Optimizing Conditions for Scandium Extraction from Bauxite Residue Using Taguchi Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Characterization
2.2. Preliminary Tests
2.3. Experimental Procedure
2.4. Taguchi Method
2.5. Analysis of Experimental Data
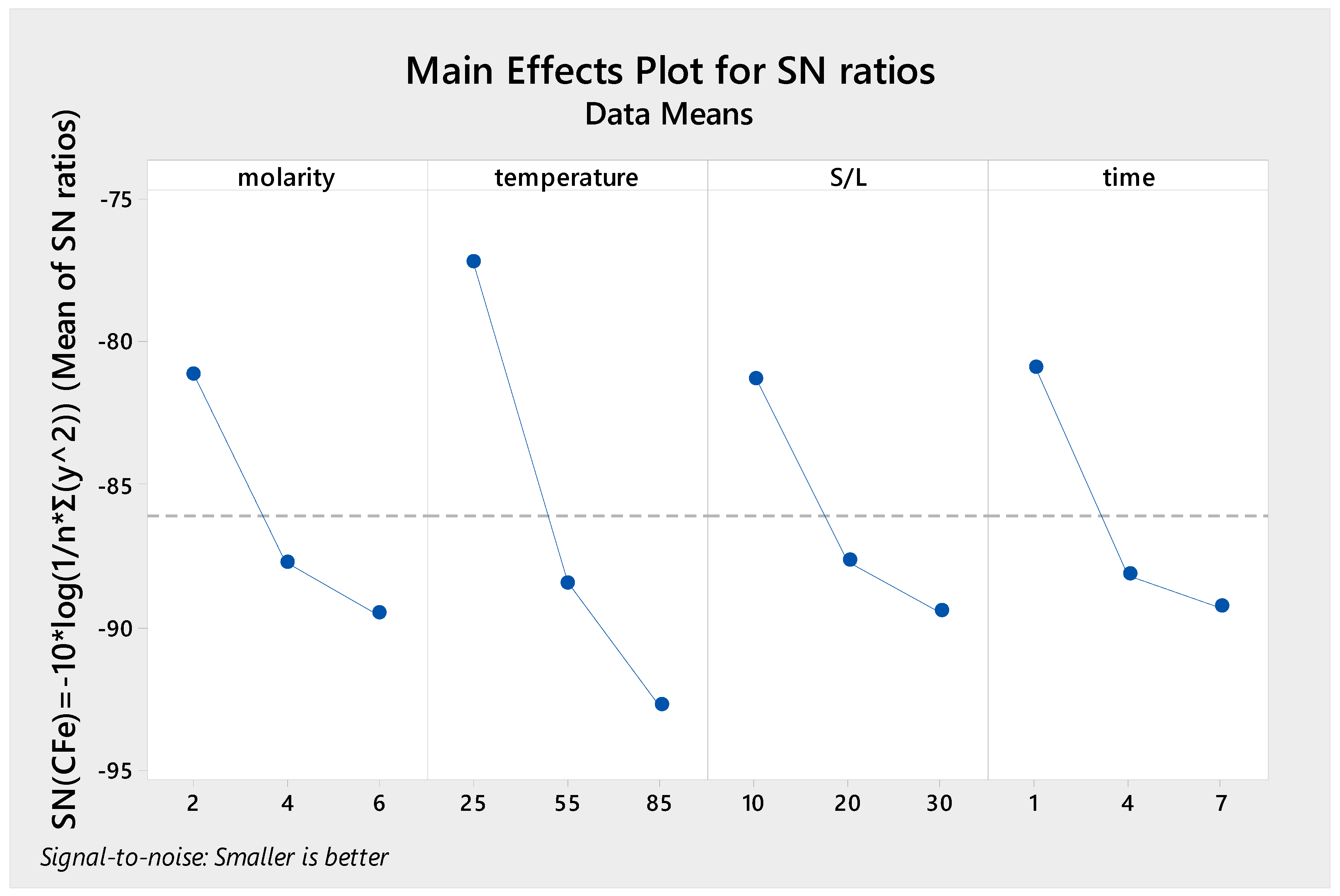
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Kumar, R.; Bandopadhyay, A. Innovative methodologies for the utilisation of wastes from metallurgical and allied industries. Resour. Conserv. Recycl. 2006, 48, 301–314. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N. Utilization of red mud in cement production: A review. Waste Manag. Res. 2011, 29, 1053–1063. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, S.C.; Sahoo, S.K.; Chakraverty, A.P.; Maharana, H.S. Progress of red mud utilization: An overview. Am. Chem. Sci. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Mishra, B.; Gostu, S. Materials sustainability for environment: Red-mud treatment. Front. Chem. Sci. Eng. 2017, 11, 483–496. [Google Scholar] [CrossRef]
- Samal, S.; Ray, A.K.; Bandopadhyay, A. Proposal for resources, utilization and processes of red mud in india—A review. Int. J. Miner. Process. 2013, 118, 43–55. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Nuamah, R.A.; Agyei-Tuffour, B.; Obada, D.O.; Yaya, A. Awaso bauxite red mud-cement based composites: Characterisation for pavement applications. Case Stud. Constr. Mater. 2017, 7, 45–55. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, J.; Li, H.; Zhao, P.; Chen, Q. Recycling red mud from the production of aluminium as a red cement-based mortar. Waste Manag. Res. 2017, 35, 500–507. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Konadu, D.S.; Annan, E.; Buabeng, F.P.; Yaya, A.; Agyei-Tuffour, B. Fabrication and characterisation of ghanaian bauxite red mud-clay composite bricks for construction applications. Am. J. Mater. Sci. 2013, 3, 110–119. [Google Scholar]
- Lavanya, P.M.; Kumar, K.S. Characterization of red mud as a construction material using bioremediation. Int. J. Res. Sci. Adv. Eng. 2017, 2, 132–139. [Google Scholar]
- Liao, C.Z.; Shih, K. Chapter 20—Thermal behavior of red mud and its beneficial use in glass-ceramic production. In Environmental Materials and Waste; Prasad, M.N.V., Shih, K., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 525–542. [Google Scholar]
- Kim, Y.; Kim, M.; Sohn, J.; Park, H. Applicability of gold tailings, waste limestone, red mud, and ferronickel slag for producing glass fibers. J. Clean. Prod. 2018, 203, 957–965. [Google Scholar] [CrossRef]
- Ascensão, G.; Seabra, M.P.; Aguiar, J.B.; Labrincha, J.A. Red mud-based geopolymers with tailored alkali diffusion properties and ph buffering ability. J. Clean. Prod. 2017, 148, 23–30. [Google Scholar] [CrossRef]
- Ma, M.; Lu, Y.; Chen, R.; Ma, L.; Wang, Y. Hexavalent chromium removal from water using heat-acid activated red mud. Open J. Appl. Sci. 2014, 4, 275–284. [Google Scholar] [CrossRef]
- Pepper, R.A.; Couperthwaite, S.J.; Millar, G.J. Re-use of waste red mud: Production of a functional iron oxide adsorbent for removal of phosphorous. J. Water Process Eng. 2018, 25, 138–148. [Google Scholar] [CrossRef]
- Alighardashi, A.; Gharibi, H.R.; Raygan, S.; Akbarzadeh, A. Study of novel mechano-chemical activation process of red mud to optimize nitrate removal from water. Water Sci. Technol. 2015, 73, 899–908. [Google Scholar] [CrossRef]
