A pXRF In Situ Study of 16th–17th Century Fresco Paints from Sviyazhsk (Tatarstan Republic, Russian Federation)
Abstract
:1. Introduction
2. Fresco Technique
3. Materials and Methods
4. Results and Discussion
4.1. Chemistry of the Paints
4.1.1. White Paint
4.1.2. Yellow Paint
4.1.3. Orange Paint
4.1.4. Pink Paint
4.1.5. Brown Paint
4.1.6. Red Paint
4.1.7. Grey Paint
4.1.8. Black Paint
4.1.9. Green Paint
4.1.10. Blue Paint
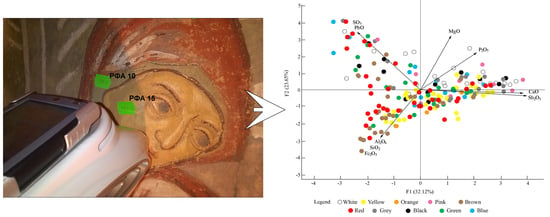
4.2. Statistical Processing
4.3. General Remarks on Pigments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgement
Conflicts of Interest
References
- Deneckere, A.; Schudel, W.; Van Bos, M.; Wouters, H.; Bergmans, A.; Vandenabeele, P.; Moens, L. In situ investigations of vault paintings in the Antwerp cathedral. Spectrochim. Acta Part A 2010, 75, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.C.; Capogrosso, V.; Gironda, M.; Alberti, R.; Mazzei, C.; Nodari, L.; Gambirasi, A.; Zendria, E.; Nevin, A. Multi-analytical non-invasive study of modern yellow paints from postwar Italian paintings from the International Gallery of Modern Art Cà Pesaro, Venice. X-Ray Spectrom. 2015, 44, 296–304. [Google Scholar] [CrossRef]
- Samanian, K. Identification of pigment used in Persian wall paintings (AD 1501-1736) using PLM, FT-IR, SEM/EDX and GC-MS techniques. Archaeometry 2015, 57, 740–758. [Google Scholar] [CrossRef]
- Hein, A.; Karatasios, I.; Mourelatos, D. Byzantine wall paintings from Mani (Greece): Microanalytical investigation of pigments and plasters. Anal. Bioanal. Chem. 2009, 395, 2061–2071. [Google Scholar] [CrossRef]
- Linn, R. Layered pigments and painting technology of the Roman wall paintings of Caesarea Maritima. J. Archaeol. Sci. Rep. 2017, 11, 774–781. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I.; Rozenberg, S. X-ray diffraction and SEM investigation of wall paintings found in the Roman Temple Complex at Horvat Omrit, Israel. Archaeometry 2015, 57, 773–787. [Google Scholar] [CrossRef]
- Holclajtner-Antunović, I.; Stojanović-Marić, M.; Bajuk-Bogdanović, D.; Žikić, R.; Uskoković-Marković, S. Multi-analytical study of techniques and palettes of wall paintings of the monastery of Žiča, Serbia. Spectrochim. Acta Part A 2016, 156, 78–88. [Google Scholar] [CrossRef]
- Marić-Stojanović, M.; Bajuk-Bogdanović, D.; Uskoković-Marković, S.; Holclajtner-Antunović, I. Spectroscopic analysis of XIV century wall paintings from Patriarchate of Peć Monastery, Serbia. Spectrochim. Acta Part A 2018, 191, 469–477. [Google Scholar] [CrossRef]
- Mohanu, I.; Mohanu, D.; Gomoiu, I.; Barbu, O.H.; Fechet, R.M.; Vlad, N.; Voicu, G.; Truşcă, G. Study of the frescoes in Ioneştii Govorii wooden church (Romania) using multi-technique investigations. Microchem. J. 2016, 126, 332–340. [Google Scholar] [CrossRef]
