Corundum Anorthosites-Kyshtymites from the South Urals, Russia: A Combined Mineralogical, Geochemical, and U-Pb Zircon Geochronological Study
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Petrology and Mineralogy of Kyshtymites
4.2. Mineralogy, Geochemistry, Solid Inclusions, and UV-Vis-NIR-Spectroscopy of Corundum–Sapphire
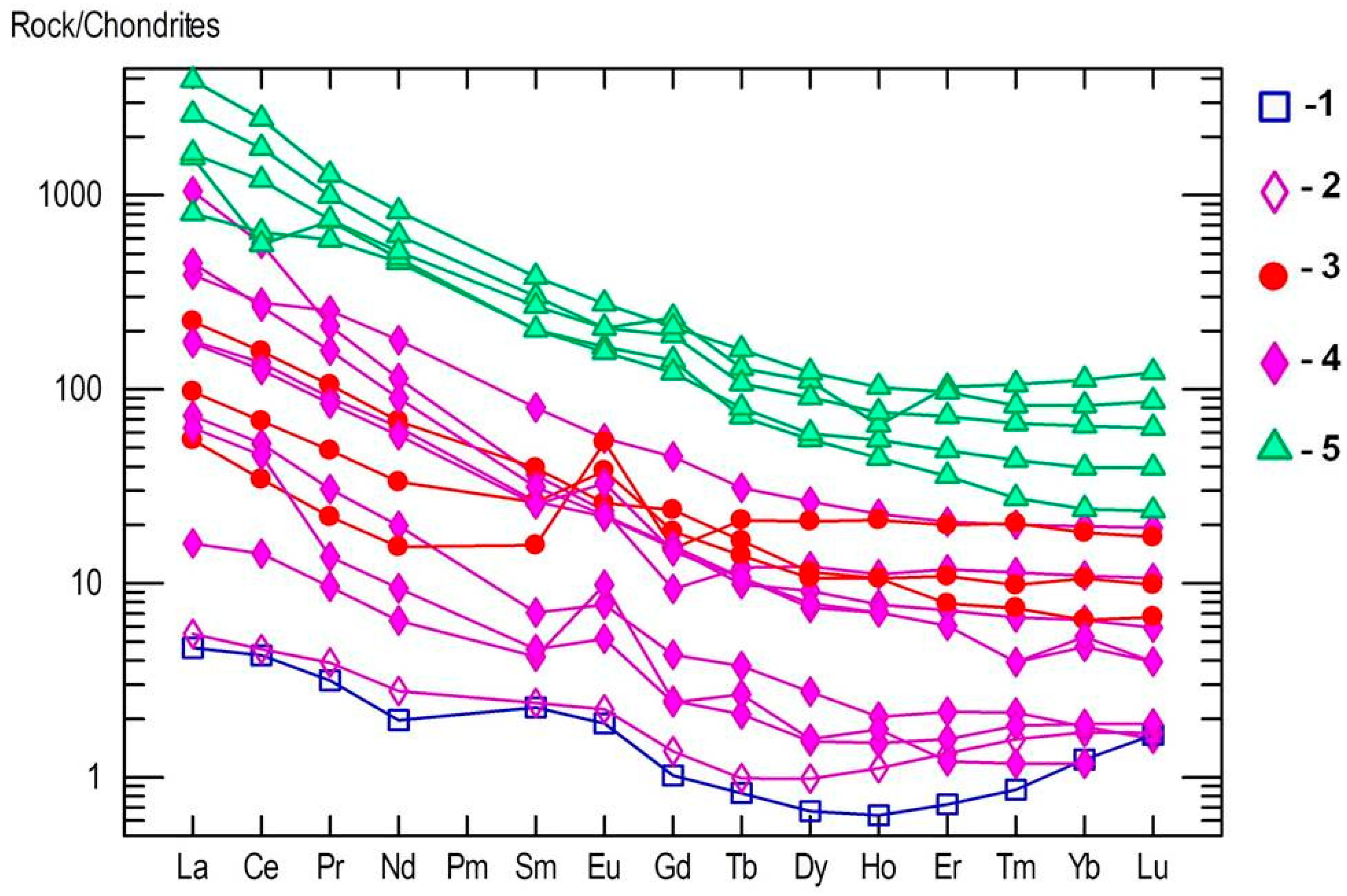
4.3. Whole Rock Geochemistry of Kyshtymites and Elements Mobility
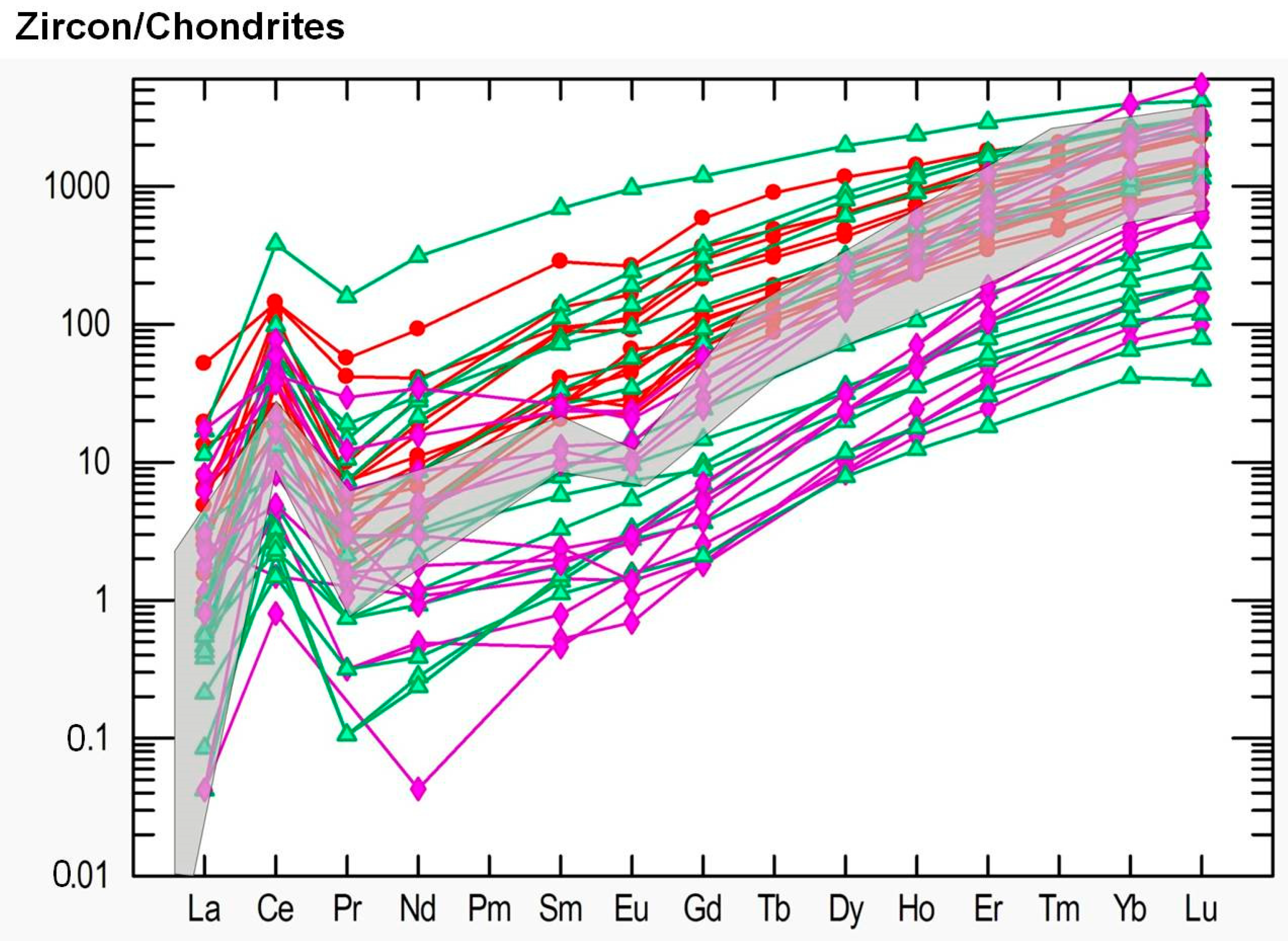
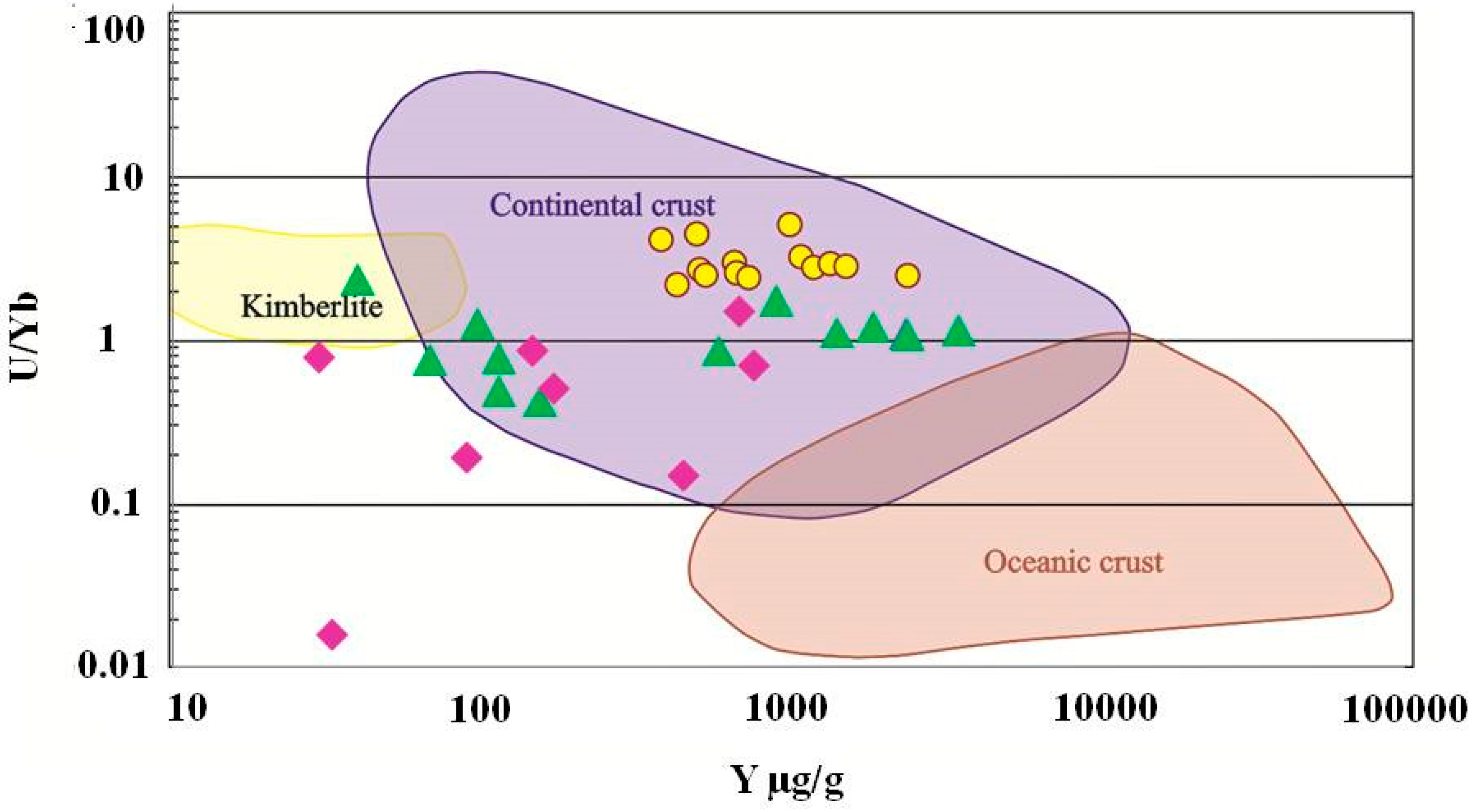
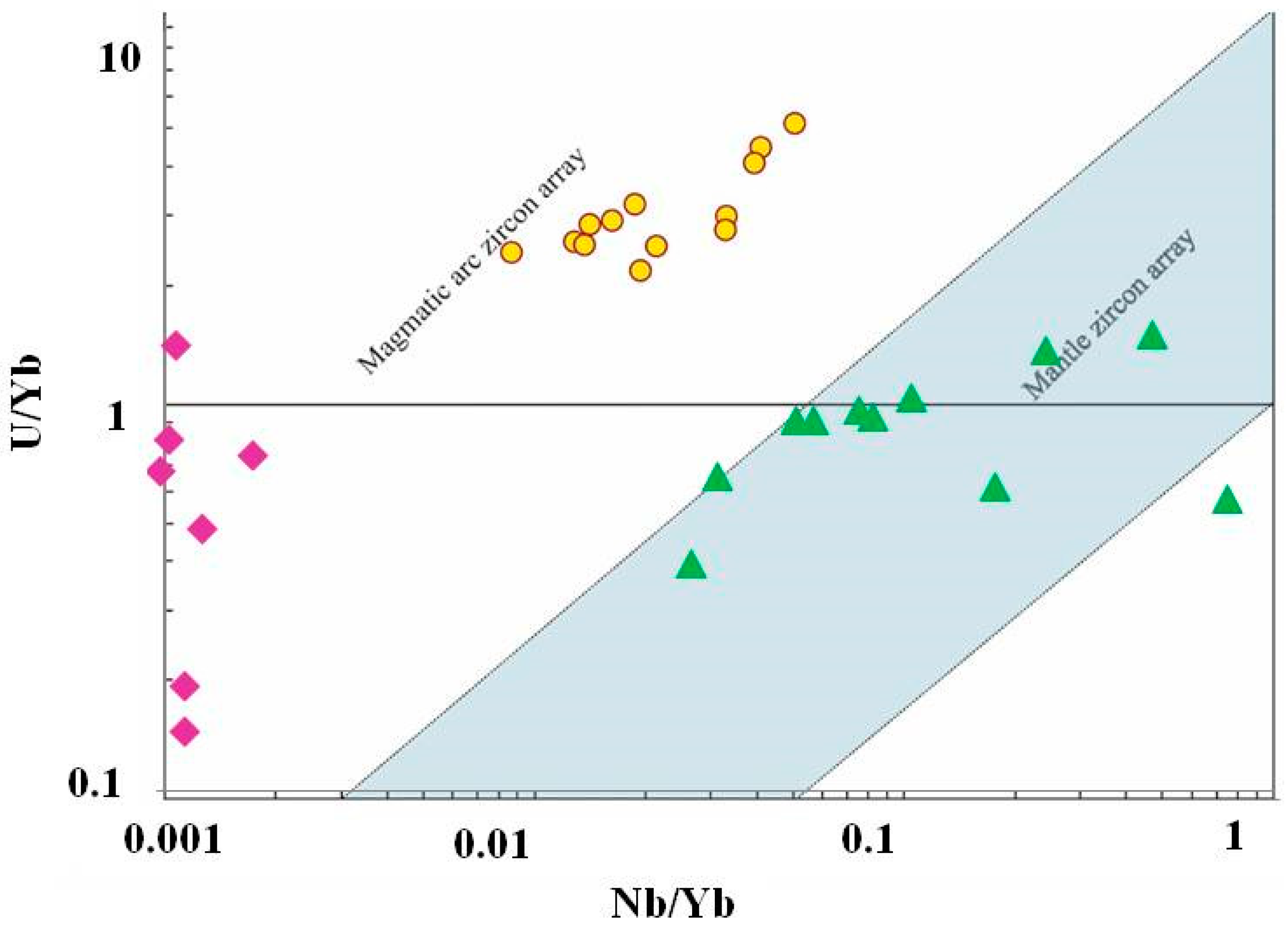
4.4. Trace Element Chemistry of Zircons and In Situ LA-ICP-MS U-Pb Zircon Geochronology
5. Discussion
5.1. Genetic Models of Corundum Anorthosites-Kyshtymites
5.2. Magmatic vs. Metamorphic Origin of Kyshtymites
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Groat, L.; Fagan, A.G. The geology and genesis of gem corundum deposits. In Geology of Gem Deposits, 2nd ed.; Groat, L.A., Ed.; Mineralogical Association of Canada Short Course Series; Mineralogical Association of Canada: Tucson, AZ, USA, 2014; Volume 44, pp. 29–112. [Google Scholar]
- Sorokina, E.S.; Karampelas, S.; Nishanbaev, T.P.; Nikandrov, S.N.; Semiannikov, B.S. Sapphire Megacrysts in Syenite Pegmatites from the Ilmen Mountains, South Urals, Russia: New Mineralogical Data. Can. Mineral. 2017, 55, 823–843. [Google Scholar] [CrossRef]
