1. Introduction
An important topic in salt research are geochemical characteristics of brines, their host rocks, and the interaction processes between salt rocks and solutions. For this reason, the origin and genesis of brines in Permian Zechstein salt deposits have been the focus of many studies, e.g., by References [
1,
2,
3,
4]. For example, occurrences of saline solutions were detected in all North German salt mines [
5]. Most of these solutions have been classified as relicts of Permian seawater, which were trapped and stored within the salt during sedimentation and subsequent processes [
6]. Due to their potentially hazardous influence on mining activities, they are handled with high priority. In this context, potential migration paths, genesis, as well as the origin of the brines, are of interest.
Apart from saline solutions, gas and hydrocarbon occurrences are also observed in salt deposits [
5,
7,
8]. However, the present study focuses on brines and their geochemical properties.
Various geochemical signatures can be used for genetic interpretations of salt minerals and brines [
9], the most important are the trace elements Br and Rb. Therefore, substantial knowledge exists about the origin, the distribution in brines and minerals, and the thermodynamic properties of these elements [
3,
10,
11,
12,
13,
14,
15,
16,
17]. However, due to locally occurring brines with high Li concentrations in salt deposits, Li gets increasing attention in salt research.
High contents of Li (up to 7000 ppm, [
8]) are found and currently mined in different salt deposits in South America, e.g., the salt deposits of the Salar de Atacama, Chile, the Salar de Hombre Muerto, Argentinia [
8,
18] and the Salar de Uyuni, Bolivia [
8,
19,
20]. The main source of Li is related to water–rock interactions with volcanic country rock (Bolivia) [
21]. These brines result from evaporation. Li concentrations of some oil field brines (e.g., Smackover Formation, Gulf Coast (TX and FL, USA [
22,
23]) may reach >100 mg/L [
23]. However, apart from these general observations, only little knowledge exists about the principle geochemical behaviour of Li in evaporites, especially in relation to the interaction between brines and minerals.
In contrast to highly concentrated Li brines in salt deposits, there is no evidence of naturally occurring Li salts or hints of significant Li contents in naturally formed salt minerals. The occurrence of Li-carnallite, interpreted to have formed in salt lakes in South America, motivated the first experimental and crystallographic studies [
24,
25]. The authors of Reference [
26] published a probable Li
2SO
4 formation in Salar de Uyuni. In order to examine the behaviour of Li in this system, the authors of Reference [
26] evaporated these brines for up to 54 days. They found that during the first 34 days, the Li and SO
4 concentrations in the brine increased, but from the 35th day, the concentration in the solution decreased, which was interpreted as a result of LiSO
4 precipitation (indirect proof).
Currently, it is unknown if the detection of very low quantities of Li in the lower ppm range originate from fluid inclusions, or whether Li is incorporated in the crystal lattice of naturally formed salt minerals.
