass Ceram. 1987, 44, 314–316. [Google Scholar] [CrossRef]
- Yamuna, A.; Devanarayanan, S.; Lalithambika, M. Mullite-β-spodumene composites from aluminosilicates. J. Am. Ceram. Soc. 2001, 84, 1703–1709. [Google Scholar] [CrossRef]
- Low, I.M.; Mathews, E.; Garrod, T.; Zhou, D.; Phillips, D.N.; Pillai, X.M. Processing of spodumene-modified mullite ceramics. J. Mater. Sci. 1997, 32, 3807–3812. [Google Scholar] [CrossRef]
- Oberžan, M.; Holc, J.; Buh, M.; Kuščer, D.; Lavrač, I.; Kosec, M. High-alumina porcelain with the addition of a Li2O-bearing fluxing agent. J. Eur. Ceram. Soc. 2009, 29, 2143–2152. [Google Scholar] [CrossRef]
- Bayuseno, A.P.; Latella, B.A.; O’Connor, B.H. Resistance of alumina spodumene ceramics to thermal shock. J. Am. Ceram. Soc. 1999, 82, 819–824. [Google Scholar] [CrossRef]
- Zhou, J.E.; Liu, K.; Dong, W.X.; Bao, Q.F.; Zhao, T.G.; Wang, Y.Q. Effects of CaO-Li2O-K2O-Na2O fluxing agents on the properties of porcelain ceramic tiles. Key Eng. Mater. 2015, 655, 258–262. [Google Scholar] [CrossRef]
- Sheng, Y. Business Club: Spodumene Price of Zhongjin Taifu Mining Co. Ltd. Stayed Stable on July 12. Available online: http://www.100ppi.com/news/detail-20190712-1481629.html (accessed on 12 July 2019).
- Sánchez, E.; García-Ten, J.; Sanz, V.; Moreno, A. Porcelain tile: Almost 30 years of steady scientific-technological evolution. Ceram. Int. 2010, 36, 831–845. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Yliniemi, J.; Ismailov, A.; Levanenb, E.; Tanskanenc, P.; Kinnunena, P.; Roningd, J.; Illikainena, M. Spodumene tailings for porcelain and structural materials: Effect of temperature (1050–1200 °C) on the sintering and properties. Miner. Eng. 2019, 141, 105843. [Google Scholar] [CrossRef]
- Dhanapandian, S.; Manoharan, C.; Ramkumar, T.; Gnanavela, B.; Sutharsana, P.; Shanthi, M. Effect of incorporation of granite and marble rejects in clay brick products: Physico-mechanical analysis. Acta Phys. Pol. A 2010, 118, 688–694. [Google Scholar] [CrossRef]
- Saboya, F., Jr.; Xavier, G.C.; Alexandre, J. The use of the powder marble by-product to enhance the properties of brick ceramic. Constr. Build. Mater. 2007, 21, 1950–1960. [Google Scholar] [CrossRef]
- Souza, A.J.; Pinheiro, B.C.A.; Holanda, J.N.F. Recycling of gneiss rock waste in the manufacture of vitrified floor tiles. J. Environ. Manag. 2010, 91, 685–689. [Google Scholar] [CrossRef]
- Pinheiro, B.C.A.; Holanda, J.N.F. Obtainment of porcelain floor tiles added with petroleum oily sludge. Ceram. Int. 2013, 39, 57–63. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.; Ma, S.; Liu, C.; Wang, X. Ceramic tiles derived from coal fly ash: Preparation and mechanical characterization. Ceram. Int. 2017, 43, 11953–11966. [Google Scholar] [CrossRef]
- Ji, R.; Zhang, Z.T.; Yan, C.; Zhu, M.G.; Li, Z.M. Preparation of novel ceramic tiles with high Al2O3 content derived from coal fly ash. Constr. Build. Mater. 2016, 114, 888–895. [Google Scholar] [CrossRef]
- Ke, S.J.; Wang, Y.M.; Pan, Z.D.; Ning, C.Y.; Zheng, S.L. Recycling of polished tile waste as a main raw material in porcelain tiles. J. Clean. Prod. 2016, 115, 238–244. [Google Scholar] [CrossRef]
- Dana, K.; Dey, J.; Das, S.K. Synergistic effect of fly ash and blast furnace slag on the mechanical strength of traditional porcelain tiles. Ceram. Int. 2005, 31, 147–152. [Google Scholar] [CrossRef]
- Das, S.K.; Pal, M.; Ghosh, J.; Pathi, K.V.; Mondal, S. The effect of basic oxygen furnace slag and fly ash additions in triaxial porcelain composition: Phase and micro structural evolution. Trans. Indian Inst. Met. 2013, 66, 213–220. [Google Scholar] [CrossRef]