- Sorokina, E.S.; Rassomakhin, M.A.; Nikandrov, S.N.; Karampelas, S.; Kononkova, N.N.; Nikolaev, A.G.; Anosova, M.O.; Orlova, A.V.; Kostitsyn, Y.A.; Kotlyarov, V.A. Origin of blue sapphire in newly discovered spinel–chlorite–muscovite rocks within meta-ultramafites of Ilmen Mountains, South Urals of Russia: Evidence from mineralogy, geochemistry, Rb-Sr and Sm-Nd isotopic data. Minerals 2019, 9, 36. [Google Scholar] [CrossRef]
- Simonet, C.; Paquette, J.L.; Pin, C.; Lansner, B.; Fritsch, E. The Dusi (Garba Tula) sapphire deposit, Central Kenya–a unique Pan-African corundum-bearing monzonite. J. Afr. Earth Sci. 2004, 38, 401–410. [Google Scholar] [CrossRef]
- Monchoux, P.; Fontan, F.; De Parseval, P.; Martin, R.F.; Wang, R.C. Igneous albitite dikes in orogenic lherzolites, western Pyrenees, France: a possible source for corundum and alkali feldspar xenocrysts in basaltic terranes. I. Mineralogical associations. Can. Mineral. 2006, 44, 817–842. [Google Scholar] [CrossRef]
- Kan-Nyunt, H.P.; Karampelas, S.; Link, K.; Thu, K.; Kiefert, L.; Hardy, P. Blue sapphires from the Baw Mar Mine in Mogok. Gems Gemol. 2013, 49, 223–232. [Google Scholar] [CrossRef]