- Čechák, T.; Gerndt, J.; Musílek, L.; Kopecká, I. Analysis of fresco paintings by X-ray fluorescence method. Radiat. Phys. Chem. 2001, 61, 717–719. [Google Scholar] [CrossRef]
- Cesareo, R.; Castellano, A.; Buccolieri, G.; Quarta, S.; Marabelli, M.; Santopadre, P.; Ioele, M.; Gigante, G.E.; Ridolfi, S. From Giotto to De Chirico: Analysis of paintings with portable EDXRF equipment. In Cultural Heritage Conservation and Environmental Impact Assessment by Non-Destructive Testing and Micro-Analysis; Van Grieken, R., Janssens, K., Eds.; Taylor and Francis Group, A.A. Balkema Publ.: Leiden, The Netherlands, 2004; pp. 183–196. ISBN 9789058096814. [Google Scholar]
- Zucchiatti, A.; Prati, P.; Bouquillon, A.; Giuntini, L.; Massi, M.; Migliori, A.; Cagnana, A.; Roascio, S. Characterisation of early medieval frescoes by μ-PIXE, SEM and Raman spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B 2004, 219–220, 20–25. [Google Scholar] [CrossRef]
- Nord, A.G.; Tronner, K.; Billström, K.; Strandberg Zerpe, B. Analysis of mediaeval Swedish paintings influenced by Russian-Byzantine art. J. Cult. Herit. 2017, 23, 162–169. [Google Scholar] [CrossRef]
- Miriello, D.; Bloise, A.; Crisci, G.M.; De Luca, R.; De Nigris, B.; Martellone, A.; Osanna, M.; Pace, R.; Pecci, A.; Ruggieri, N. Non-destructive multi-analytical approach to study the pigments of wall painting fragments reused in mortars from the archaeological site of Pompeii (Italy). Minerals 2018, 8, 134. [Google Scholar] [CrossRef]
- Duran, A.; Perez-Rodriguez, J.L.; Jimenez de Haro, M.C.; Franquelo, M.L.; Robador, M.D. Analytical study of Roman and Arabic wall paintings in the Patio De Banderas of Reales Alcazares’ Palace using non-destructive XRD/XRF and complementary techniques. J. Archaeol. Sci. 2011, 38, 2366–2377. [Google Scholar] [CrossRef]
- Vornicu, N.; Bibire, C.; Murariu, E.; Ivanov, D. Analysis of mural paintings using in situ non-invasive XRF, FTIR spectroscopy and optical microscopy. X-Ray Spectrom. 2013, 42, 380–387. [Google Scholar] [CrossRef]
- Mendoza Cuevas, A.; Bernardini, F.; Gianoncelli, A.; Tuniz, C. Energy dispersive X ray diffraction and fluorescence portable system for cultural heritage applications. X-Ray Spectrom. 2015, 44, 105–115. [Google Scholar] [CrossRef]
- Simsek, G.; Unsalan, O.; Bayraktar, K.; Colomban, P. On-site pXRF analysis of glaze composition and coloring agents of “Iznik” tiles at Edirne mosques (15th and 16th-centuries). Ceram. Int. 2019, 45, 595–605. [Google Scholar] [CrossRef]
- Simsek, G.; Casadio, F.; Colomban, P.; Bellot-Gurlet, L.; Faber, K.T.; Zelleke, G.; Milande, V.; Moinet, E. On-Site Identification of early BÖTTGER red stoneware made at Meissen using portable XRF: 1, Body Analysis. J. Am. Ceram. Soc. 2014, 97, 2745–2754. [Google Scholar] [CrossRef]