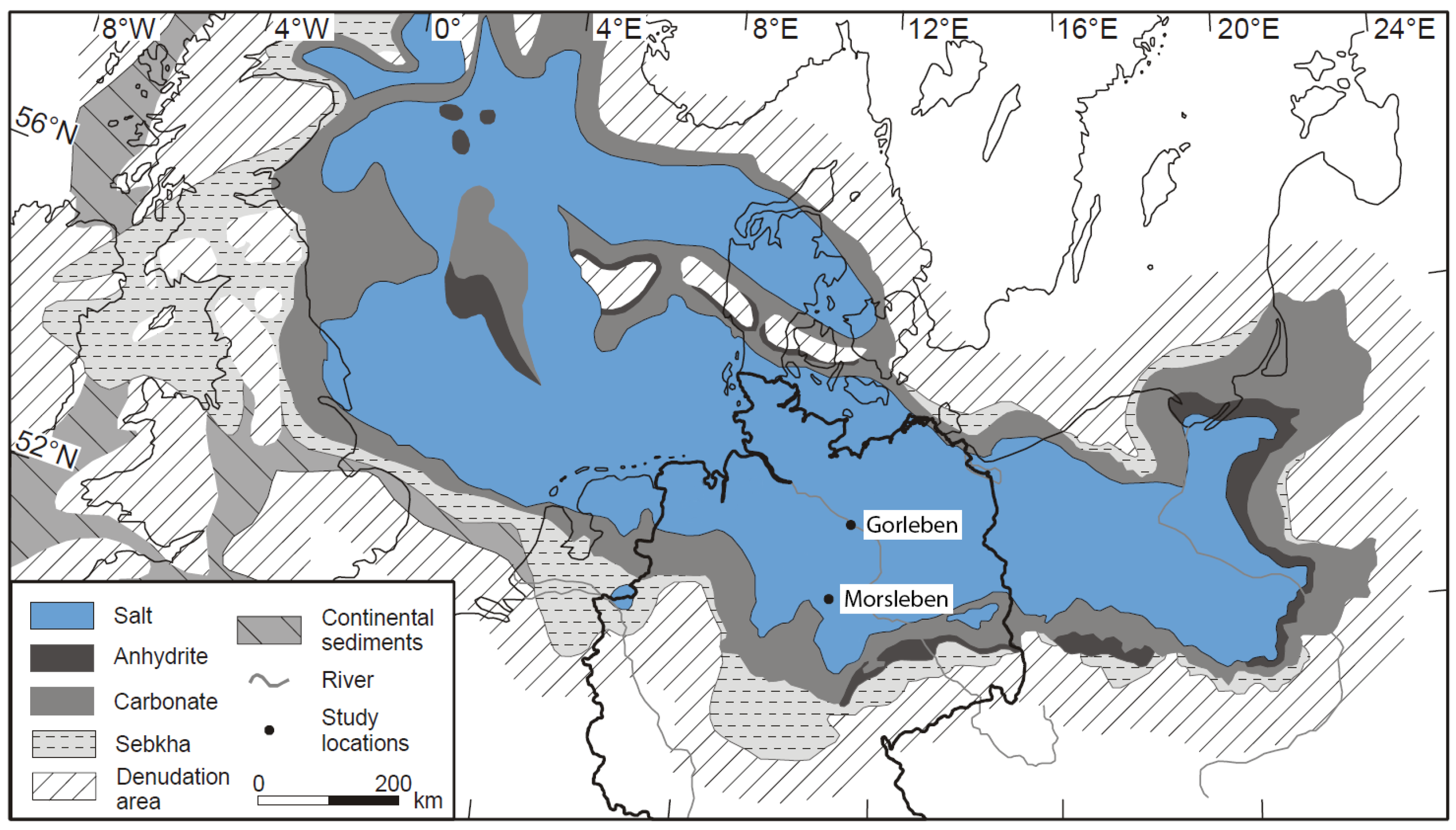
In brines and rocks of the Upper Permian salt deposits of the Gorleben salt dome and the Morsleben salt structure, both located in the Southern Permian Basin, northern Germany (
Figure 1), high Li concentrations of up to 401 µg/g in brines [
27] and 161 µg/g in bulk rock samples [
28] were measured. The Gorleben salt dome consists of Upper Permian (Zechstein) rock salt formations. The salt dome is aligned in the NE–SW direction and is ca. 14 km long. The salt table is located ca. 250 m below ground level [
5]. The salt movement started in the Early Triassic and during Upper Jurassic periods to the Lower Cretaceous period, and the salt rocks penetrated the overburden and created a salt dome. The final stages of salt rise occurred during the Upper Cretaceous and Paleogen periods [
29,
30]. The Gorleben salt dome was investigated for its suitability to construct a repository for high-level radioactive waste between 1979–2000 and 2010–2012. In the Gorleben exploration mine, saline solutions were collected continuously at different sites between ca. 1996 and 2012, all of the solutions originating from anhydrite rocks [
5,
31].