- Khoi, N.N.; Hauzenberger, C.A.; Sutthirat, C.; Tuan, D.A.; Häger, T.; Van Nam, N. Corundum with Spinel Corona from the Tan Huong–Truc Lau Area in Northern Vietnam. Gems Gemol. 2018, 54, 4. [Google Scholar] [CrossRef]
- Voudouris, P.; Mavrogonatos, C.; Graham, I.; Giuliani, G.; Melfos, V.; Karampelas, S.; Karantoni, V.; Wang, K.; Tarantola, A.; Zaw, K.; et al. Gem Corundum Deposits of Greece: Geology, Mineralogy and Genesis. Minerals 2018, 9, 49. [Google Scholar] [CrossRef]
- Keulen, N.; Kalvig, P. Fingerprinting of corundum (ruby) from Fiskenæsset, West Greenland. Geol. Surv. Denmark Greenland 2013, 25, 53–56. [Google Scholar]
- Karmakar, S.; Mukherjee, S.; Sanyal, S.; Sengupta, P. Origin of peraluminous minerals (corundum, spinel, and sapphirine) in a highly calcic anorthosite from the Sittampundi Layered Complex, Tamil Nadu, India. Contrib. Mineral. Petrol. 2017, 172, 67. [Google Scholar] [CrossRef]
- Gibson, G.M. Margarite in Kyanite- and Corundum-Bearing Anorthosite, Amphibolite, and Hornblendite from Central Fiordland, New Zealand. Contrib. Miner. Petrol. 1979, 68, 171–179. [Google Scholar] [CrossRef]
- Pratt, G.H. Corundum and Its Occurrence and Distribution in the United States; US Government Printing Office: Washington, DC, USA, 1906.