- Ridolfi, S. Portable EDXRF in a multi-technique approach for the analyses of paintings. Insight Non-Destruct. Test. Condit. Mon. 2017, 59, 273–275. [Google Scholar] [CrossRef]
- Gabdrakhmanov, N.K.; Ulengov, R.A.; Gorelova, Y.N. The historic city of the Republic of Tatarstan as a basis for regional tourism cluster. J. Organ. Cult. Commun. Confl. 2016, 20, 46. [Google Scholar]
- Hussey, J.M. The Orthodox Church in the Byzantine Empire; Oxford University Press: Oxford, UK, 2010; p. 456. ISBN 9780199582761. [Google Scholar]
- Bikiaris, D.; Daniilia, S.; Sotiropoulou, S.; Katsimbiri, O.; Pavlidou, E.; Moutsatsou, A.P.; Chryssoulakis, Y. Ochre-differentiation through micro-Raman and micro-FTIR spectroscopies: Application on wall paintings at Meteora and Mount Athos, Greece. Spectrochim. Acta Part A 1999, 56, 3–18. [Google Scholar] [CrossRef]
- Khramchenkova, R.H.; Batalin, G.A.; Gareev, B.I.; Kaplan, P.J.; Kosushkin, V.F.; Nuzhdin, E.V.; Safina, I.R.; Sitdikov, A.G. Investigation of the Layer of Fresco Painting. In Kazan. Assumption Cathedral. Study and Preservation; Valeeva, R.M., Sitdikova, A.G., Hajrutdinova, R.R., Eds.; Glavdizajn OOO: Kazan, Tatarstan, 2016; Volume 2, pp. 123–141. (In Russian) [Google Scholar]
- Valeev, R.M.; Sitdikov, A.G.; Hajrutdinov, R.R. (Eds.) Kazan. Assumption Cathedral. Study and Preservation; Glavdizajn OOO: Kazan, Tatarstan, 2016; Volume 2, pp. 94–181. (In Russian) [Google Scholar]
- Kotulanová, E.; Bezdicka, P.; Hradil, D.; Hradilová, J.; Svarcová, S.; Grygar, T. Degradation of lead-based pigments by salt solutions. J. Cult. Herit. 2009, 10, 367–378. [Google Scholar] [CrossRef]
- Unković, N.; Ljaljević Grbić, M.; Subakov-Simić, G.; Stupar, M.; Vukojević, J.; Jelikić, A.; Stanojević, D. Biodeteriogenic and toxigenic agents on 17th century mural paintings and façade of the old church of the Holy Ascension (Veliki Krčimir, Serbia). Indoor. Built. Environ. 2015, 25, 826–837. [Google Scholar] [CrossRef]
- Karpovich-Tate, N.; Rebrikova, N.L. Microbial communities on damaged frescoes and building materials in the cathedral of the Nativity of the Virgin in the Pafnutii-Borovskii Monastery, Russia. Intern. Biodeter. 1991, 27, 281–296. [Google Scholar] [CrossRef]
- Brysbaert, A.; Melessanaki, K.; Anglos, D. Pigment analysis in Bronze Age Aegean and Eastern Mediterranean painted plaster by laser-induced breakdown spectroscopy (LIBS). J. Archaeol. Sci. 2006, 33, 1095–1104. [Google Scholar] [CrossRef]
- Jones, R.E.; Photos-Jones, E. Source: Technical studies of Aegean Bronze Age wall painting: Methods, results and future prospects. Br. Sch. Athens Stud. 2005, 13, 199–228. [Google Scholar]
- Westlake, P.; Siozos, P.; Philippidis, A.; Apostolaki, C.; Derham, B.; Terlixi, A.; Perdikatsis, V.; Jones, R.; Anglos, D. Studying pigments on painted plaster in Minoan, Roman and Early Byzantine Crete. A multi-analytical technique approach. Anal. Bioanal. Chem. 2012, 402, 1413–1432. [Google Scholar] [CrossRef]
