Scrubbing and Inhibiting Coagulation Effect on the Purification of Natural Powder Quartz
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification Methods
2.3. Equipment
3. Results and Discussion
3.1. Impurity Analysis of NPQ
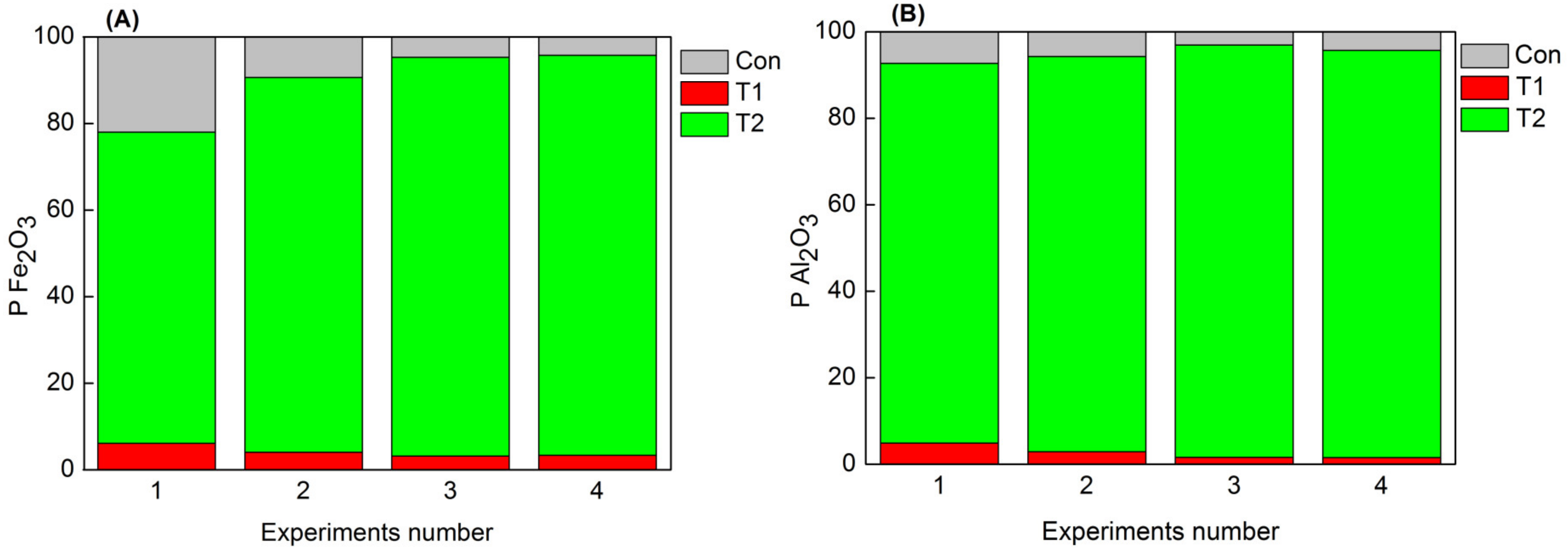
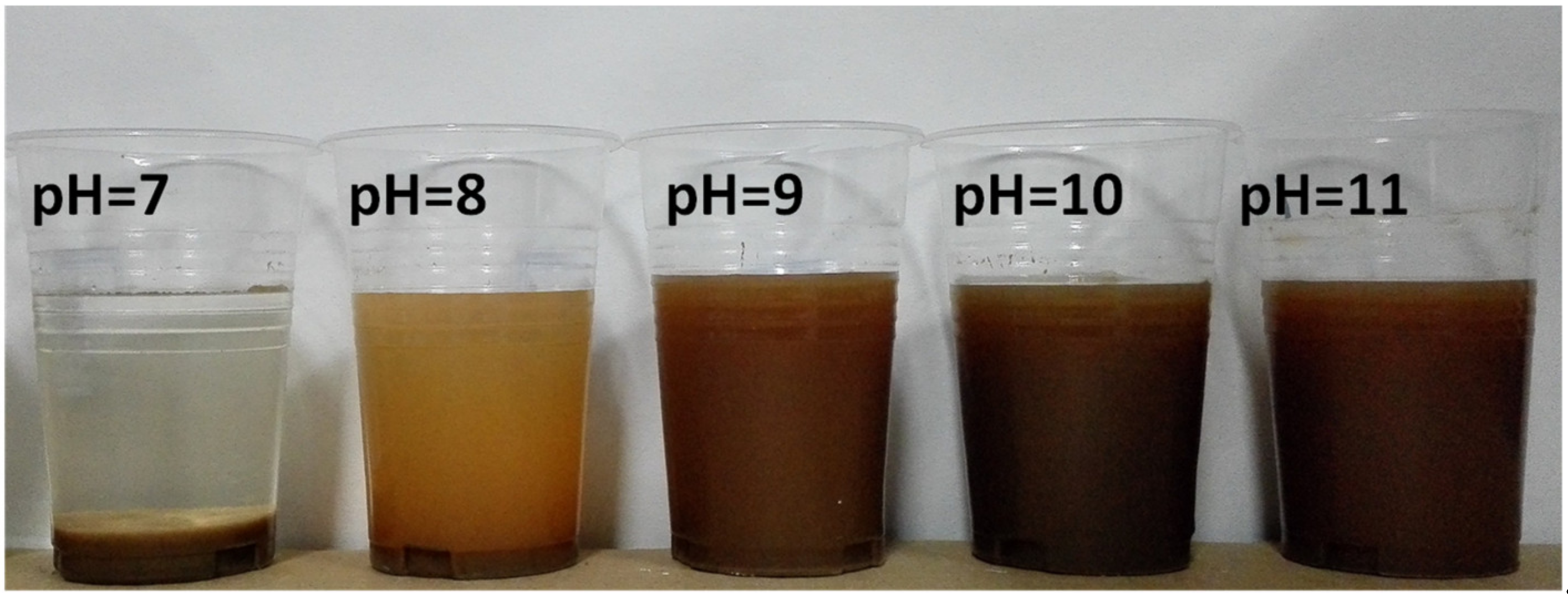
3.2. Effects of Scrubbing and Regulators on Impurities Removal
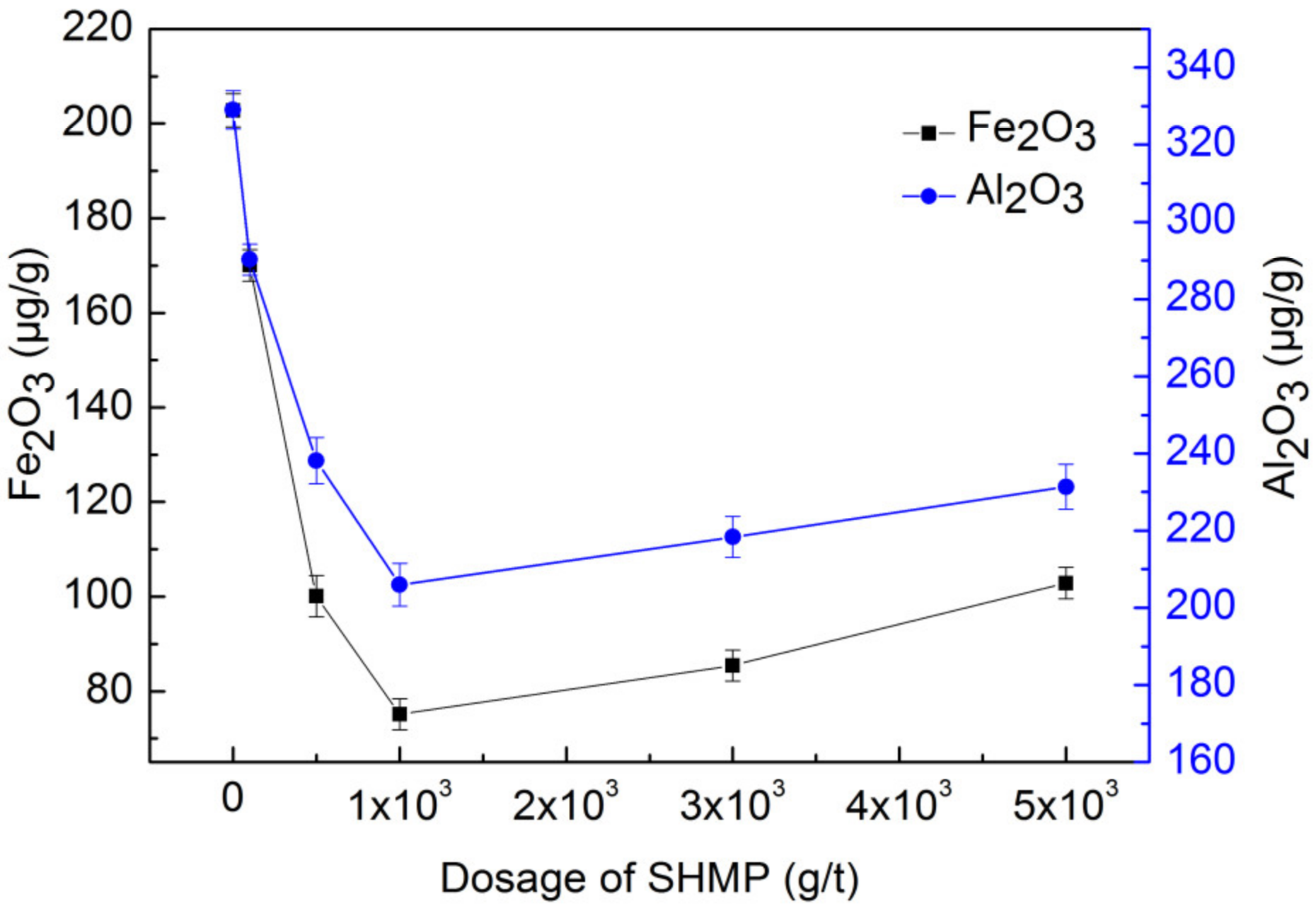
3.3. Effects of pH and Dosage of SHMP on Impurities Removal
4. Purification Mechanism
4.1. Effects of pH on the Zeta Potential
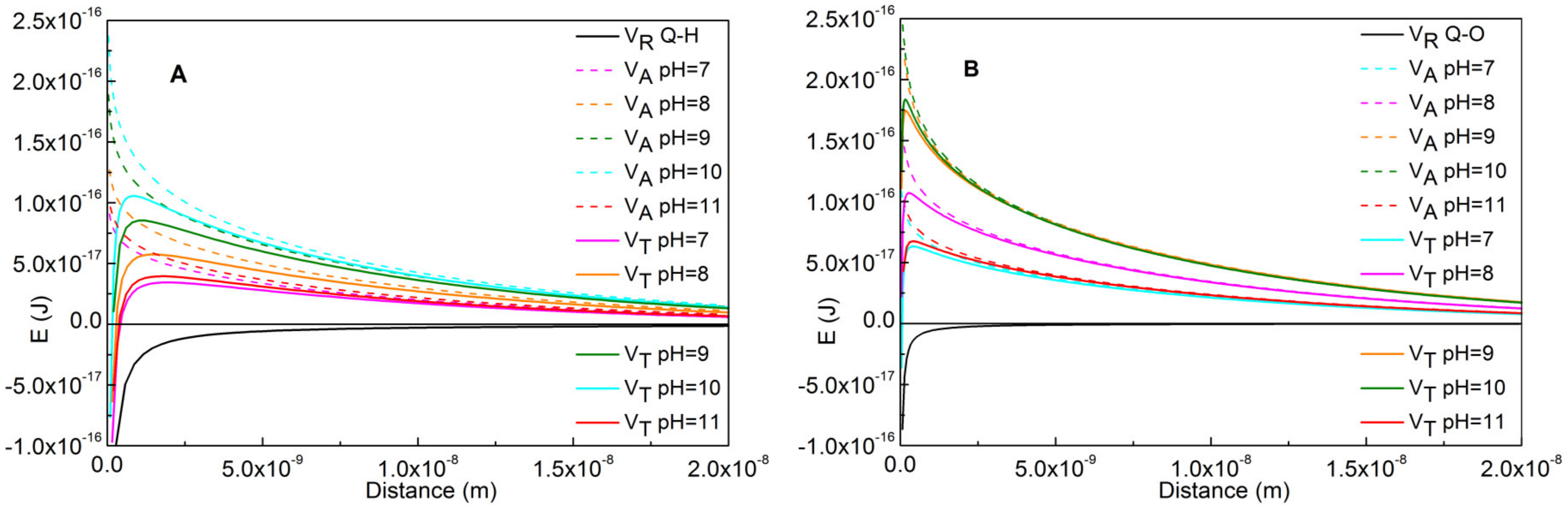
4.2. Heterocoagulation between Quartz and Impurities
4.3. Coagulation of Fine Impurity Particles
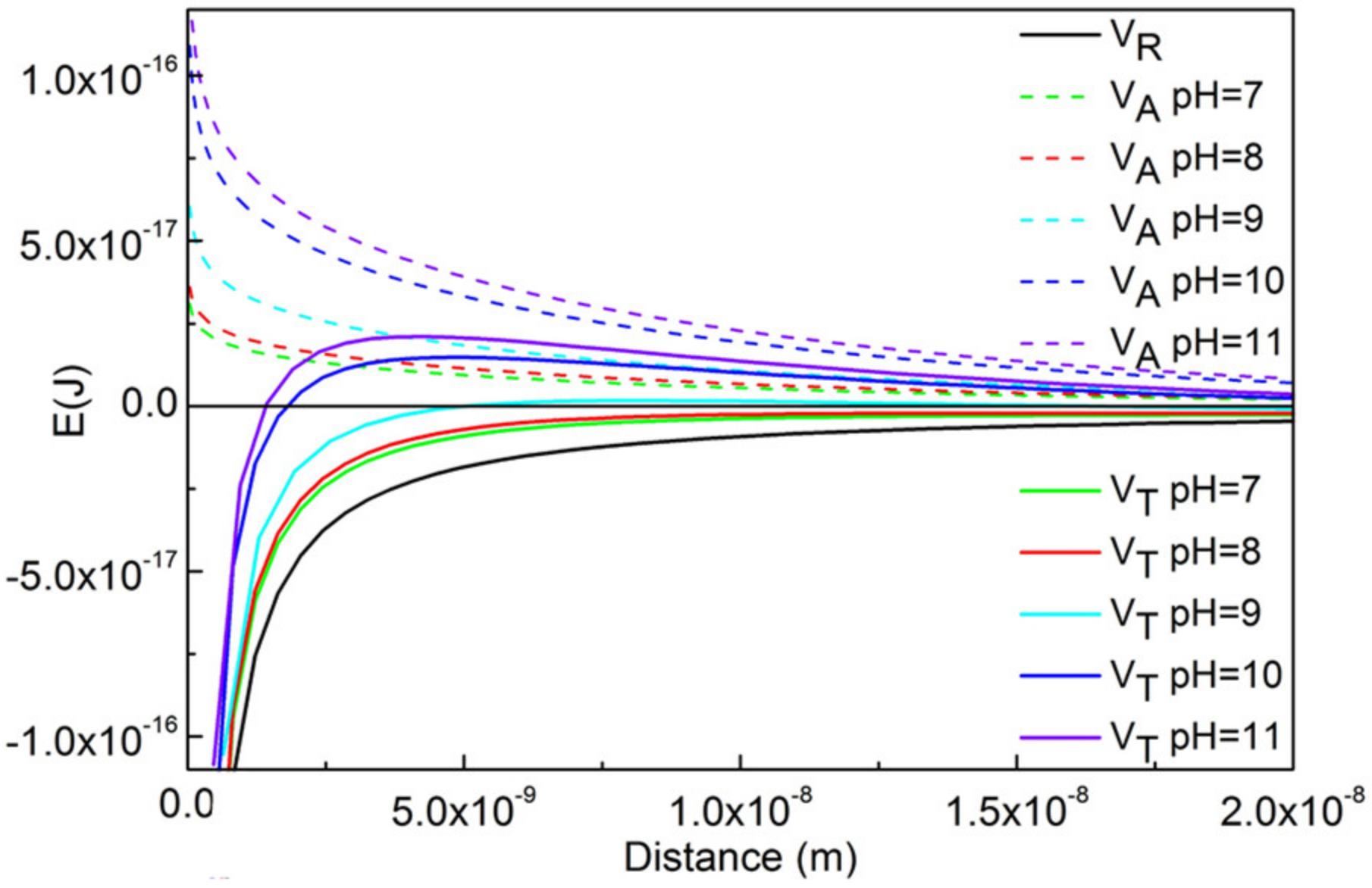
4.4. Effects of SHMP on the Zeta Potential and Desliming
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santos, M.F.M.D.; Fujiwara, E.; Schenkel, E.A.; Enzweiler, J.; Suzuki, C.K. Quartz sand resources in the Santa Maria Eterna formation, Bahia, Brazil: A geochemical and morphological study. J. South Am. Earth Sci. 2015, 62, 176–185. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Li, X.; Zhang, Z. Iron removal from extremely fine quartz and its kinetics. Sep. Purif. Technol. 2013, 108, 45–50. [Google Scholar] [CrossRef]