The Morsleben salt structure is located in the northeastern part of the Subherzynian basin, at the southern rim of the Zechstein basin. In this region, Zechstein salt migrated into the NW–SE trending Allertal fault zone [
33] and underwent various types of deformation. The main salt migration took place from the Upper Triassic to Cretaceous periods with material inflow mainly from the west to the east [
34]. The salt body is primarily regarded as a tectonic structure and not a halokinetic one [
34]. In the Morsleben mine, brine samples of two influxes have been collected and analysed sporadically since 1907, and continuously since 1991 [
35].
References [
10,
36] already linked elevated Li concentrations in brines to be originated from phyllosilicate-bearing strata (also see References [
8,
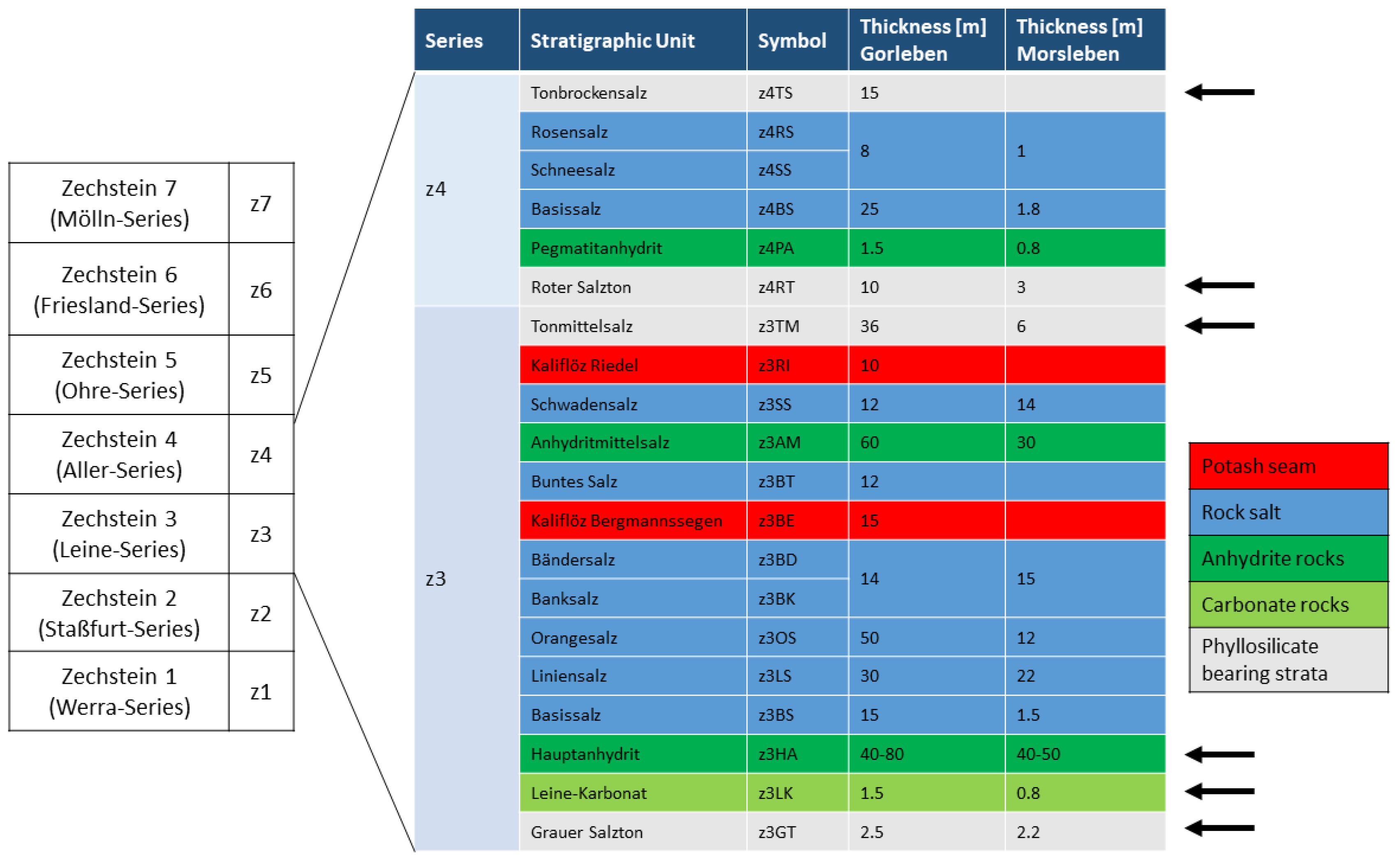
37]). Typical lithostratigraphic units of this type are, for example, the Grauer Salzton (z3GT), Leinekarbonat (z3LK), Hauptanhydrit (z3HA), Tonmittelsalz (z3TM), Roter Salzton (z4RT) and the Tonbrockensalz (z4TS) (
Figure 2).