- Elsen, J. Microscopy of historic mortars—A review. Cement Concr. Res. 2006, 36, 1416–1424. [Google Scholar] [CrossRef]
- Stulik, D. Paint. In The Science of Paintings; Taft, W.S., Mayer, J.W., Eds.; Springer: New York, NY, USA, 2000; pp. 12–25. ISBN 0-387-98722-3. [Google Scholar]
- Bläuer-Böhm, C.; Jägers, E. Analysis and recognition of dolomitic lime mortars. In Roman Wall Painting Materials, Techniques, Analysis and Conservation; Béarat, H., Fuchs, M., Maggetti, M., Paunier, D., Eds.; Institute of Mineralogy and Petrography Fribourg: Fribourg, Switzerland, 1997; pp. 223–235. [Google Scholar]
- Taft, W.S.; Mayer, J.W. The Science of Paintings; Springer: New York, NY, USA, 2000; p. 236. ISBN 0-387-98722-3. [Google Scholar]
- Coccato, A.; Moens, L.; Vandenabeele, P. On the stability of mediaeval inorganic pigments: A literature review of the effect of climate, material selection, biological activity, analysis and conservation treatments. Herit. Sci. 2017, 5, 25. [Google Scholar] [CrossRef]
- Horgnies, M.; Darque-Ceretti, E.; Bayle, M.; Gueita, E.; Aucouturier, M. An exceptionally perennial surface artwork: Fresco on lime or on cement. Surf. Interface Anal. 2014, 46, 791–795. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Farrel, D.W. The conservational heritage of wall paintings and buildings: An FT-Raman spectroscopic study of prehistoric, Roman, mediaeval and Renaissance lime substrates and mortars. J. Raman Spectrosc. 2008, 39, 985–992. [Google Scholar] [CrossRef]
- Konta, J. Clay and man: Clay raw materials in the service of man. Appl. Clay Sci. 1995, 10, 275–335. [Google Scholar] [CrossRef]
- Hradil, D.; Grygar, T.; Hradilová, J.; Bezdička, P. Clay and iron oxide pigments in the history of painting. Appl. Clay Sci. 2003, 22, 223–236. [Google Scholar] [CrossRef]
- IMA (International Mineralogical Association). List of Minerals. Available online: http://nrmima.nrm.se//IMA_Master_List_%282018-11%29.pdf (accessed on 1 December 2018).
- Neuendorf, K.K.E.; Mehl, J.P., Jr.; Jackson, J.A. (Eds.) Glossary of Geology, 5th ed. revised.; American Geosciences Institute Alexandria: Alexandria, VA, USA, 2011; p. 783. [Google Scholar]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. The Pigment Compendium: A Dictionary of Historical Pigments; Elsevier Butterworth-Heinemann: Amsterdam, The Netherlands, 2004; p. 499. ISBN 100750689803. [Google Scholar]
- Petushkova, J.P.; Lyalikova, N.N. Microbiological degradation of lead-containing pigments in mural paintings. Stud. Conserv. 1986, 31, 65–69. [Google Scholar] [CrossRef]
- Aze, S.; Vallet, J.M. Chromatic degradation processes of red lead pigment. Chem. Preprint Arch. 2002, 6, 236–243. Available online: http://preprint.chemweb.com/inorgchem/0206001 (accessed on 14 February 2019).