- Prakash, S.; Das, B.; Mohanty, J.K.; Venugopal, R. The recovery of fine iron minerals from quartz and corundum mixtures using selective magnetic coating. Int. J. Miner. Process. 1999, 57, 87–103. [Google Scholar] [CrossRef]
- Zhong, L.L.; Lei, S.M.; Wang, E.W.; Pei, Z.Y.; Li, L.; Yang, Y.Y. Research on Removal Impurities from Vein Quartz Sand with Complexing Agents. Appl. Mech. Mater. 2013, 454, 194–199. [Google Scholar] [CrossRef]
- Andrews, P.R.A.; Collings, R.K. Canadian silica resources for glass and foundry sand production: Processing studies at CANMET. Int. J. Miner. Process. 1989, 25, 311–317. [Google Scholar] [CrossRef]
- Tuncuk, A.; Akcil, A. Iron removal in production of purified quartz by hydrometallurgical process. Int. J. Miner. Process. 2016, 153, 44–50. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, X.; Huang, H.; Zhou, L.; Xiong, T. High efficiency iron removal from quartz sand using phosphoric acid. Int. J. Miner. Process 2012, 114–117, 30–34. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, P.; Hou, Q.; Duan, H.; Xie, Y. Study on removal fluid inclusions in quartz sand by microwave explosion. Nanosci. Nanotechnol. Lett. 2017, 9, 151–154. [Google Scholar] [CrossRef]