- Guo, T.; Yang, H.; Liu, Q.; Gu, H.; Wang, N.; Yu, W.; Dai, Y. Adsorptive removal of phosphate from aqueous solutions using different types of red mud. Water Sci. Technol. 2018, 2017, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, L.; Sun, P.; Zhai, P.; Chen, X.; Zhang, H.; Zhang, Z.; Zhu, W. Novel application of red mud: Facile hydrothermal-thermal conversion synthesis of hierarchical porous Alooh and Al2O3 microspheres as adsorbents for dye removal. Chem. Eng. J. 2017, 321, 622–634. [Google Scholar] [CrossRef]
- Oliveira, E.H.C.; Mendonça, É.T.R.; Barauna, O.S.; Ferreira, J.M.; Motta Sobrinho, M.A. Study of variables for optimization of the dye indosol adsorption process using red mud and clay as adsorbents. Adsorption 2016, 22, 59–69. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lu, R.; Zhou, F.; An, Q.; Meng, Z.; Fei, B.; Lv, F. Novel multiple coagulant from bayer red mud for oily sewage treatment. Desalin. Water Treat. 2015, 54, 690–698. [Google Scholar] [CrossRef]
- Kulishenko, A.E.; Klimenko, N.A.; Grechanik, S.V.; Kravchenko, T.B.; Kostyuk, V.A.; Avramenko, L.P.; Kosogina, I.V. The use of products of recycling waste of aluminum manufacturing as a coagulant when purifying highly colored natural water. J. Water Chem. Technol. 2018, 40, 265–271. [Google Scholar] [CrossRef]
- Ni, F.; He, J.; Wang, Y.; Luan, Z. Preparation and characterization of a cost-effective red mud/polyaluminum chloride composite coagulant for enhanced phosphate removal from aqueous solutions. J. Water Process Eng. 2015, 6, 158–165. [Google Scholar] [CrossRef]
- Tao, L.; Wu, H.; Wang, J.; Li, B.; Wang, X.-Q.; Ning, P. Removal of SO2 from flue gas using bayer red mud: Influence factors and mechanism. J. Cent. South Univ. 2019, 26, 467–478. [Google Scholar] [CrossRef]
- Thang, N.H.; Quyen, P.V.T.H.; Nhung, L.T.; Phong, D.T.; Tuyen, N.N.K. Utilization of red mud and bagasse for production of gas absorption materials. Aip Conf. Proc. 2018, 1954, 040010. [Google Scholar] [CrossRef]
- Thang, N.H.; Ngoc, N.H.L.; Uyen, V.T.N.; Kien, P.T. Novel materials synthesized from red mud, bagasse, and bentonite for gas treatment by CO2 absorption. Matec Web Conf. 2018, 207, 03005. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.; Wang, M.; Wang, H.; Xian, P. Recovery of iron from red mud by selective leach with oxalic acid. Hydrometallurgy 2015, 157, 239–245. [Google Scholar] [CrossRef]
- Urík, M.; Bujdoš, M.; Milová-Žiaková, B.; Mikušová, P.; Slovák, M.; Matúš, P. Aluminium leaching from red mud by filamentous fungi. J. Inorg. Biochem. 2015, 152, 154–159. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Tsakiridis, P.E.; Potiriadis, K. Greek “red mud” residue: A study of microwave reductive roasting followed by magnetic separation for a metallic iron recovery process. J. Hazard. Mater. 2013, 254–255, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Kaußen, F.; Friedrich, B. Reductive smelting of red mud for iron recovery. Chem. Ing. Tech. 2015, 87, 1535–1542. [Google Scholar] [CrossRef]
- Zhu, X.-f.; Zhang, T.-a.; Wang, Y.-X.; Lü, G.-z.; Zhang, W.-g. Recovery of alkali and alumina from bayer red mud by the calcification–carbonation method. Int. J. Miner. Metall. Mater. 2016, 23, 257–268. [Google Scholar] [CrossRef]