- McElhaney, M.S.; McSween, H.Y. Petrology of the Chunky Gal Mountain mafic-ultramafic complex, North Carolina. GSA Bull. 1983, 94, 855–874. [Google Scholar] [CrossRef]
- Koptev-Dvornikov, V.S.; Kuznetsov, E.A. Borzovskoe Corundum Deposit: Petrological Study; State Technical Publishing House: Moscow, Russia, 1931; p. 320. (In Russian) [Google Scholar]
- Claire, M.O. Corundum and emery on the Urals. Uralskiy Technik. 1918, 7, 1–17. (In Russian) [Google Scholar]
- Kolesnik, N.Y. High-Temperature Metasomatism in Ultrabasic Massifs; Science Publishing House: Novosibirsk, Russia, 1976; p. 240. (In Russian) [Google Scholar]
- Kolesnik, N.Y.; Korolyuk, V.N.; Lavrent’ev, Y.G. Spinels and ore minerals of the Borzovsk deposit of corundum plagioclasites. Notes Russian Mineral. Soc. 1974, 103, 373–378. (In Russian) [Google Scholar]
- Nedosekova, I.L.; Vladykin, N.V.; Pribakin, S.V.; Bayanova, T.B. The structure of the Ilmenogorsky-Vishnevogorsky Miaskite-Carbonatite Complex: Origin, ore-bearing. Sources of matter (Ural, Russia). Geol. Ore Depos. 2009, 51, 157–181. (In Russian) [Google Scholar] [CrossRef]
- Rusin, A.I.; Krasnobaev, A.A.; Valizer, P.M. Geology of the Ilmen Mountains: situation and problems. In Geology and Mineralogy of the Ilmenogorsky Complex: Situation and Problems; IGZ UB RAS: Miass, Russia, 2006; pp. 3–19. (In Russian) [Google Scholar]
- Krasnobaev, A.A.; Puzhakov, B.A.; Petrov, V.I.; Busharina, S.V. Zirconology of metamorphites of the Kyshtym-Arakulian strata of the Sysert-Ilmenogorsky complex. Proc. Zavaritsky Inst. Geol. Geochem. (Trudy Instituta Geologii i Geokhimii im. Akademika A.N. Zavaritskogo) 2009, 156, 264–268. (In Russian) [Google Scholar]
- Kramm, U.; Blaxland, A.B.; Kononova, V.A.; Grauert, B. Origin of the Ilmenogorsk-Vishnevogorsk nepheline syenites, Urals, USSR, and their time of emplacement during the history of the Ural fold belt: A Rb-Sr study. J. Geol. 1983, 91, 427–435. [Google Scholar] [CrossRef]
- Kramm, U.; Chernyshev, I.V.; Grauert, S. Zircon typology and U-Pb systematics: A case study of zircons from nefeline syenite of the Il’meny Mountains, Ural. Petrology 1993, 1, 474–485. [Google Scholar]
- Chernyshev, I.V.; Kononova, V.A.; Kramm, U. Isotope geochronology of alkaline rocks of the Urals in the light of zircon uranium-lead data. Geochemistry 1987, 3, 323–338. [Google Scholar]
- Ivanov, K.S.; Erokhin, Y.V. About the Age and nature of the metamorphic complexes of the Ilmenogorsk zone of the Urals. Rep. Acad. Sci. 2015, 461, 312–315. [Google Scholar]
- Filina, M.I.; Sorokina, E.S.; Rassomakhin, M.A.; Kononkova, N.N.; Kostitsyn, Y.A.; Orlova, A.V. Genetic linkage of corundum plagioclazite-kyshtymite and miaskites of Ilmensky-Vishnevogorsky complex, Southern Urals, Russia: new data on Rb-Sr and Sm-Nd isotopic composition, geochemistry and mineralogy. Geochem. Int. 2019. (accepted). [Google Scholar]
- Arslanova, K.A.; Golubchina, M.N.; Iskanderova, A.D. Geological Dictionary; Nedra Publishing House: Moscow, Russia, 1978; Volume 2, p. 456. (In Russian) [Google Scholar]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of reference values for NIST SRM 610-617 glasses following ISO Guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Jochum, K.P.; Scholz, D.; Stoll, B.; Weis, U.; Wilson, S.A.; Yang, Q.; Schwalb, A.; Börner, N.; Jacob, D.E.; Andreae, M.O. Accurate trace element analysis of speleothems and biogenic calcium carbonates by LA-ICP-MS. Chem. Geol. 2012, 318, 31–44. [Google Scholar] [CrossRef]