- Iordanidis, A.; Garcia-Guinea, J.; Strati, A.; Gkimourtzina, A.; Papoulidou, A. Byzantine wall paintings from Kastoria, northern Greece: Spectroscopic study of pigments and efflorescing salts. Spectrochim. Acta Part A 2011, 78, 874–887. [Google Scholar] [CrossRef]
- Gettens, R.J.; Fitzhugh, E.W.; Feller, R.L. Calcium carbonate whites. Stud. Conserv. 1974, 19, 157–184. [Google Scholar] [CrossRef]
- Berke, H. The invention of blue and purple pigments in ancient times. Chem. Soc. Rev. 2007, 36, 15–30. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals; Pearson Prentice Hall: London, UK, 1992; p. 696. [Google Scholar]
- Colomban, P. Lapis lazuli as unexpected blue pigment in Iranian Lâjvardina ceramics. J. Raman Spectrosc. 2003, 34, 420–423. [Google Scholar] [CrossRef]
- Hernanz, A.; Gavira-Vallejo, J.M.; Ruiz-López, J.F.; Edwards, H.G.M. A comprehensive micro-Raman spectroscopic study of prehistoric rock paintings from the Sierra de las Cuerdas, Cuenca, Spain. J. Raman Spectrosc. 2008, 39, 972–984. [Google Scholar] [CrossRef]
- Mason, B.; Vitaliano, C.J. The mineralogy of the antimony oxides and antimonates. Miner. Mag. 1953, 30, 100–112. [Google Scholar] [CrossRef]
- Taylor, B.N. The International System of Units (SI); USA National Institute of Standards and Technology Special Publication 330: Washington, DC, USA, 2001; p. 75. [Google Scholar]
- Fieshbeck, K. Logarithmische Rechentafeln für Chemiker, Pharmazeuten, Medziner und Physiker; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1972; p. 81. [Google Scholar]
- Bro, R.; Smilde, A.K. Principal Component analysis. J. Anal. Methods Chem. 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Kolovrat (Mihajlova), N. Fresco technique. Himija i zhizn 1969, 6, 30–33. Available online: http://www.domarchive.ru/gallery/freska/3474 (accessed on 14 February 2019). (In Russian).
- Makita, M.; Esperón, M.; Pereyra, B.; López, A.; Orrantia, E. Reduction of arsenic content in a complex galena concentrate by Acidithiobacillus ferrooxidans. BMC Biotechnol. 2004, 4, 22. [Google Scholar] [CrossRef]
- Molera, J.; Coll, J.; Labrador, A.; Pradell, T. Manganese brown decorations in 10th to 18th century Spanish tin glazed ceramics. Appl. Clay Sci. 2013, 82, 86–90. [Google Scholar] [CrossRef]
- Piovesan, R.; Siddall, R.; Mazzoli, C.; Nodari, L. The Temple of Venus (Pompeii): A study of the pigments and painting techniques. J. Archaeol. Sci. 2011, 38, 2633–2643. [Google Scholar] [CrossRef]
- Germinario, C.; Francesco, I.; Mercurio, M.; Langella, A.; Sali, D.; Kakoulli, I.; De Bonis, A.; Grifa, C. Multi-analytical and non-invasive characterization of the polychromy of wall paintings at the Domus of Octavius Quartio in Pompeii. Eur. Phys. J. Plus 2018, 133, 359. [Google Scholar] [CrossRef]
- Amato, S.R.; Bersani, D.; Lottici, P.P.; Pogliani, P.; Pelosi, C. A multi-analytical approach to the study of the mural paintings in the presbytery of Santa Maria Antiqua al Foro Romano in Rome. Archaeometry 2017, 59, 1050–1064. [Google Scholar] [CrossRef]
- Khramchenkova, R.; Gubaidullin, A.; Safina, I.; Nuzdin, E. Chemical composition of the fragment of Islamic enamel glass vessel 13th centuries. In Proceedings of the Technart Symposium on Non-destructive and Microanalytical Techniques in Art and Cultural Heritage, Catania, Italy, 27–30 April 2015; p. 141. [Google Scholar]