- Alkan, G.; Xakalashe, B.; Yagmurlu, B.; Kaußen, F.; Friedrich, B. Conditioning of red mud for subsequent titanium and scandium recovery—a conceptual design study. World Metall.-Erzmetall 2017, 70, 5–12. [Google Scholar]
- Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.E.; Markopoulos, C. Titanium leaching from red mud by diluted sulfuric acid at atmospheric pressure. J. Hazard. Mater. 2008, 157, 579–586. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropoulou, M.; Hatzilyberis, K.S.; Mendrinos, L.N.; Salmas, C.E. Pilot-plant investigation of the leaching process for the recovery of scandium from red mud. Ind. Eng. Chem. Res. 2002, 41, 5794–5801. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Ochsenkühn, K.M.; Parissakis, G. Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, D.; Liao, C.; Deng, Y.; Zhang, T.; Shih, K. Red mud powders as low-cost and efficient catalysts for persulfate activation: Pathways and reusability of mineralizing sulfadiazine. Sep. Purif. Technol. 2016, 167, 136–145. [Google Scholar] [CrossRef]
- Wu, J.; Gong, Z.; Lu, C.; Niu, S.; Ding, K.; Xu, L.; Zhang, K. Preparation and performance of modified red mud-based catalysts for selective catalytic reduction of NOX with NH3. Catalysts 2018, 8, 35. [Google Scholar] [CrossRef]
- Zou, Q.; An, W.; Wu, C.; Li, W.; Fu, A.; Xiao, R.; Chen, H.; Xue, S. Red mud-modified biochar reduces soil arsenic availability and changes bacterial composition. Environ. Chem. Lett. 2018, 16, 615–622. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Song, J.; Xiao, R.; Luo, L.; Yang, Z.; Chai, L. Stabilization of cd-, pb-, cu- and zn-contaminated calcareous agricultural soil using red mud: A field experiment. Environ. Geochem. Health 2018, 40, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Kim, Y.-K. Stabilization of heavy metal contaminated marine sediments with red mud and apatite composite. J. Soils Sediments 2016, 16, 726–735. [Google Scholar] [CrossRef]
- Luo, H.L.; Zhu, Q.; Zhou, Y.; Liu, Z.L.; Ou, W.Q. Stabilization of cd in ore soil using modified red mud materials. Adv. Mater. Res. 2017, 1142, 296–299. [Google Scholar] [CrossRef]
- Lymperopoulou, T. Determination and Recovery of Rare Earths from Bauxites and Red Mud. Ph.D. Thesis, National Technical University of Athens, Athens, Greece, 1996. [Google Scholar]
- Tsakanika, L.-A. Separation and Recovery of Lanthanides from Red Mud by Use of Selective Extraction and Chromatographic Techniques. Ph.D. Thesis, National Technical University of Athens, Athens, Greece, 2013. [Google Scholar]
- Ochsenkühn-Petropoulou, M.; Tsakanika, L.-A.; Lymperopoulou, T. Process Control of an Innovative Method for the Recovery and Separation of rare Earths from Red Mud by Different analytical Techniques. In Proceedings of the 1st European Rare Earth Resources Conference (ERES 2014), Milos Island, Greece, 4–7 September 2014; pp. 28–29. [Google Scholar]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Parissakis, G. Direct determination of landthanides, yttrium and scandium in bauxites and red mud from alumina production. Anal. Chim. Acta 1994, 296, 305–313. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials. Third List of Critical Raw Materials for the EU of 2017. Available online: https://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en (accessed on 28 February 2019).