- Patrick, D.O.; Kefas, H.M.; John, Y.M.; Ameh, V.I. Investigation of the physical properties of tiles produced with Otukpo clay. Leonardo Electron. J. Pract. Technol. 2015, 27, 162–176. [Google Scholar]
- Manoharan, C.; Sutharsan, P.; Dhanapandian, S.; Venkatachalapathy, R.; MohamedAsanulla, R. Analysis of temperature effect on ceramic brick production from alluvial deposits, Tamilnadu, India. Appl. Clay Sci. 2011, 54, 20–25. [Google Scholar] [CrossRef]
- Liu, J.; Peng, L.; Qin, S. Structure and properties of the fused quartz crucibles for silicon ingots in sintering and casting process. J. Synth. Cryst. 2016, 45, 1261–1265, (In Chinese with English abstract). [Google Scholar]
- Zhou, J.; Ma, G.Y.; Ma, R. Quantitative analysis on amorphous phase content in blast furnace slag. Phys. Test Chem. Anal. Part A 2015, 51, 711–716. [Google Scholar]
- Peng, L.; Qin, S. Mechanical behaviour and microstructure of an artificial stone slab prepared using a waste SiO2 crucible and quartz. Constr. Build. Mater. 2018, 50, 273–280. [Google Scholar] [CrossRef]
- GB/T 1548-2015, Test Method for Linear Shrinkage of Ceramic Body Slurry; Ministry of Industry and Information Technology of People’s Republic of China: Beijing, China, 2015. (In Chinese)
- GB/T 3810.3-2006, Test Methods of Ceramic Tiles: Determination of Water Absorption, Apparent Porosity, Apparent Relative Porosity and Bulk Density; Standardization Administration of the People’s Republic of China: Beijing, China, 2006. (In Chinese)
- GB/T 3810.4-2006, Test Methods of Ceramic Tiles-Part 4: Determination of Modulus of Rupture and Breaking Strength; Standardization Administration of the People’s Republic of China: Beijing, China, 2006. (In Chinese)
- QB/T 1010-2015, Test Methods of for True Density of Ceramic Materials and Pigment; Ministry of Industry and Information Technology of People’s Republic of China: Beijing, China, 2015. (In Chinese)
- Stevens, S.J.; Hand, R.J.; Sharp, J.H. Polymorphism of silica. J. Mater. Sci. 1997, 32, 2929–2935. [Google Scholar] [CrossRef]
- Marians, C.S.; Hobbs, L.W. Network properties of crystalline polymorphs of silica. J. Non-Cryst. Solids 1990, 124, 242–253. [Google Scholar] [CrossRef]
- Castelein, O.; Guinebretiere, R.; Bonnet, J.P.; Blanchart, P. Shape, size and composition of mullite nanocrystals from rapidly sintered kaolin. J. Eur. Ceram. Soc. 2001, 21, 2369–2376. [Google Scholar] [CrossRef]
- Iqbal, Y.; Lee, W.E. Fired porcelain microstructures revisited. J. Am. Ceram. Soc. 1999, 82, 3384–3390. [Google Scholar] [CrossRef]
- Iqbal, Y.; Lee, W.E. Microstructural evolution in triaxial porcelain. J. Am. Ceram. Soc. 2000, 83, 3121–3127. [Google Scholar] [CrossRef]
- Lu, H.Y.; Wang, W.L.; Tuan, W.H.; Lin, M.H. Acicular mullite crystals in vitrified kaolin. J. Am. Ceram. Soc. 2004, 87, 1843–1847. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Mugoni, C.; Guandalini, S.; Cattini, A.; Mazzini, D.; Alboni, C.; Siligardi, C. Glass recycling in the production of low-temperature stoneware tiles. J. Clean. Prod. 2018, 197, 1531–1539. [Google Scholar] [CrossRef]
- Luz, A.P.; Ribeiro, S. Use of glass waste as a raw material in porcelain stoneware tile mixtures. Ceram. Int. 2007, 33, 761–765. [Google Scholar] [CrossRef]
- Tucci, A.; Esposito, L.; Malmusi, L.; Rambaldi, E. New body mixes for porcelain stoneware tiles with improved mechanical characteristics. J. Eur. Ceram. Soc. 2007, 27, 1875–1881. [Google Scholar] [CrossRef]
- Falamaki, C.; Afarani, M.S.; Aghaie, A. Initial sintering stage pore growth mechanism applied to the manufacture of ceramic membrane supports. J. Eur. Ceram. 2004, 24, 2285–2292. [Google Scholar] [CrossRef]
- ISO 13006, Ceramic Tiles-Definitions, Classification, Characteristics and Marking; Technical Committee: ISO/TC 189 Ceramic Tile; ISO: Geneva, Switzerland, 2012.