The “Hauptanhydrit” (z3HA) is an anhydrite rock unit, with a thickness of ca. 40–80 m. The unit is subdivided into 13 zones, distinguishable by differences in composition, sedimentary-diagenetic structures, grain size and thickness. The base of the Hauptanhydrit is characterized by ca. 4 wt. % carbonate (mainly magnesite, minor dolomite and calcite) and traces of quartz and mica. In certain areas, the magnesite content can increase to 22 wt. %, caused by algal layers [
5,
39]. At the top of the layer, the magnesite content decreases to ca. 2 wt. % [
39].
The footwall of the Hauptanhydrit consists of the “Grauer Salzton” (z3GT), a maximum of 2.5 m thick phyllosilicate-bearing rock and the “Leine-Karbonat” (z3LK), a carbonate rock of maximum 1.5 m thickness. The composition of these stratigraphic units differs, depending on the sedimentary conditions and the position within the Zechstein basin due to the transport distance of clastic material from the backcountry.
In Gorleben, in the centre of the basin, the composition is more homogeneous. The main components of the z3GT are anhydrite (ca. 55 wt. %), hydrotalcite, chlorite and quartz, and the minor components are magnesite, halite, illite and kaolinite [
5]. The z3LK consists of magnesite and anhydrite, only trace amounts of hydrotalcite were observed [
5].
In Morsleben, at the rim of the basin, the main components of the z3GT are quartz, muscovite-illite (ca. 30 wt. % each), with additional trace amounts of tourmaline, chlorite, serpentine and halite [
39]. The z3LK consists of magnesite (ca. 56 wt. %), anhydrite (ca. 20 wt. %) and minor amounts of quartz, muscovite-illite, koenenite, chlorite and calcite [
39].
The transition between the Leine- and the Aller-Series is characterised by the Tonmittelsalz (z3TM) and the Roter Salzton (z4RT). Close to the top of the Aller-Series, the Tonbockensalz (z4TS) is developed. These units are characterized by a high amount of halite of ca. 80–90 wt. % [
39], with minor amounts of quartz, anhydrite, muscovite, chlorite-smectite, kaolinite and magnesite [
28]. Clay rock occurrences are interrupted by intercalations of anhydrite and halite layers. The z4TS could not be observed in the Morsleben salt structure.
Organic matter is contained in all stratigraphic units described, often enriched in thin layers associated with carbonate and quartz/phyllosilicates.
Depending on the lithology of the samples, Li concentrations are highly variable [
28]. Potentially Li-bearing minerals within phyllosilicate-containing strata are muscovite and chlorites.