- Jackson, S.E.; Pearson, N.J.; Griffin, W.L.; Belousova, E.A. The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U–Pb zircon geochronology. Chem. Geol. 2004, 211, 47–69. [Google Scholar] [CrossRef]
- Kooijman, E.; Berndt, J.; Mezger, K. U-Pb dating of zircon by laser ablation ICP-MS: Recent improvements and new insights. Eur. J. Miner. 2012, 24, 5–21. [Google Scholar] [CrossRef]
- Wiedenbeck, M.; Alle, P.; Corfu, F.; Griffin, W.L.; Meier, M.; Oberli, F.; von Quadt, A.; Roddick, J.C.; Spiegel, W. Three natural zircon standards for U-Th-Pb, Lu-Hf, trace-element and REE analyses. Geostand. Newsl. 1995, 19, 1–23. [Google Scholar] [CrossRef]
- Ludwig, K.R. A User’s Manual; Barkeley Geochonology Center: Berkeley, CA, USA, 2009; p. 100. [Google Scholar]
- Platonov, A.N.; Taran, M.N.; Balitsky, V.S. The Nature of the Coloring of Gems; Nedra Publishing House: Moscow, Russia, 1984; p. 196. (In Russian) [Google Scholar]
- Sorokina, E.S.; Koivula, J.I.; Muyal, J.; Karampelas, S. Multiphase fluid inclusions in blue sapphires from the Ilmen Mountains, southern Urals. Gems Gemol. 2016, 52, 209–211. [Google Scholar]
- Peucat, J.J.; Ruffault, P.; Fritch, E.; Bouhnik-Le Coz, M.; Simonet, C.; Lasnier, B. Ga/Mg ratio as a new geochemical tool to differentiate magmatic from metamorphic blue sapphires. Lithos 2007, 98, 261–274. [Google Scholar] [CrossRef]
- Sutherland, F.L.; Zaw, K.; Meffre, S.; Giuliani, G.; Fallick, A.E.; Graham, I.T.; Webb, G.B. Gem-corundum megacrysts from east Australian basalt fields: trace elements, oxygen isotopes and origins. Aust. J. Earth Sci. 2009, 56, 1003–1022. [Google Scholar] [CrossRef]
- Zwaan, J.C.; Buter, E.; Merty-Kraus, R.; Kane, R.E. The origin of Montana’s alluvial sapphires. Gems Gemol. 2015, 51, 370–391. [Google Scholar]
- Uher, P.; Giuliani, G.; Szaka, L.L.S.; Fallick, A.; Strunga, V.; Vaculovic, T.; Ozdin, D.; Greganova, M. Sapphires related to alkali basalts from the Cerov’a Highlands, Western Carpathians (southern Slovakia): Composition and origin. Geologica Carpathica 2012, 63, 71–82. [Google Scholar] [CrossRef]
- Palke, A.C.; Wong, J.; Verdel, C.; Avila, J.N. A common origin for Thai/Cambodian rubies and blue and violet sapphires from Yogo Gulch, Montana, U.S.A.? Am. Mineral. 2018, 103, 469–479. [Google Scholar] [CrossRef]
- Giuliani, G.; Caumon, G.; Rakotosamizanany, S.; Ohnenstetter, D.; Rakototondrazafy, M. Classification chimique des corindons par analyse factorielle discriminante: application a la typologie des gisements de rubis et saphirs. Chapter mineralogy, physical properties and geochemistry. Revue Gemmol. 2014, 188, 14–22. [Google Scholar]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. London Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Fowler, A.; Prokoph, A.; Stenr, R.; Dupuis, C. Organization of oscillatory zoning in zircon: Analysis, scaling, geochemistry, and model of a zircon from Kipawa, Quebec, Canada. Geochim. Cosmochim. Acta. 2002, 66, 311–328. [Google Scholar] [CrossRef]