- Štyriakov, A.I.; Štyriak, I.; Kraus, I.; Uhlík, P.; Madejov, A.J.; Orolínov, A.Z. Bioleaching of clays and iron oxide coatings from quartz sands. Appl. Clay Sci. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- He, M.S.; Yao, Q.; Yi, Y.Q. Analysis of the development and utilization and the supply and demand situation on Natural powder quartz at Jiang Xi province. China Nonmet. Miner. Ind. 2003, 6, 52–55. (In Chinese) [Google Scholar]
- Richard, B.; John, M.M. Geology of Microcrcrystalline Silica (Tripoli) Deposits, Southernmost Illinois. Illinois State Geological Survey. 1994, pp. 4–8. Available online: https://core.ac.uk/download/pdf/17355415.pdf (accessed on 27 January 2019).
- Tan, J.; Zhou, H.; Wang, M.; Zhu, B.Q.; Gao, X.Q.; Zheng, C.H. Study of the purification and whitening of Natural powder quartz. Acta Mineral. Sin. 2014, 34, 7–12. (In Chinese) [Google Scholar]
- Zhang, Y.P.; Huang, K.L.; Liu, S.Q. Separation of clinochlore from powder quartz by reverse flotation and its mechanism. J. Cent. South Univ. Technol. (Sci. Technol.) 2007, 2, 285–290. (In Chinese) [Google Scholar]
- Bayley, R.W.; Biggs, C.A. Characterisation of an attrition scrubber for the removal of high molecular weight contaminants in sand. Chem. Eng. J. 2005, 111, 71–79. [Google Scholar] [CrossRef]
