Surface Properties and Floatability Comparison of Aegirite and Specularite by Density Functional Theory Study and Experiment
Abstract
:1. Introduction
2. Materials, Reagents, and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Computational Method
2.2.2. Optimization of Aegirite and Specularite Bulk Structure
2.2.3. Optimization of Aegirite and Specularite Surfaces
2.2.4. X-ray Diffraction (XRD) Measurements
2.2.5. Flotation Tests
2.2.6. Zeta Potential Measurements
3. Results and Discussion
3.1. Preparation of Aegirite and Specularite Bulk Crystals
3.2. Surface Slab Model Optimization
3.3. Relationship between Surface Property and Floatability
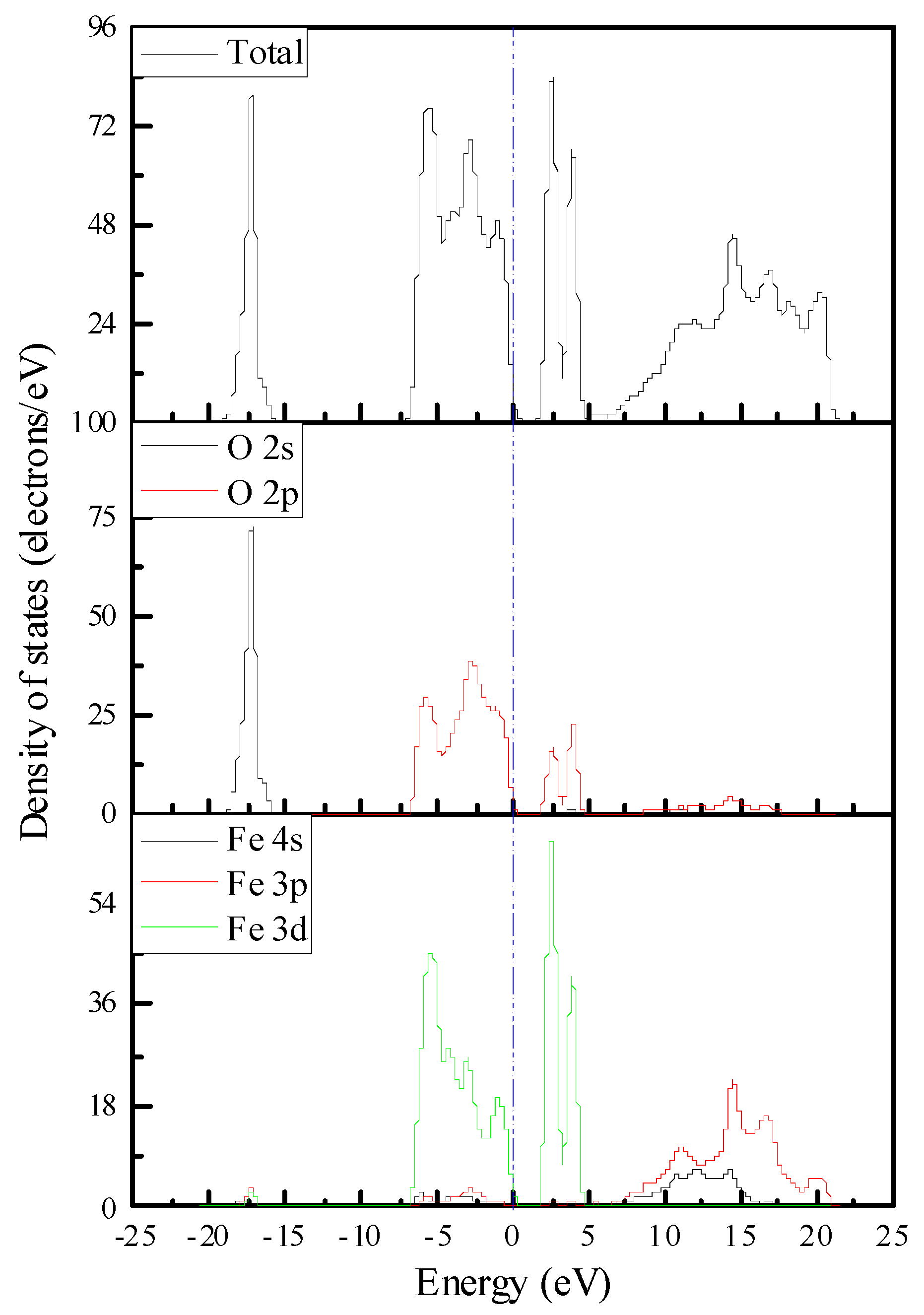
3.3.1. Electronic Structure and Properties of Mineral Surface
3.3.2. Unsatisfied Bond Properties of Mineral Surface
3.3.3. Surface Charge of Mineral Surface
3.3.4. Floatability of Aegirite and Specularite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakhaei, F.; Irannajad, M. Reagents types in flotation of iron oxide minerals: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 89–124. [Google Scholar] [CrossRef]
- Sedov, A. Overview of Steel and Iron Market; Deloitte CIS Research Center: Moscow, Russia, 2017. [Google Scholar]
- Hao, H.; Li, L.; Yuan, Z.; Liu, J. Comparative effects of sodium silicate and citric acid on the dispersion and flotation of carbonate-bearing iron ore. J. Mol. Liq. 2018, 271, 16–23. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Wang, B.; Duan, H.; Peng, X.; Chen, X.; Zhao, Q. Novel hydroxy polyamine surfactant N-(2-hydroxyethyl)-N-dodecyl-ethanediamine: Its synthesis and flotation performance study to quartz. Miner. Eng. 2019, 142, 105894. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Severov, V.V. The use of collectors mixture in the reverse cationic flotation of magnetite ore: The role of Fe-bearing silicates. Miner. Eng. 2010, 23, 91–98. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Hu, Y.; He, J.; Tian, M.; Zhou, J.; Zhou, Q.; Chen, S.; Chen, D.; Chen, P.; et al. Novel insights into the hydroxylation behaviors of α-quartz (101) surface and its effects on the adsorption of sodium oleate. Minerals 2019, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Zhou, Y.; Zhang, Y. The effect of new modified fatty acid (CY-23) collector on chlorite/hematite separation. J. Chem. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Filippov, L.O.; Severov, V.V.; Filippova, I.V. Mechanism of starch adsorption on Fe-Mg-Al-bearing amphiboles. Int. J. Miner. Process. 2013, 123, 120–128. [Google Scholar] [CrossRef]
- Sahoo, H.; Rath, S.S.; Rao, D.S.; Mishra, B.K.; Das, B. Role of silica and alumina content in the flotation of iron ores. Int. J. Miner. Process. 2016, 148, 83–91. [Google Scholar] [CrossRef]
- Luo, X.M.; Yin, W.Z.; Wang, Y.F.; Sun, C.Y.; Ma, Y.Q.; Liu, J. Effect and mechanism of siderite on reverse anionic flotation of quartz from hematite. J. Cent. South Univ. 2016, 23, 52–58. [Google Scholar] [CrossRef]