Due to relatively low Li concentrations in seawater (0.17 µg/g; [
40]) and evaporated seawater, from which the salts originate, it is unclear where the high Li concentrations derive from. The Li concentrations of brines detected in salt deposits are in the range of bulk rock analyses of Upper Permian (Zechstein) phyllosilicate-bearing strata [
28], both of which are some magnitudes higher than the Li concentrations of the most strongly evaporated seawater. Lepidolite was used to investigate leaching effects between brines and phyllosilicates because lepidolite is comparable with muscovites and chlorites that are common components of the phyllosilicate-bearing strata. Another advantage of using lepidolite is its very high Li content, advantageous for getting a measurable leaching effect in a manageable timescale for laboratory experiments. Experimental studies on the leaching behaviour were performed by exposing lepidolite to 18 saline solutions of different composition for the duration of ca. three years.
2. Analytical Methods and Experimental Setup
2.1. Sampling of Natural Brines
In the Gorleben salt dome, the inflow of solutions was linked to mining activity. During excavation of galleries, at certain points, connected to changes in lithology and lithostratigraphic boundaries, saline solutions entered the mine. However, most solution influxes had a limited volume of few dm
3 to several m
3 [
5]. Brine that originated from more extensive and long-lasting solution influx were collected by a system of tailraces, tubes and accumulation bins. The gathered brine was sampled on a regular basis.
In the Morsleben mine, a similar construction is used to collect brine at mining claim 1A. The volume of inflowing brine is ca. 1.4 m
3/a [
35]. The influx rate has been sporadically analysed since 1962, and systematic analyses of the composition and trace element content have been carried out in monthly intervals since 1991. At the second solution influx, mining claim H, the brine inflow started in 1907 due to extensive mining activities. Consequently, a protection embankment was built, thus the exact position of the influx is not clear. Furthermore, the volume is higher (ca. 10 m
3/a, [
35]), therefore the brine is collected in a pool. The influx rate has been monitored since 1907, and systematic analyses of the composition and trace element content have also been carried out since 1991.
2.2. Experimental Setup
For each experimental run, 8 g of lepidolite (from Minas Gerais, Brazil; stoichiometric formula: K(Li,Al)
2–3((OH,F)
2/Si
3AlO
10)) with a lithium concentration of 2.42 wt. % (for more details see
Section 3.2.1) was ground to a grain size <200 µm and added to 100 g solution (mass ratio 1
(rock):12.5
(solution)). The compositions of the solutions vary from double distilled H
2O, NaCl, KCl, MgCl
2 solutions, modern seawater to artificial solutions (
Table 1), approximately comparable to the composition of the so-called solutions Q, R and Z (according to References [
10,
41]). These solutions are basically halite, anhydrite and partly polyhalite saturated. Solution Q is saturated with respect to sylvite, carnallite and kainite. Solution R is saturated with respect to kieserite, carnallite and kainite. Solution Z is saturated with respect to kieserite, carnallite and bischofite (
Figure 3). With the exception of seawater and double distilled H
2O, the solutions were prepared using pure NaCl (Emsure ACS, ISO for analysis, Merck), KCl (pro analysi, Merck), MgCl
2·6H
2O (extra pure for table water, Merck) and MgSO
4·H
2O (Sigma-Aldrich). The first solution used for the experiments was double distilled H
2O, representing the largest difference in concentration with an electrical conductivity of 0.055 µS/cm (sample 1).
Three pure NaCl solutions were used for the experiments:
0.42 mol NaCl/kg H2O is a typical NaCl-content of fresh seawater (sample 2);
4.96 mol NaCl/kg H2O assigns first halite precipitation from evaporating seawater (sample 3);
5.74 mol NaCl/kg H2O is close to the theoretical halite saturation at 6.11 mol/kg H2O (sample 4) in a pure NaCl solution.
Six pure artificial KCl solutions were created:
0.01 mol KCl/kg H2O represents the KCl content of fresh seawater (sample 5);
0.02 mol KCl/kg H2O almost corresponds to solution Z at the point of bischofite formation at the end of seawater evaporation (sample 6);
0.19 mol KCl/kg H2O is typical at halite formation during seawater evaporation (sample 7);
0.37 mol KCl/kg H2O assigns KCl concentration of evaporating seawater at polyhalite saturation (sample 8);
0.60 mol KCl/kg H2O represents almost solution Q, equilibrium with sylvite, carnallite and kainite at 25 °C (sample 9), and
4.29 mol KCl/kg H2O at sylvite saturation in a pure KCl solution (sample 10).