- Stražišar, J.; Sešelj, A. Attrition as a process of comminution and separation. Powder Technol. 1999, 105, 205–209. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, X.; Zhang, G.; Zheng, S.; Frost, R.L. A novel method for purification of low grade diatomite powders in centrifugal fields. Int. J. Miner. Process. 2013, 125, 18–26. [Google Scholar] [CrossRef]
- Batalović, V. Centrifugal separator, the new technical solution, application in mineral processing. Int. J. Miner. Process. 2011, 100, 86–95. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Petruk, W. Automatic Image Analysis for Mineral Beneficiation. JOM 1988, 40, 29–31. [Google Scholar] [CrossRef]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Min. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Houta, N.; Lecomte-Nana, G.L.; Tessier-Doyen, N.; Peyratout, C. Dispersion of phyllosilicates in aqueous suspensions: Role of the nature and amount of surfactant. J. Colloid Interface Sci. 2014, 425, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ma, L.; Xu, H. DLVO theoretical analyses between montmorillonite and fine coal under different pH and divalent cations. Powder Technol. 2018, 330, 147–151. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: New York, NY, USA, 1985; p. 262. [Google Scholar]
- Berg, J.C. An Introduction to Interfaces & Colloids: The Bridge to Nanoscience; World Scientific: Singapore, 2010. [Google Scholar]
- Robinson, D. Measurement of the solid dielectric permittivity of clay minerals and granular samples using a time domain reflectometry immersion method. Vadose Zone J. 2004, 3, 705–713. [Google Scholar] [CrossRef]
- Marland, S.; Merchant, A.; Rowson, N. Dielectric properties of coal. Fuel 2001, 80, 1839–1849. [Google Scholar] [CrossRef]
- Kaya, A.; Yukselen, Y. Zeta potential of clay minerals and quartz contaminated by heavy metal. Can. Geotech. J. 2005, 42, 1280–1289. [Google Scholar] [CrossRef]
- Yukselen-Aksoy, Y.; Kaya, A. A study of factors affecting on the zeta potential of kaolinite and quartz powder. Environ. Earth Sci. 2011, 62, 697–705. [Google Scholar] [CrossRef]
- Rohem Peçanha, E.; da Fonseca de Albuquerque, M.D.; Antoun Simão, R.; de Salles Leal Filho, L.; de Mello Monte, M.B. Interaction forces between colloidal starch and quartz and hematite particles in mineral flotation. Colloid Surf. A Physicochem. Eng. Asp. 2019, 562, 79–85. [Google Scholar] [CrossRef]