- Moreira, G.F.; Peçanha, E.R.; Monte, M.B.M.; Leal Filho, L.S.; Stavale, F. XPS study on the mechanism of starch-hematite surface chemical complexation. Miner. Eng. 2017, 110, 96–103. [Google Scholar] [CrossRef]
- Quast, K. Literature review on the use of natural products in the flotation of iron oxide ores. Miner. Eng. 2017, 108, 12–24. [Google Scholar] [CrossRef]
- Li, M.; Huangfu, M.; Hu, Y.; Liu, J.; Gao, X.; Liu, W. Effect and mechanism of iron ion on flotation of aegirite in dodecylamine system. Chin. J. Process. Eng. 2019, 19, 1014–1021. (In Chinese) [Google Scholar]
- Silvester, E.J.; Bruckard, W.J.; Woodcock, J.T. Surface and chemical properties of chlorite in relation to its flotation and depression. Miner. Process. Extr. Metall. 2011, 120, 65–70. [Google Scholar] [CrossRef]
- Kar, B.; Sahoo, H.; Rath, S.S.; Das, B. Investigations on different starches as depressants for iron ore flotation. Miner. Eng. 2013, 49, 1–6. [Google Scholar] [CrossRef]
- Sahoo, H.; Rath, S.S.; Das, B.; Mishra, B.K. Flotation of quartz using ionic liquid collectors with different functional groups and varying chain lengths. Miner. Eng. 2016, 95, 107–112. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Wang, B.; Zhao, Q.; Duan, H.; Chen, X. Molecular-level insights into the adsorption of a hydroxy-containing tertiary amine collector on the surface of magnesite ore. Powder Technol. 2019, 355, 700–710. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A Phys. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Sun, W.; Hu, Y.H.; Liu, X.W. Anisotropic surface broken bond properties and wettability of calcite and fluorite crystals. Trans. Nonferrous Met. Soc. China 2012, 22, 1203–1208. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Liu, X.; Yu, F.; Lu, D. The cleavage and surface properties of wet and dry ground spodumene and their flotation behavior. Appl. Surf. Sci. 2015, 357, 333–339. [Google Scholar] [CrossRef]
- Han, C.; Li, T.; Zhang, W.; Zhang, H.; Zhao, S.; Ao, Y.; Wei, D.; Shen, Y. Density functional theory study on the surface properties and floatability of hemimorphite. Minerals 2018, 8, 542. [Google Scholar] [CrossRef] [Green Version]
- He, G.; Xiang, H.; Jiang, W.; Kang, J.; Chen, J. First-principles theory on electronic structure and floatability of spodumene. Rare Metals. 2014, 33, 742–748. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Guo, J. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite. Miner. Eng. 2010, 23, 1120–1130. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Z.; Zheng, G.; Zhu, Y.; Wu, W. The design of a macromolecular depressant for galena based on DFT studies and its application. Miner. Eng. 2017, 112, 50–56. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Wu, H.; Tian, J.; Liu, J.; Gao, Z.; Wang, L. Surface crystal chemistry of spodumene with different size fractions and implications for flotation. Sep. Purif. Technol. 2016, 169, 33–42. [Google Scholar] [CrossRef]