- Bersani, D.; Berzioli, M.; Caglio, S.; Casoli, A.; Lottici, P.P.; Medeghini, L.; Poldi, G.; Zannini, P. An integrated multi-analytical approach to the study of the dome wall paintings by Correggio in Parma cathedral. Microchem. J. 2014, 114, 80–88. [Google Scholar] [CrossRef]
- Kovács, J.; Kovácsné-Székely, I. Understanding the spatio-temporal samples: A practical view for geologists. Földt. Közl. 2006, 136, 139–146, (In Hungarian, in original). [Google Scholar]
- Gettens, R.J.; Stout, G.L. A monument of Byzantine wall painting: The method of construction. Stud. Conserv. 1958, 3, 107–119. [Google Scholar]
- Baraldi, P.; Bonazzi, A.; Giordani, N.; Paccagnella, F.; Zannini, P. Analytical characterization of Roman plasters of the ‘Domus Farini’ in Modena. Archaeometry 2006, 48, 481–499. [Google Scholar] [CrossRef]
- Avlonitou, L. Pigments and colors: An inside look at the painted decoration of the Macedonian funerary monuments. J. Archaeol. Sci. 2016, 7, 668–678. [Google Scholar] [CrossRef]
- Bakiler, M.; Kırmızı, B.; Öztürk, Ö.O.; Hanyalı, Ö.B.; Dağ, E.; Çağlar, E.; Köroğlu, G. Material characterization of the Late Roman wall painting samples from Sinop Balatlar Church Complex in the black sea region of Turkey. Microchem. J. 2016, 126, 263–273. [Google Scholar] [CrossRef]
- Lepot, L.; Denoël, S.; Gilbert, B. The technique of the mural paintings of the Tournai Cathedral. J. Raman Spectrosc. 2006, 37, 1098–1103. [Google Scholar] [CrossRef]
- Hernanz, A.; Bratu, I.; Marutoiu, O.F.; Marutoiu, C.; Gavira-Vallejo, J.M.; Edwards, H.G.M. Micro-Raman spectroscopic investigation of external wall paintings from St. Dumitru’s Church, Suceava, Romania. Anal. Bioanal. Chem. 2008, 392, 263–268. [Google Scholar] [CrossRef]
- Buzgar, N.; Buzatu, A.; Apopei, A.-I.; Cotiugă, V. In situ Raman spectroscopy at the Voroneţ Monastery (16th century, Romania): New results for green and blue pigments. Vib. Spectrosc. 2014, 72, 142–148. [Google Scholar] [CrossRef]
- Cheilakou, E.; Troullinos, M.; Koui, M. Identification of pigments on Byzantine wall paintings from Crete (14th century AD) using non-invasive Fiber Optics Diffuse Reflectance Spectroscopy (FORS). J. Archaeol. Sci. 2014, 41, 541–555. [Google Scholar] [CrossRef]
- Barbu, O.-H.; Zahariade, A. Noninvasive in situ study of pigments in artworks by means of VIS, IRFC image analysis, and X-ray fluorescence spectrometry. COLOR Res. Appl. 2016, 41, 321–324. [Google Scholar] [CrossRef]
- Forbes, R.J. Metallurgy in Antiquity. A Notebook for Archaeologists and Technologists; Brill Archive: Leiden, The Netherlands, 1950; p. 489. [Google Scholar]
- Wang, C.Y. Antimony: Its History, Chemistry, Mineralogy, Geology, Mettalurgy, Uses, Preparations, Analysis, Production, and Valuation; with Complete Bibliographies, 2nd ed.; Charles Griffin & Co. Ltd.: London, UK, 1919; p. 225. [Google Scholar]
- Puchkov, V.N. General features relating to the occurrence of mineral deposits in the Urals: What, where, when and why. Ore Geol. Rev. 2017, 85, 4–29. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V.; Herrington, R.J.; Ayupova, N.R.; Zaykov, V.V.; Lein, A.Y.; Tseluyko, A.S.; Melekestseva, I.Y. Chimneys in Paleozoic massive sulfide mounds of the Urals VMS deposits: Mineral and trace element comparison with modern black, grey, white and clear smokers. Ore Geol. Rev. 2017, 85, 64–106. [Google Scholar] [CrossRef]