- James, R.O.; Healy, T.W. Adsorption of hydrolyzable metal ions at the oxide—Water interface. II. Charge reversal of SiO2 and TiO2 colloids by adsorbed Co(II), La(III), and Th(IV) as model systems. J. Colloid Interface Sci. 1972, 40, 53–64. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Xu, R.-K.; Li, J.-Y.; Hong, Z.-N. Effect of clay colloids on the zeta potential of Fe/Al oxide-coated quartz: A streaming potential study. J. Soils Sediments 2016, 16, 2676–2686. [Google Scholar] [CrossRef]
- Rodríguez, K.; Araujo, M. Temperature and pressure effects on zeta potential values of reservoir minerals. J. Colloid Interface Sci. 2006, 300, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Espinozaortega, O. Role of Sodium Hexametaphosphate in the Flotation of Acanthite Fines from Finely Disseminated Ores. Sep. Sci. Technol. 2009, 44, 2971–2982. [Google Scholar]
- Zhang, T.; Vandeperre, L.J.; Cheeseman, C.R. Formation of magnesium silicate hydrate (M-S-H) cement pastes using sodium hexametaphosphate. Cem. Concr. Res. 2014, 65, 8–14. [Google Scholar] [CrossRef]
- Tiller, C.L.; O’Melia, C.R. Natural organic matter and colloidal stablility: Models and measurements. Colloid Surf. A Physicochem. Eng. Asp. 1993, 73, 89–102. [Google Scholar] [CrossRef]