- Dzade, N.Y.; Roldan, A.; Leeuw, N.H.; De, A. Density functional theory study of the adsorption of benzene on hematite (α-Fe2O3) surfaces. Minerals 2014, 4, 89–115. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Hao, H.; Yuan, Z.; Liu, J. Molecular dynamics simulation of siderite-hematite-quartz flotation with sodium oleate. Appl. Surf. Sci. 2017, 419, 557–563. [Google Scholar] [CrossRef]
- De Oliveira, C.; Duarte, H.A. Disulphide and metal sulphide formation on the reconstructed (0 0 1) surface of chalcopyrite: A DFT study. Appl. Surf. Sci. 2010, 257, 1319–1324. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The american mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Cameron, M.; Sueno, S.; Prewitt, C.T.; Papike, J.J. High-temperature crystal chemistry of acmite, diopside, hedenbergite jadeite, spodumene and ureyite. Am. Mineral. J. Earth Planet. Mater. 1973, 58, 594–618. [Google Scholar]
- Maslen, E.N.; Streltsov, A.V.; Streltsova, R.N.; Ishizawa, N. Synchrotron X-ray study of the electron density in alpha-Fe2O3 Locality: Synthetic. Acta Crystallogr. Sect. B Struct.Sci. 1994, 50, 435–441. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.; Wu, B.; Long, X. Density functional theory study on natural hydrophobicity of sulfide surfaces. Trans. Nonferrous Met. Soc. China 2014, 24, 491–498. [Google Scholar] [CrossRef]
- Tong, Y.Y.; Renouprez, A.J.; Martin, G.A.; Van der Klink, J.J. Electron availability and the surface Fermi level local density of states: An alternative way to see catalytic activity of metals. Stud. Surf. Sci. Catal. 1996, 101, 901–910. [Google Scholar]
- Baum, M.; Komarek, A.C.; Holbein, S.; Fernández-Díaz, M.T.; André, G.; Hiess, A.; Sidis, Y.; Steffens, P.; Becker, P.; Bohatý, L.; et al. Magnetic structure and multiferroic coupling in pyroxene NaFeSi2O6. Phys. Rev. B 2015, 91, 214415. [Google Scholar] [CrossRef]
- Kinkar Roy, R.; Hirao, K.; Krishnamurty, S.; Pal, S. Mulliken population analysis based evaluation of condensed Fukui function indices using fractional molecular charge. J. Chem. Phys. 2001, 115, 2901–2907. [Google Scholar] [CrossRef]
- Gao, X.; Li, M.; Zhao, Y.; Zhang, Y. Mechanistic study of selective adsorption of Hg2+ ions by porous alginate beads. Chem. Eng. J. 2019, 278, 122096. [Google Scholar] [CrossRef]
- Wei, D.Z. Solid Material Sorting, 3rd ed.; Metallurgical Industry Press: Beijing, China, 2015. [Google Scholar]
- Shrimali, K.; Jin, J.; Hassas, B.V.; Wang, X.; Miller, J.D. The surface state of hematite and its wetting characteristics. J. Colloid Interface Sci. 2016, 477, 16–24. [Google Scholar] [CrossRef]
- Yin, W.; Sun, H.; Hong, J.; Cao, S.; Yang, B.; Won, C.; Song, M. Effect of Ca selective chelator BAPTA as depressant on flotation separation of magnesite from dolomite. Mineral. Eng. 2019, 144, 106050. [Google Scholar] [CrossRef]