- Belousova, E.A.; Griffin, W.L.; O’Reilly, S.Y.; Fisher, N.I. Igneous zircon: trace element compositon as an indicator of source rock type. Contrib. Mineral. Petrol. 2002, 143, 602–622. [Google Scholar] [CrossRef]
- Watson, E.B.; Wark, D.A.; Thomas, J.B. Crystallization thermometers for zircon and rutile. Contrib. Mineral. Petrol. 2006, 151, 413–433. [Google Scholar] [CrossRef]
- Nedosekova, I.L.; Belyatsky, B.V.; Belousova, E.A. Trace elements and Hf isotope composition as indicator of zircon genesis due to the evolution of alkaline-carbonatite magmatic system (Il’meny–Vishnevogorsky complex, Urals, Russia). Geol. Geophys. 2016, 57, 1135–1154. [Google Scholar] [CrossRef]
- Fersman, L.E. Pegmatites; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1940; p. 712. (In Russian) [Google Scholar]
- Lodochnikov, V.N. Serpentines and Serpentinites Ilchirsk and Other Petrological Issues Associated with Them; United Scientific and Technical Publishing: Moscow, Russia, 1936; p. 817. (In Russian) [Google Scholar]
- Korzhinskiy, D.S. Essay on metasomatic processes. In The Main Problems in the Theory of Magmatic Ore Deposits; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1953; pp. 332–450. (In Russian) [Google Scholar]
- Eskova, E.M.; Zhabin, A.G.; Mukhitdinov, G.N. Mineralogy and Geochemistry of Rare Elements of the Vishnevogorsky Mountains; Science Publishing House: Moscow, Russia, 1964; p. 318. (In Russian) [Google Scholar]
- Nedosekova, I.L. U-Pb age and Lu-Hf isotopic systems of zircons Ilmenogorsky-Vishnevogorsky alkaline-carbonatitic complex, South Ural. Lithosphere 2014, 5, 19–31. (In Russian) [Google Scholar]
- Sutherland, F.L.; Abduriym, A. Geographic typing of gem corundum: a test case from Australia. J. Gemmol. 2009, 31, 203–210. [Google Scholar] [CrossRef]
- Giuliani, G.; Fallick, A.E.; Garnier, V.; France-Lanord, C.; Ohnenstetter, D.; Schwarz, D. Oxygen isotope composition as a tracer for the origins of rubies and sapphires. Geology 2005, 33, 249–252. [Google Scholar] [CrossRef]
- Vysotsky, S.V.; Nechaev, V.P.; Kissin, A.Y.; Yakovlenko, V.V.; Velivetskaya, T.A.; Sutherland, F.L.; Agoshkov, A.I. Oxygen isotopic composition as an indicator of ruby and sapphire origin: A review of Russian occurrences. Ore Geol. Rev. 2015, 68, 164–170. [Google Scholar] [CrossRef]
- Sutherland, F.L.; Giuliani, G.; Fallick, A.E.; Garland, M.; Webb, G. Sapphire-ruby characteristics, West Pailin, Cambodia: Clues to their origin based on trace element and O isotope analysis. Aust. Gemmol. 2008, 23, 329–368. [Google Scholar]
- Sutherland, F.L.; Schwarz, D.; Jobbins, E.A.; Coenraads, R.R.; Webb, G. Distinctive gem corundum suites from discrete basalt fields: a comparative study of Barrington, Australia, and west Pailin, Cambodia, gemfields. J. Gemmol. 1998, 26, 65–85. [Google Scholar] [CrossRef]
- Saeseaw, S.; Sangsawong, S.; Vertriest, W.; Atikarnsakul, U. An In-Depth Study of Blue Sapphires from Pailin, Cambodia; Gemological Institute of America report: Carlsbad, CA, USA, 2017; p. 45. [Google Scholar]
- Grimes, C.B.; John, B.E.; Kelemen, P.B.; Mazdab, F.K.; Wooden, J.L.; Cheadle, M.J.; Hanghoj, K.; Schwartz, J.J. Trace element chemistry of zircons from oceanic crust: A method for distinguishing detrital zircon provenance. Geology 2007, 35, 643–646. [Google Scholar] [CrossRef]