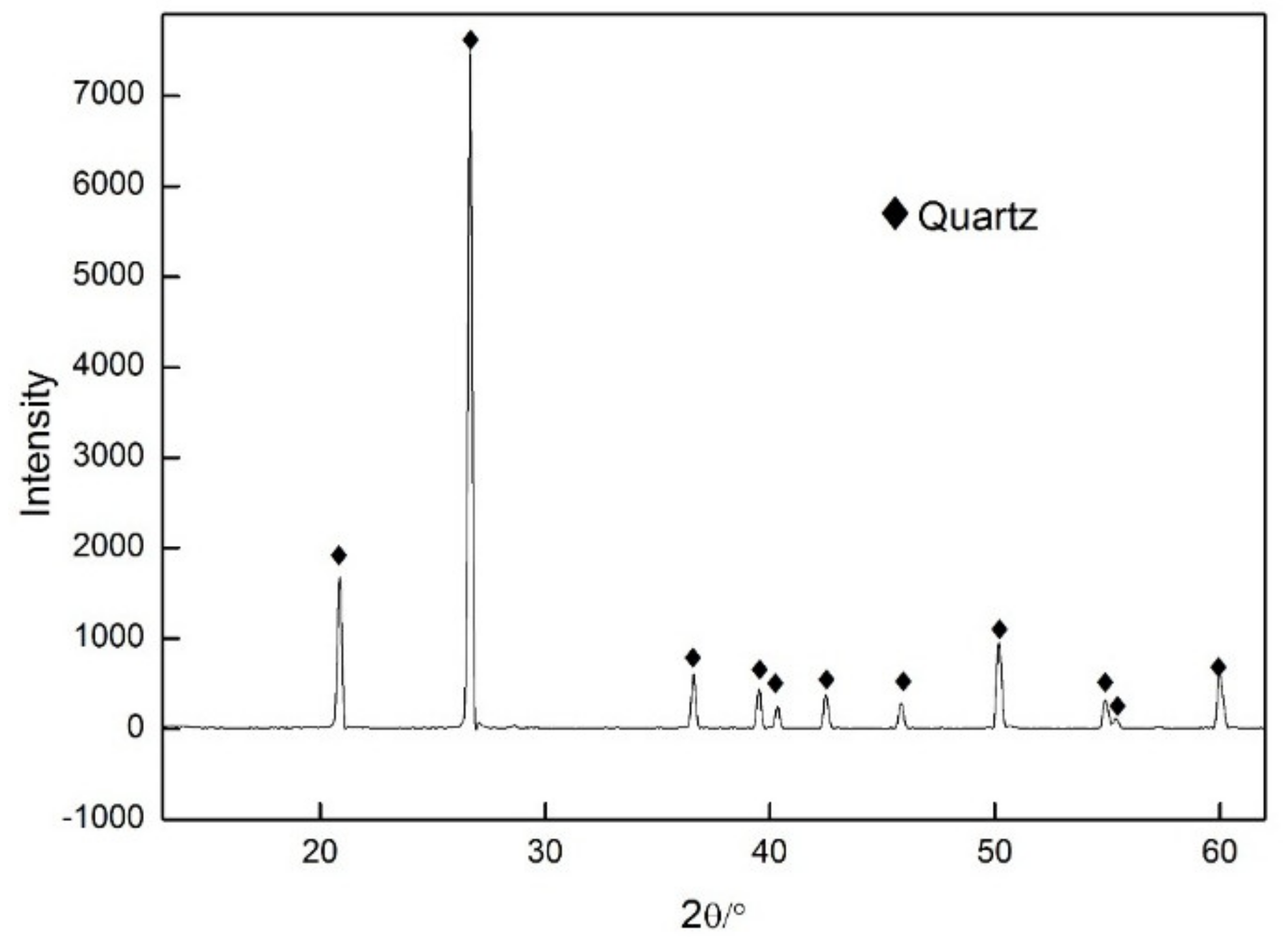

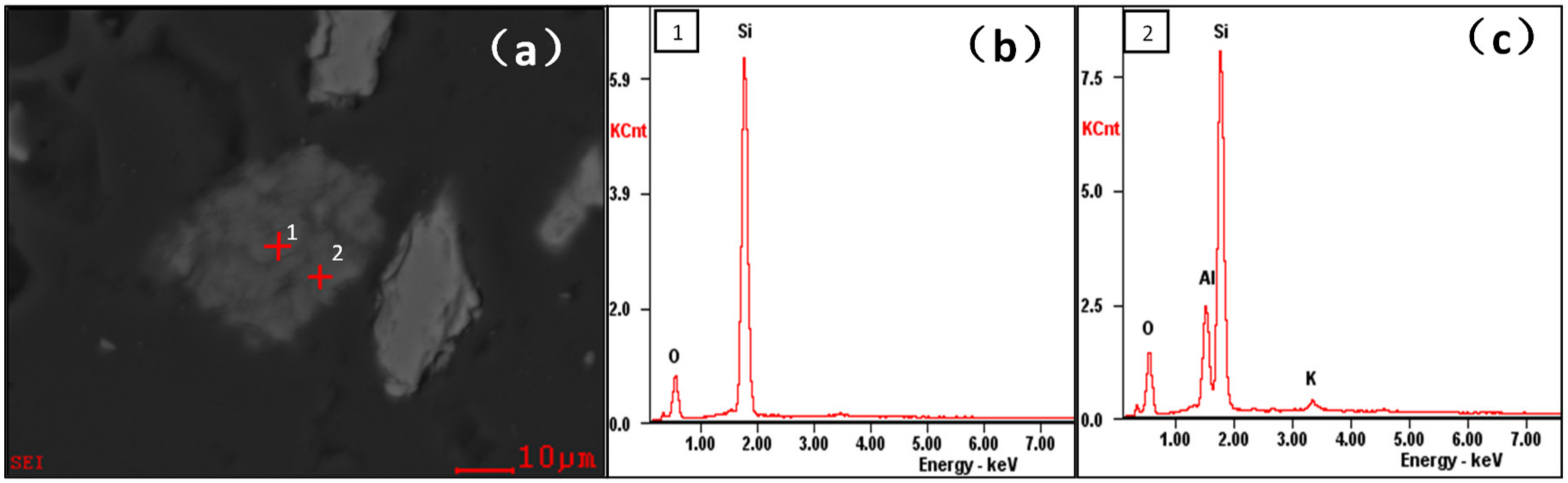





| Compositions | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | L.O.I. a |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt %) | 98.88 | 0.48 | 0.19 | 0.03 | 0.06 | 0.04 | 0.04 | 0.05 | 0.24 |
| Particle Size/Mesh | Yield (%) | Content (%) | ||
|---|---|---|---|---|
| Fe2O3 | Al2O3 | |||
| +100 | 1.96 | 0.301 | 0.862 | |
| −100~+200 | 4.51 | 0.132 | 0.157 | |
| −200~+325 | 26.14 | 0.040 | 0.031 | |
| −325 | sediments | 61.95 | 0.053 | 0.043 |
| suspensions | 5.44 | 2.576 | 8.790 | |
| Impurities | Hematite | Orthoclase | Anorthose | Mica | Total |
|---|---|---|---|---|---|
| Fe2O3 | 92.17 | 0 | 0 | 7.83 | 100 |
| Al2O3 | 0 | 64.42 | 26.53 | 9.06 | 100 |
| No. | Regulator | Dosage /(g·t-1) | Product | Yield (%) | Impurity Content (%) | |
|---|---|---|---|---|---|---|
| Fe2O3 | Al2O3 | |||||
| 1 | / | T1 | 6.47 | 0.182 | 0.365 | |
| / | T2 | 5.44 | 2.551 | 7.76 | ||
| Con | 88.09 | 0.048 | 0.04 | |||
| 2 | / | T1 | 4.52 | 0.173 | 0.311 | |
| / | T2 | 5.85 | 2.857 | 7.51 | ||
| Con | 89.63 | 0.020 | 0.033 | |||
| 500 | T1 | 4.33 | 0.141 | 0.179 | ||
| 3 | NaOH | T2 | 6.03 | 2.948 | 7.602 | |