- Yue, T.; Wu, X. Depressing iron mineral by metallic-starch complex (MSC) in reverse flotation and its mechanism. Minerals 2018, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Yang, S.; Liu, C.; Li, H. Effects of the calcite on quartz flotation using the reagent scheme of starch/dodecylamine. Colloid Surf. A Physicochem. Eng. Asp. 2019, 583, 123983. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Yuan, Z.; Xu, X.; Song, Z. AFM and DFT study of depression of hematite in oleate-starch-hematite flotation system. Appl. Surf. Sci. 2019, 480, 749–758. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, B.; Sun, C.; Liu, J.; Sun, H.; Li, Y.; Han, Y. Density functional theory study of α-Bromolauric acid adsorption on the α-quartz (1 0 1) surface. Miner. Eng. 2016, 92, 72–77. [Google Scholar] [CrossRef]
- Tang, M.; Liu, Q. The acidity of caustic digested starch and its role in starch adsorption on mineral surfaces. Int. J. Miner. Process. 2012, 112, 94–100. [Google Scholar] [CrossRef]









| Mineral | Property | |||||
|---|---|---|---|---|---|---|
| Aegirite | Slab depth (Å) | 6.272 | 12.543 | 18.814 | 25.085 | 31.356 |
| Surface energy(J/m2) | 0.317 | 0.476 | 0.503 | 0.521 | 0.524 | |
| Vacuum thickness (Å) | 8 | 10 | 12 | 14 | 16 | |
| Surface energy(J/m2) | 0.517 | 0.521 | 0.526 | 0.528 | 0.529 | |
| Specularite | Slab depth (Å) | 13.772 | 27.544 | 41.316 | 55.088 | |
| Surface energy(J/m2) | 1.116 | 1.231 | 1.233 | 1.234 | ||
| Vacuum thickness (Å) | 8 | 10 | 12 | 14 | 16 | |
| Surface energy(J/m2) | 1.219 | 1.232 | 1.235 | 1.237 | 1.238 | |
| Mineral | Displacement (Å) | ||
|---|---|---|---|
| ∆Fe | ∆O1 | ∆O2 | |
| Aegirine | −0.2300 | 0.0016 | 0.0156 |
| Specularite | −0.2807 | 0.0028 | 0.0161 |
| Mineral | Interior Value | Surface Value |
|---|---|---|
| Aegirine (∑ (Fe–O, Si–O Bond)) | 4.41 | 3.95 |
| Specularite (∑ Fe–O Bond) | 1.62 | 1.29 |
| Mineral | ∑ Negative Charge (×10−19 C) | ∑ Positive Charge (×10−19 C) | R (Negative/Positive) |
|---|---|---|---|
| Aegirine | −1.49 | 0.74 | 2.01 |
| Specularite | −1.29 | 0.81 | 1.59 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Liu, J.; Gao, X.; Hu, Y.; Tong, X.; Zhao, F.; Yuan, Q. Surface Properties and Floatability Comparison of Aegirite and Specularite by Density Functional Theory Study and Experiment. Minerals 2019, 9, 782. https://doi.org/10.3390/min9120782
Li M, Liu J, Gao X, Hu Y, Tong X, Zhao F, Yuan Q. Surface Properties and Floatability Comparison of Aegirite and Specularite by Density Functional Theory Study and Experiment. Minerals. 2019; 9(12):782. https://doi.org/10.3390/min9120782
Chicago/Turabian StyleLi, Mingyang, Jun Liu, Xiangpeng Gao, Yiming Hu, Xiong Tong, Fugang Zhao, and Qidong Yuan. 2019. "Surface Properties and Floatability Comparison of Aegirite and Specularite by Density Functional Theory Study and Experiment" Minerals 9, no. 12: 782. https://doi.org/10.3390/min9120782
APA StyleLi, M., Liu, J., Gao, X., Hu, Y., Tong, X., Zhao, F., & Yuan, Q. (2019). Surface Properties and Floatability Comparison of Aegirite and Specularite by Density Functional Theory Study and Experiment. Minerals, 9(12), 782. https://doi.org/10.3390/min9120782