- Grimes, C.B.; Wooden, J.L.; Cheadle, M.J.; John, B.E. “Fingerprinting” tectono-magmatic provenance using trace elements in igneous zircon. Contrib. Mineral. Petrol. 2015, 170, 46. [Google Scholar] [CrossRef]
- Botcharnikov, R.E.; Almeev, R.R.; Koepke, J.; Holtz, F. Phase relations and liquid lines of descent in hydrous ferrobasalt—Implications for the Skaergaard Intrusion and Columbia River flood basalts. J. Petrol. 2008, 49, 1687–1727. [Google Scholar] [CrossRef]
- Arndt, N. The formation of massif anorthosite: Petrology in reverse. Geosci. Front. 2013, 7, 875–889. [Google Scholar] [CrossRef]
- Leet, J.K.; Williams, I.S.; Ellis, D.J. Pb, U and Th diffusion in natural zircon. Nature 1997, 390, 159–162. [Google Scholar]
- Biondi, J.C. Neoproterozoic Cana Brava chrysotile deposit (Goiás, Brazil): Geology and geochemistry of chrysotile vein formation. Lithos 2014, 184, 132–154. [Google Scholar] [CrossRef]
- Varlakov, A.S.; Kuznetsov, G.P.; Korablev, G.G. Hyperbasites of the Ilmenogorsky-Vishnevogorsky complex Complex (Southern Urals); Publishing House of the Institute of Mineralogy, Ural Branch RAS: Miass, Russia, 1998; p. 195. (In Russian) [Google Scholar]
- Yakymchuk, C.; Kristoffer, S. Corundum formation by metasomatic reactions in Archean metapelite, SW Greenland: Exploration vectors for ruby deposits within high-grade greenstone belts. Geosci. Front. 2017, 9, 1–24. [Google Scholar] [CrossRef]
- Hofmann, A.W. Mantle geochemistry: The message from oceanic volcanism. Nature 1997, 385, 219–229. [Google Scholar] [CrossRef]


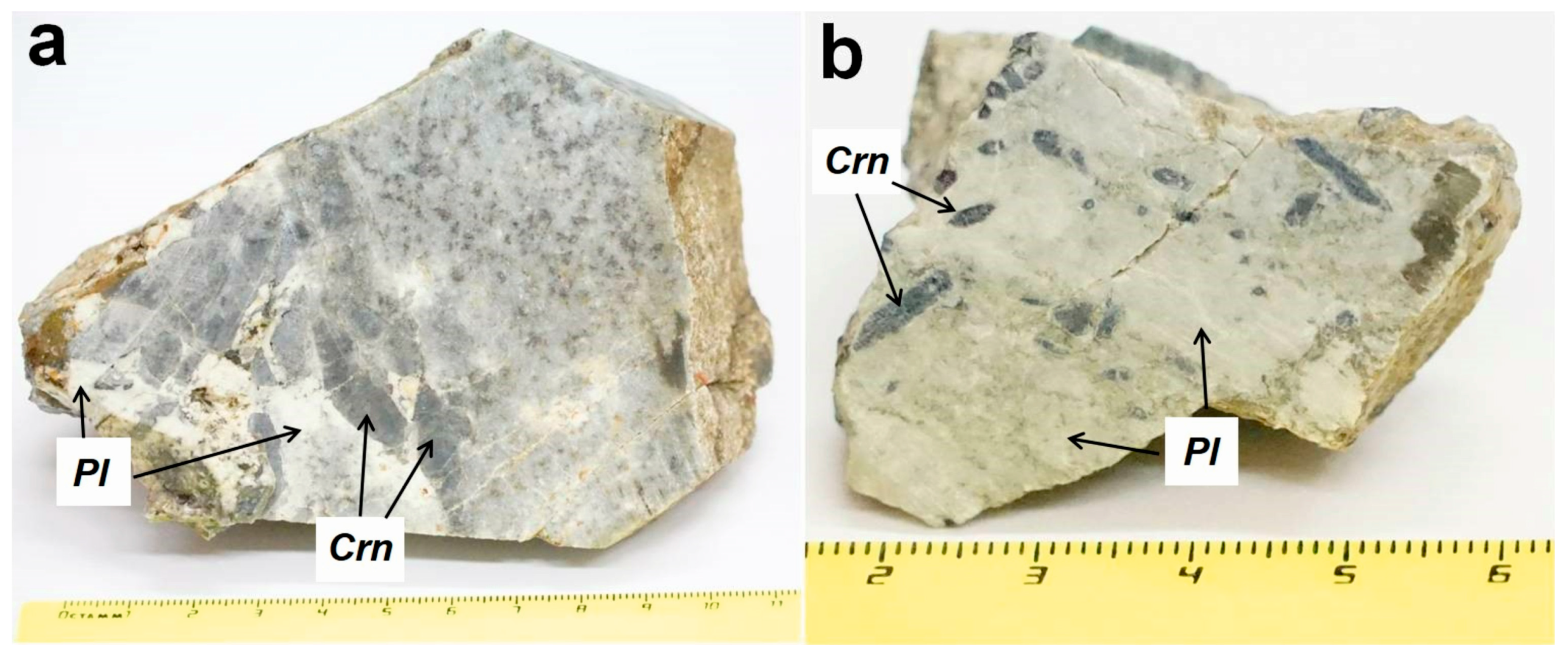



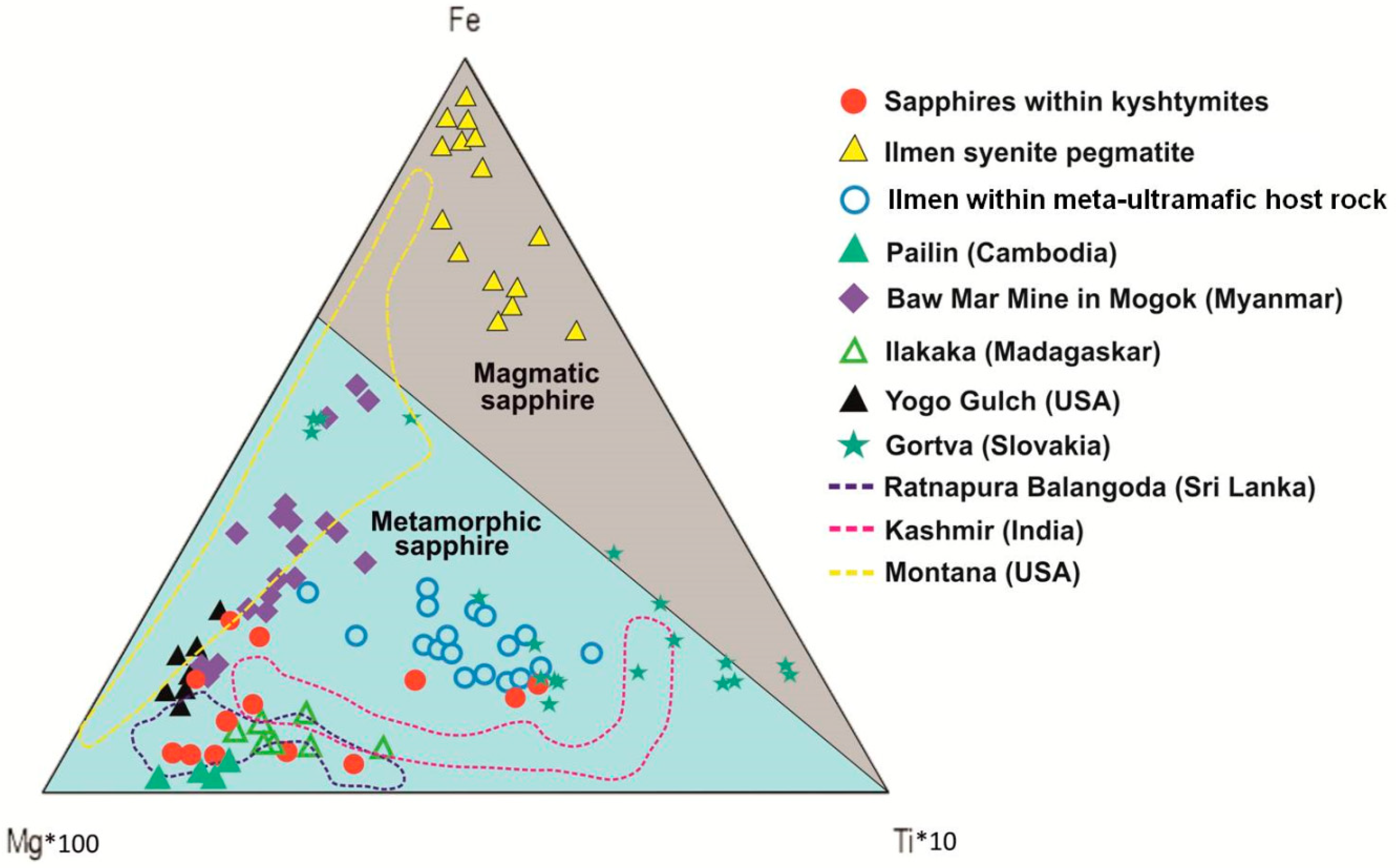


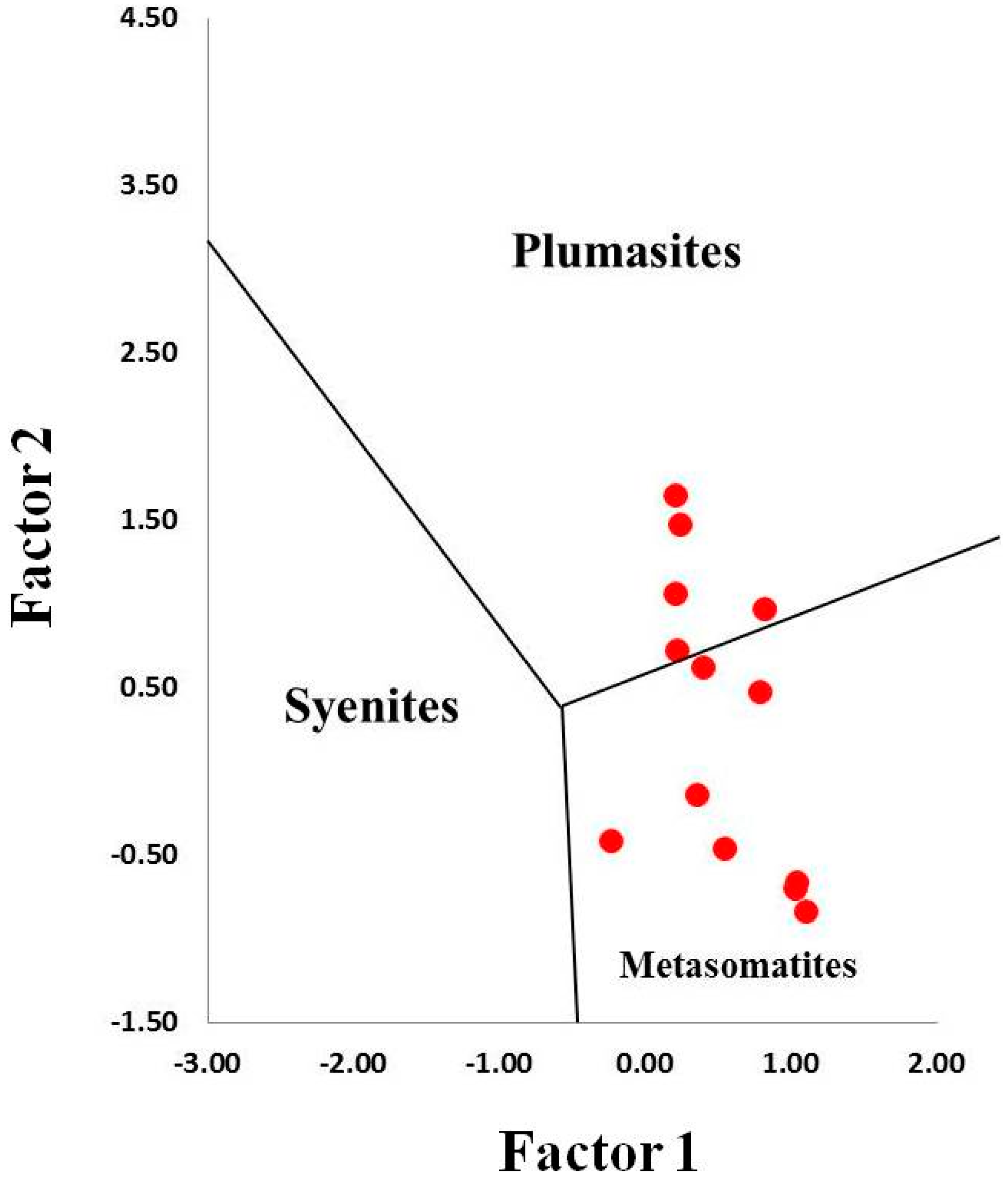
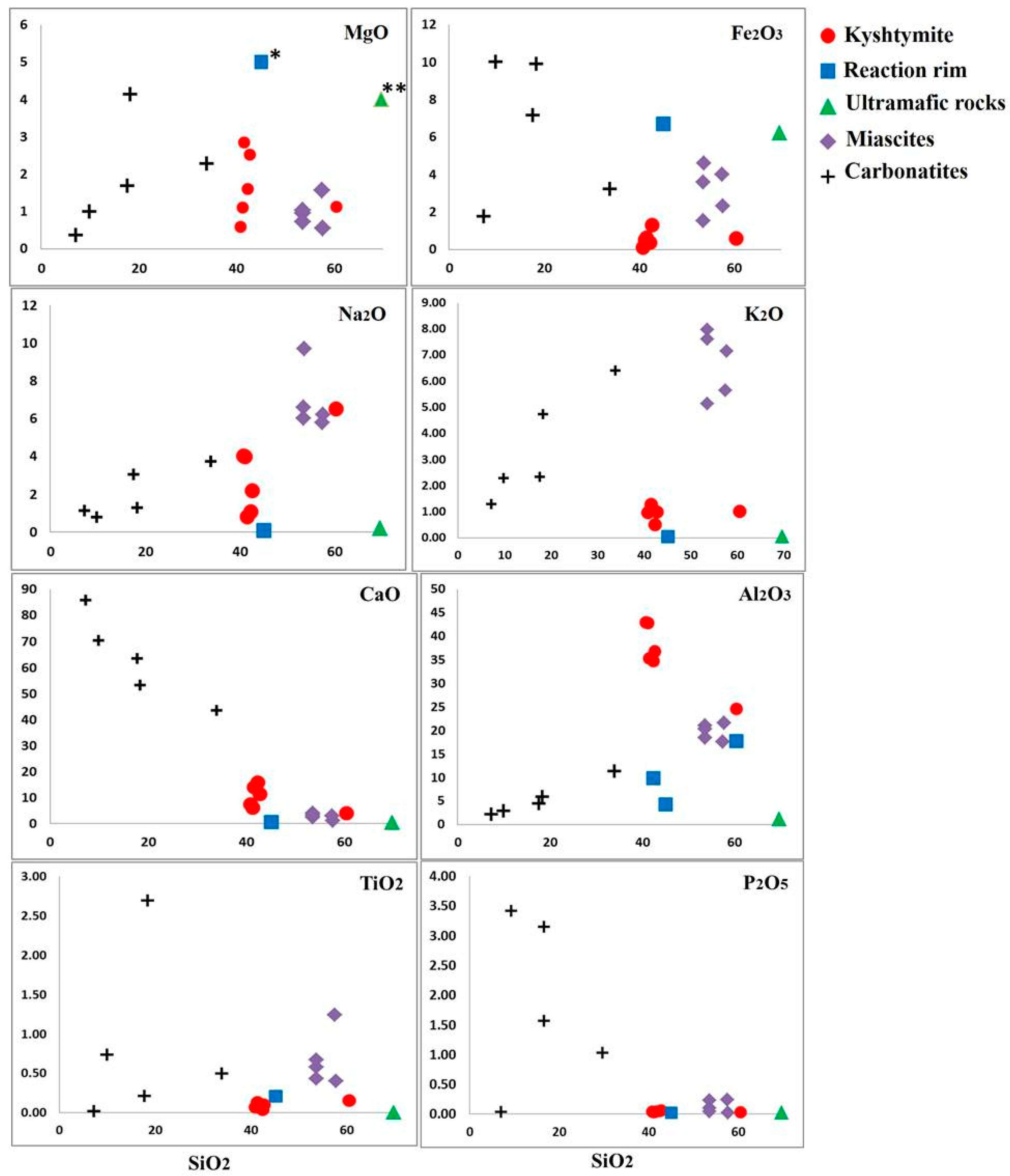

| Sample/No. of Spot | Color | Li | Be | Mg | Ti | V | Cr | Fe* | Ga | Ga/Mg | Fe/Ti | Cr/Ga | Fe/Mg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K-8-1 | 1 | Wt | 32.45 | 1.85 | 199.85 | 972.77 | 5.59 | 3.18 | 1166.20 | 36.40 | 0.18 | 1.20 | 0.09 | 5.84 |
| 2 | Bl | 8.34 | 1.67 | 183.70 | 700.93 | 5.96 | 12.34 | 1477.19 | 38.57 | 0.21 | 2.11 | 0.32 | 8.04 | |
| 3 | Bl | bdl | bdl | 334.58 | 782.98 | 6.49 | 2.59 | 2099.17 | 36.90 | 0.11 | 2.68 | 0.07 | 6.27 | |
| 4 | Wt | bdl | bdl | 74.20 | 702.78 | 6.35 | 3.31 | 855.22 | 36.53 | 0.49 | 1.22 | 0.09 | 11.53 | |
| 5 | Wt | bdl | 2.20 | 69.22 | 231.35 | 7.54 | 2.59 | 1399.44 | 33.72 | 0.49 | 6.05 | 0.08 | 20.22 | |
| 6 | Wt | bdl | 3.12 | 67.05 | 115.41 | 8.52 | 1.61 | 1710.43 | 33.38 | 0.50 | 14.82 | 0.05 | 25.51 | |
| 7 | Bl | bdl | 2.51 | 66.78 | 101.64 | 9.08 | 2.44 | 2332.41 | 33.03 | 0.49 | 22.95 | 0.07 | 34.92 | |
| 8 | Bl | bdl | bdl | 291.17 | 458.20 | 5.35 | 3.86 | 1865.92 | 32.00 | 0.11 | 4.07 | 0.12 | 6.41 | |
| 9 | Wt | bdl | bdl | 388.58 | 297.79 | 4.39 | 7.07 | 1710.43 | 30.36 | 0.08 | 5.74 | 0.23 | 4.40 | |
| 10 | Wt | bdl | bdl | 741.16 | 585.52 | 4.74 | 6.51 | 855.22 | 30.76 | 0.04 | 1.46 | 0.21 | 1.15 | |
| K-8-2 | 1 | Wt | bdl | bdl | 131.29 | 986.27 | 5.53 | 6.04 | 1788.18 | 32.77 | 0.25 | 1.81 | 0.18 | 13.62 |
| 2 | Bl | bdl | bdl | 59.29 | 782.19 | 6.27 | 1.89 | 2021.42 | 36.16 | 0.61 | 2.58 | 0.05 | 34.09 | |
| 3 | Bl | bdl | bdl | 46.67 | 706.48 | 6.38 | 1.60 | 2021.42 | 37.30 | 0.80 | 2.86 | 0.04 | 43.32 | |