| Con | 89.64 | 0.010 | 0.018 | |||
| 500 | T1 | 4.38 | 0.148 | 0.169 | ||
| 4 | SHMP | T2 | 6.02 | 2.963 | 7.521 | |
| Con | 89.60 | 0.010 | 0.024 | |||
| ζ | Dosage of SHMP /g·t-1 | ||
|---|---|---|---|
| 0 | 1000 | 5000 | |
| Quartz | −51.31 | −56.03 | −38.57 |
| Hematite | −27.01 | −61.83 | −51.23 |
| Orthoclase | −25.56 | −75.07 | −67.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Liang, C.; Hou, D.; Sun, Z.; Zheng, S. Scrubbing and Inhibiting Coagulation Effect on the Purification of Natural Powder Quartz. Minerals 2019, 9, 140. https://doi.org/10.3390/min9030140
Du X, Liang C, Hou D, Sun Z, Zheng S. Scrubbing and Inhibiting Coagulation Effect on the Purification of Natural Powder Quartz. Minerals. 2019; 9(3):140. https://doi.org/10.3390/min9030140
Chicago/Turabian StyleDu, Xin, Chao Liang, Donglai Hou, Zhiming Sun, and Shuilin Zheng. 2019. "Scrubbing and Inhibiting Coagulation Effect on the Purification of Natural Powder Quartz" Minerals 9, no. 3: 140. https://doi.org/10.3390/min9030140
APA StyleDu, X., Liang, C., Hou, D., Sun, Z., & Zheng, S. (2019). Scrubbing and Inhibiting Coagulation Effect on the Purification of Natural Powder Quartz. Minerals, 9(3), 140. https://doi.org/10.3390/min9030140