Four pure MgCl2 solutions were used:
0.03 mol MgCl2/kg H2O, representative for fresh seawater (sample 11);
3.50 mol MgCl2/kg H2O, as in solution Q at 25 °C (sample 12);
4.26 mol MgCl2/kg H2O, as in solution R at 25 °C (sample 13), and
5.51 mol MgCl2/kg H2O near bischofite saturation, almost representing solution Z at 25 °C (sample 14).
Sample 15 represents seawater from the North Sea. Additionally, three artificial solutions that correspond approximately to the solutions at the invariant points are Q (sample 16), R (sample 17) and Z (sample 18). All three solutions are comparable to the natural analogue with the exception of the trace components (Br, Rb, Li and Si).
The lepidolite samples in the solutions were shaken at a temperature of 22 °C to 25 °C on a shaking table with a frequency of 150 shakes/min. During the first year, the process was interrupted only shortly after 24 to 26 days for measuring the electrical conductivity. After one year, the solutions were separated from the lepidolite by filtration with Sartorius membrane filters (pore size <0.45 µm and <0.1 µm, Sartorius AG, Göttingen, Germany), washed with double distilled H2O, cleaned with ethanol and dried at room temperature. In a second step, half of the reacted lepidolite and half of the reaction solution were merged again (in the same rock-water-mass-relation of 1(rock):12.5(solution)), and the experiments continued for two more years following the same conditions described above. The entire experiment lasted for ca. three years.
2.3. Analytical Methods
The initial, unaltered lepidolite was analysed using XRD (PANalytical MPD Pro, Malvern Panalytical GmbH, Kassel, Germany), XRF (PANalytical Axios) using the total fusion (tetra-) borate flux method, ICP-OES (Agilent Technologies 5100, LabWrench, Santa Clara, CA, USA) and ICP-MS (Thermo Fisher iCAP Q, Thermo Fisher Scientific, Waltham, MA, USA). The solutions, initial and after reaction, were analysed with respect to density (Anton PAAR DMA 38, Anton Paar GmbH, Graz, Austria), electrical conductivity (WTW Multi 3420, and WTW TetraCon 925, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany), pH (WTW MultiLine P4 and WTW SenTix 81) as well as main, minor (ICP-OES, Spectro Arcos, Spectro Analytical instruments GmbH, Kleve, Germany) and trace components (ICP-MS, Agilent 7500/7700, LabWrench, Santa Clara, CA, USA). Furthermore, both initial and altered lepidolite were analysed by optical microscopy and SEM (FEI Quanta 650 MLP, Thermo Fisher Scientific, Waltham, MA, USA) analyses.
The standard deviation (2σ) for the ICP-OES Spectro Arcos is <1% for Na, K, Ca, Mg, Cl and SO4 of the analysed brines. The dissolved lepidolite was analysed using ICP-OES (Agilent Technologies 5100) with a RSD (relative standard deviation) <1% for Al, Ca, K, Li, Mg, SO4 and <1.5% for Na. The ICP-MS (Thermo Fisher iCAP Q) shows a standard deviation of (2σ) < 2%.
Analyses of rock samples and natural brines from the Gorleben and Morsleben mines occurred the same way [
28,
35], with certain differences of the used hardware due to the long duration of the monitoring.
Modelling of the mineral saturation of the natural brines and the seawater was performed with the software package EQ3/6v7.2 (version 7.2, Lawrence Livermore National Laboratory, Livermore, CA, USA) [
42] special version c [
16,
17], using the thermodynamic data base hmw (version R10, Lawrence Livermore National Laboratory, Livermore, CA, USA) [
43], modified according to [
16,
17].