| 4 | Bl | bdl | bdl | 296.46 | 559.58 | 6.17 | 2.40 | 1865.92 | 37.19 | 0.13 | 3.33 | 0.06 | 6.29 | |
| 5 | Wt | bdl | 3.47 | 127.59 | 164.38 | 8.63 | 2.52 | 1166.20 | 34.76 | 0.27 | 7.09 | 0.07 | 9.14 | |
| 6 | Wt | bdl | 2.67 | 94.50 | 122.82 | 8.55 | 2.90 | 1166.20 | 34.46 | 0.36 | 9.50 | 0.08 | 12.34 | |
| 7 | Bl | bdl | 2.33 | 92.62 | 133.67 | 8.60 | 1.86 | 1943.67 | 35.50 | 0.38 | 14.54 | 0.05 | 20.99 | |
| 8 | Bl | bdl | bdl | 77.21 | 583.13 | 6.59 | 0.92 | 2410.15 | 39.81 | 0.52 | 4.13 | 0.02 | 31.21 | |
| 9 | Wt | 6.83 | bdl | 95.29 | 656.99 | 5.98 | 0.73 | 1943.67 | 37.72 | 0.40 | 2.96 | 0.02 | 20.40 | |
| 10 | Wt | bdl | bdl | 91.85 | 711.78 | 5.17 | 0.93 | 1399.44 | 36.40 | 0.40 | 1.97 | 0.03 | 15.24 | |
| K-12-1 | 1 | Bl | bdl | bdl | 164.38 | 939.95 | 3.97 | bdl | 1010.71 | 42.99 | 0.26 | 1.08 | - | 6.15 |
| 2 | Wt | bdl | 2.81 | 167.82 | 294.08 | 6.70 | bdl | 621.97 | 56.25 | 0.34 | 2.11 | - | 3.71 | |
| 3 | Wt | bdl | 1.96 | 188.47 | 210.70 | 6.22 | bdl | 1321.70 | 61.33 | 0.33 | 6.27 | - | 7.01 | |
| 4 | Wt | bdl | 3.60 | 147.68 | 216.79 | 6.49 | bdl | 4198.33 | 57.49 | 0.39 | 19.37 | - | 28.43 | |
| 5 | Wt | bdl | 3.81 | 94.97 | 125.20 | 6.11 | bdl | 855.22 | 48.65 | 0.51 | 6.83 | - | 9.00 | |
| 6 | Wt | bdl | bdl | 222.08 | 205.14 | 3.71 | bdl | 1554.94 | 50.95 | 0.23 | 7.58 | - | 7.00 | |
| 7 | Wt | bdl | bdl | 127.77 | 156.44 | 6.14 | bdl | 1321.70 | 46.38 | 0.36 | 8.45 | - | 10.34 | |
| 8 | Bl | bdl | 1.85 | 157.23 | 374.82 | 8.07 | bdl | 5209.04 | 52.41 | 0.33 | 13.90 | - | 33.13 | |
| 9 | Bl | bdl | bdl | 190.32 | 524.11 | 8.71 | 0.03 | 3265.37 | 54.58 | 0.29 | 6.23 | - | 17.16 | |
| 10 | Bl | bdl | bdl | 237.44 | 555.61 | 7.91 | 0.35 | 3109.87 | 50.90 | 0.21 | 5.60 | 0.01 | 13.10 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filina, M.I.; Sorokina, E.S.; Botcharnikov, R.; Karampelas, S.; Rassomakhin, M.A.; Kononkova, N.N.; Nikolaev, A.G.; Berndt, J.; Hofmeister, W. Corundum Anorthosites-Kyshtymites from the South Urals, Russia: A Combined Mineralogical, Geochemical, and U-Pb Zircon Geochronological Study. Minerals 2019, 9, 234. https://doi.org/10.3390/min9040234
Filina MI, Sorokina ES, Botcharnikov R, Karampelas S, Rassomakhin MA, Kononkova NN, Nikolaev AG, Berndt J, Hofmeister W. Corundum Anorthosites-Kyshtymites from the South Urals, Russia: A Combined Mineralogical, Geochemical, and U-Pb Zircon Geochronological Study. Minerals. 2019; 9(4):234. https://doi.org/10.3390/min9040234
Chicago/Turabian StyleFilina, Maria I., Elena S. Sorokina, Roman Botcharnikov, Stefanos Karampelas, Mikhail A. Rassomakhin, Natalia N. Kononkova, Anatoly G. Nikolaev, Jasper Berndt, and Wolfgang Hofmeister. 2019. "Corundum Anorthosites-Kyshtymites from the South Urals, Russia: A Combined Mineralogical, Geochemical, and U-Pb Zircon Geochronological Study" Minerals 9, no. 4: 234. https://doi.org/10.3390/min9040234
APA StyleFilina, M. I., Sorokina, E. S., Botcharnikov, R., Karampelas, S., Rassomakhin, M. A., Kononkova, N. N., Nikolaev, A. G., Berndt, J., & Hofmeister, W. (2019). Corundum Anorthosites-Kyshtymites from the South Urals, Russia: A Combined Mineralogical, Geochemical, and U-Pb Zircon Geochronological Study. Minerals, 9(4), 234. https://doi.org/10.3390/min9040234