- Givental, E. Three Hundred Years of Glory and Gloom: The Urals Region of Russia in Art and Reality; SAGE Open: Thousand Oaks, CA, USA, 2013; pp. 1–9. [Google Scholar]
- Gimbutas, M. Bronze Age Cultures in Central and Eastern Europe; De Gruyter Mouton & Co Publ.: The Hague, The Netherlands, 1965; ISBN-10 3111283410, ISBN-13: 978-3111283418; 784p. [Google Scholar]
- Giovannoni, S.; Matteini, M.; Moles, A. Studies and developments concerning the problem of altered lead pigments in wall painting. Stud. Conserv. 1990, 35, 21–25. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Farrel, D.W.; Newton, E.M.; Perez, R.F. Minium; FT-Raman non-destructive analysis applied to an historical controversy. Analyst 1999, 124, 1323–1326. [Google Scholar] [CrossRef]
- Alekseev-Aljurvi, J.V. Colorful Raw Materials and Paints Used in Painting; AOOT Tver’: Moskow, Russia, 2004; pp. 238–247. (In Russian) [Google Scholar]




| Variables | CaO | MgO | Fe2O3 | PbO | SO3 | Sb2O3 | Al2O3 | SiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| F1 | 0.97 | 0.25 | −0.37 | −0.50 | −0.53 | 0.93 | −0.22 | −0.33 | 0.46 |
| F2 | −0.07 | 0.61 | −0.57 | 0.65 | 0.68 | −0.04 | −0.34 | −0.50 | 0.42 |
| Color Type | Dominant Chemical Element | Inferred Recipes (Main Pigments) |
|---|---|---|
| White A | Pb – Mg | Anglesite—Slaked dolomitic lime |
| White B | Ca – Mg – Sb | Slaked lime—Slaked dolomitic lime—Antimony white |
| Yellow | Ca – Mg – Fe | Slaked lime—Slaked dolomitic lime—Yellow ochre |
| Orange | Ca – Mg – Fe | Slaked lime—Slaked dolomitic lime—Red and yellow ochre |
| Pink A | Fe – Pb – Ca | Red ochre—Litharge (Minium?)—Slaked lime |
| Pink B | Pb – Mg | Litharge (Minium?)—Slaked dolomitic lime |
| Brown A | Fe – P | Yellow ochre—Bone black |
| Brown B | Pb | Massicot |
| Brown C | Ca – P – Fe | Bone black—Yellow ochre |
| Red A | Fe | Red ochre |
| Red B | Pb – Fe – As | Litharge (Minium)—Red ochre—Realgar |
| Grey A | Fe | Magnetite |
| Grey B | Ca – Sb | Slaked lime—Stibnite |
| Grey C | Pb(As) | Anglesite (As) |
| Black A | P – Sb – Ca | Bone black—Stibnite—Slaked lime |
| Black B | Pb – Fe | Galena—Magnetite |
| Black C | Pb | Plattnerite |
| Green A | Fe – Ca – Cu | Green earth—Slaked lime—Malachite? (Chrysocolla?) |
| Green B | Pb – Fe – As | Anglesite—Yellow ochre—Orpiment |
| Blue A | Fe | Blue ochre (Vivianite) |
| Blue B | Pb | White lead (Anglesite) + Blue lead (Galena) |
| Blue C | Ca – Cu – Sb | Slaked lime—Azurite(Egyptian blue?)—Stibnite |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khramchenkova, R.; Ionescu, C.; Sitdikov, A.; Kaplan, P.; Gál, Á.; Gareev, B. A pXRF In Situ Study of 16th–17th Century Fresco Paints from Sviyazhsk (Tatarstan Republic, Russian Federation). Minerals 2019, 9, 114. https://doi.org/10.3390/min9020114
Khramchenkova R, Ionescu C, Sitdikov A, Kaplan P, Gál Á, Gareev B. A pXRF In Situ Study of 16th–17th Century Fresco Paints from Sviyazhsk (Tatarstan Republic, Russian Federation). Minerals. 2019; 9(2):114. https://doi.org/10.3390/min9020114
Chicago/Turabian StyleKhramchenkova, Rezida, Corina Ionescu, Airat Sitdikov, Polina Kaplan, Ágnes Gál, and Bulat Gareev. 2019. "A pXRF In Situ Study of 16th–17th Century Fresco Paints from Sviyazhsk (Tatarstan Republic, Russian Federation)" Minerals 9, no. 2: 114. https://doi.org/10.3390/min9020114