5. Conclusions
The investigation of Li occurrences in Upper Permian (Zechstein) salt rocks and saline solutions of the Gorleben salt dome and Morsleben salt structure yield that most of the brine occurrences are connected to anhydrite rock-bearing formations and lithological boundaries. Due to their geochemical composition, especially the high Mg and Br content in relation to their mineral saturation status, this characterizes them as intrasalinar solutions, which developed during sedimentation/diagenesis and the subsequent rock–fluid interaction. Thermodynamic modelling suggests that maximum evaporated seawater shows no higher Li concentration than ca. 26 µg/g. In the Gorleben site, brines with Li concentrations of up to 401 µg/g were found, which is in line with published data for saline brines from North German salt deposits. Due to distinct higher Li concentrations in the brines, other sources of Li could be considered.
However, the origin of Li in the brines cannot be clearly defined at present. Different sources were discussed, e.g., that Li in the brines may originate from contact of saline solution with phyllosilicate-bearing strata. Other possible Li sources could be relictic metamorphic brines and organic compounds, which support enrichment of Li.
To improve the understanding of the Li origin in saline brines in an evaporitic environment, leaching experiments using a Li-bearing phyllosilicate were performed. Instead of muscovite and chlorite, which are typical phyllosilicates of the silicate-bearing strata, the more Li-containing lepidolite was used as an analogue material. Lepidolite with a Li content of 2.42 wt. % was exposed to 18 solutions of different composition (17 saline solutions and double distilled H2O) for three years. The most intensive leaching effect (53.36 µg Li/g in the brine) was observed on the interaction with a 0.03 molal MgCl2 solution and the second most by a modern seawater interaction (50.59 µgLi/gbrine), followed by low concentrated KCl solutions (0.1 to 0.60 molal KCl). The experiments show that Li was leached from the lepidolite, dependent on duration of the interaction reaction, the composition and pH value of the brines. Independent of these basic conditions, in all interaction solutions, with the exception of double distilled H2O, lepidolite was leached, resulting in minimal contents of ca. 40 µgLi/gbrine. The experiments show that the reaction progress was not finished after three years. Additional elements were leached (e.g., Si, Rb and Cs), and composition-dependent, pH changes were detected as well.
The results from the experiments, regarding principal rock–water interaction processes, are basically transferable to the natural occurrences of phyllosilicate-bearing strata of the Gorleben and Morsleben salt deposits, but do not provide resilient proof for the origin of the Li in the investigated brines. Li-bearing phyllosilicates like muscovite and chlorites are typical in the rock types [
28] and are comparable to lepidolite, which certainly shows a higher Li concentration. The Mg-containing solutions are the most effective for Li leaching by trend, which was observed in the experiments as well as in the natural brines. These Mg- and Li-enriched natural brines occurred mainly in phyllosilicate-bearing strata and anhydrite rocks or migrated through them.
The experiments lasted three years at a temperature of 22–25 °C, and it can be assumed that in geological times, much more Li might be leached due to much more reaction time and higher temperatures (maximum 150 °C, [
38]). The maximum depth of the salt deposit of Gorleben and Morsleben, regarding the Zechstein basis, was 3500 m to 4000 m. The present lithostatic pressure in Gorleben is 17.6–19.3 MPa at the mining level, and the rock temperature is 30–38 °C [
53].
Further investigations will be extended to Li-bearing phyllosilicates that are typical for the phyllosilicate-bearing strata, e.g., muscovite and chlorite.
To estimate the source of Li in the brines of the salt deposits and to study brine–rock interaction processes, stable isotopic investigations will be performed. The δ
7Li of seawater is ca. +31‰ and considerably higher compared to most other rock types [
58]. Therefore, it should be possible to distinguish the marine origin of the brine, e.g., relictic Permian pore solutions, or freshwater. Further, the interaction between brine and allochthones-originated phyllosilicates could be determined.