- Gouvêa, D.; Kaneko, T.T.; Kahn, H.; Conceição, E.S.; Antoniassi, J.L. Using bone ash as an additive in porcelain sintering. Ceram. Int. 2015, 41, 487–496. [Google Scholar] [CrossRef]


| wt.% | SCW | Calcined Kaolin | Feldspar | LO |
|---|---|---|---|---|
| SiO2 | 99.7 | 64.6 | 77.6 | 68.2 |
| Al2O3 | 0.2 | 33.3 | 12.4 | 19.9 |
| Fe2O3 | 0.1 | 0.4 | 0.6 | 0.2 |
| CaO | <0.1 | 0.8 | 0.9 | 0.1 |
| MgO | <0.1 | 0.1 | 0.2 | <0.1 |
| K2O | <0.1 | 0.1 | 5.4 | 6.1 |
| Na2O | <0.1 | <0.1 | 2.7 | 2.2 |
| MnO | <0.1 | <0.1 | <0.1 | 0.4 |
| TiO2 | <0.1 | <0.1 | <0.1 | <0.1 |
| P2O5 | <0.1 | <0.1 | <0.1 | 0.2 |
| Li2O | <0.1 | <0.1 | <0.1 | 2.7 |
| wt.% | SCW | Calcined Kaolin | Feldspar | LO |
|---|---|---|---|---|
| S10 | 20 | 30 | 40 | 10 |
| S20 | 20 | 30 | 30 | 20 |
| S30 | 20 | 30 | 20 | 30 |
| S40 | 20 | 30 | 10 | 40 |
| S50 | 20 | 30 | 0 | 50 |
| R1180–R1280 | 20 | 30 | 50 | 0 |
| Ref | Vitreous Phase | Cristobalite | Quartz | K-Feldspar | Na-Feldspar | Mullite |
|---|---|---|---|---|---|---|
| R1180 | 39 | 5 | 22 | 14 | 11 | 9 |
| R1200 | 51 | 5 | 21 | 8 | 5 | 10 |
| R1220 | 60 | 5 | 19 | 3 | 2 | 11 |
| R1240 | 61 | 4 | 21 | 2 | 0 | 13 |
| R1260 | 64 | 4 | 20 | 0 | 0 | 12 |
| R1280 | 65 | 3 | 19 | 0 | 0 | 13 |
| Ref | Water Absorption (%) | Rupture Modulus (MPa) | Firing Shrinkage (cm·m−1) | Bulk Density (g·cm−1) | True Density (g·cm−1) | Open Porosity (%) | Closed Porosity (%) | Total Porosity (%) |
|---|---|---|---|---|---|---|---|---|
| R1180 | 11.2 ± 0.8 | 24 ± 3.0 | 3.8 ± 0.1 | 1.90 ± 0.01 | 2.57 ± 0.02 | 21.3 ± 0.6 | 4.7 ± 0.4 | 26.0 ± 1.1 |
| R1200 | 7.1 ± 0.7 | 37 ± 1.4 | 5.9 ± 0.1 | 2.08 ± 0.02 | 2.35 ± 0.02 | 14.8 ± 0.8 | 3.2 ± 0.4 | 18.0 ± 1.3 |
| R1220 | 3.8 ± 0.4 | 45 ± 2.5 | 8.1 ± 0.2 | 2.25 ± 0.02 | 2.29 ± 0.01 | 8.5 ± 0.5 | 1.4 ± 0.2 | 10.0 ± 0.6 |
| R1240 | 1.2 ± 0.2 | 59 ± 1.7 | 9.2 ± 0.2 | 2.31 ± 0.01 | 2.18 ± 0.02 | 2.7 ± 0.1 | 2.8 ± 0.1 | 5.5 ± 0.2 |
| R1260 | 0.3 ± 0.1 | 61 ± 1.5 | 9.8 ± 0.3 | 2.29 ± 0.01 | 2.14 ± 0.02 | 0.5 ± 0.1 | 4.3 ± 0.2 | 4.8 ± 0.2 |
| R1280 | 0.1 ± 0.1 | 59 ± 2.6 | 7.0 ± 0.1 | 2.23 ± 0.02 | 2.11 ± 0.01 | 0.2 ± 0.1 | 8.5 ± 0.4 | 8.8 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Qin, S. Sintering Behavior and Technological Properties of Low-Temperature Porcelain Tiles Prepared Using a Lithium Ore and Silica Crucible Waste. Minerals 2019, 9, 731. https://doi.org/10.3390/min9120731
Peng L, Qin S. Sintering Behavior and Technological Properties of Low-Temperature Porcelain Tiles Prepared Using a Lithium Ore and Silica Crucible Waste. Minerals. 2019; 9(12):731. https://doi.org/10.3390/min9120731
Chicago/Turabian StylePeng, Lihua, and Shan Qin. 2019. "Sintering Behavior and Technological Properties of Low-Temperature Porcelain Tiles Prepared Using a Lithium Ore and Silica Crucible Waste" Minerals 9, no. 12: 731. https://doi.org/10.3390/min9120731