- SCANDIUM Investing News. Why Scandium Could be a Huge Opportunity. Available online: http://investingnews.com/daily/resource-investing/critical-metalsinvesting/scandiuminvesting/scandium-production-the-problem-and-the-opportunity/ (accessed on 28 February 2019).
- Williams-Jones, A.E.; Vasyukova, O.V. The economic geology of scandium, the runt of the rare earth element litter. Econ. Geol. 2018, 113, 973–988. [Google Scholar] [CrossRef]
- Samson, I.M.; Chassé, M. Scandium. In Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth; White, W.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–5. [Google Scholar]
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths, 2nd ed.; Taylor & Francis: Didcot, UK, 2015. [Google Scholar]
- Rivera, R.M.; Xakalashe, B.; Ounoughene, G.; Binnemans, K.; Friedrich, B.; Van Gerven, T. Selective rare earth element extraction using high-pressure acid leaching of slags arising from the smelting of bauxite residue. Hydrometallurgy 2019, 184, 162–174. [Google Scholar] [CrossRef]
- Bonomi, C.; Giannopoulou, I.; Panias, D. Correlation of scandium and titanium during leaching of bauxite residue (red mud) by an imidazolium ionic liquid. In Proceedings of the 2nd conference on European Rare Earth Resources (ERES2017), Santorini Island, Greece, 26 May 2017; pp. 199–201. [Google Scholar]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Qu, Y.; Lian, B. Bioleaching of rare earth and radioactive elements from red mud using penicillium tricolor rm-10. Bioresour. Technol. 2013, 136, 16–23. [Google Scholar] [CrossRef]
- Pepper, R.A.; Couperthwaite, S.J.; Millar, G.J. Comprehensive examination of acid leaching behaviour of mineral phases from red mud: Recovery of Fe, Al, Ti, and Si. Miner. Eng. 2016, 99, 8–18. [Google Scholar] [CrossRef]
- Hatzilyberis, K.; Lymperopoulou, T.; Tsakanika, L.-A.; Ochsenkühn, K.-M.; Georgiou, P.; Defteraios, N.; Tsopelas, F.; Ochsenkühn-Petropoulou, M. Process design aspects for scandium-selective leaching of bauxite residue with sulfuric acid. Minerals 2018, 8, 79. [Google Scholar] [CrossRef]
- Taguchi, G.; Konishi, S. Taguchi Methods, Orthogonal Arrays and Linear Graphs, Tools for Quality Engineering; American Supplier Institute: Dearborn, MI, USA, 1987. [Google Scholar]
- Copur, M.; Kizilca, M.; Kocakerim, M.M. Determination of the optimum conditions for copper leaching from chalcopyrite concentrate ore using taguchi method. Chem. Eng. Commun. 2015, 202, 927–935. [Google Scholar] [CrossRef]
- Mbuya, B.I.; Kime, M.B.; Tshimombo, A.M.D. Comparative study of approaches based on the taguchi and anova for optimising the leaching of copper–cobalt flotation tailings. Chem. Eng. Commun. 2017, 204, 512–521. [Google Scholar] [CrossRef]
- Ochsenkuehn-Petropoulou, M.; Tsakanika, L.-A.; Lymperopoulou, T.; Ochsenkuehn, K.-M.; Hatzilyberis, K.; Georgiou, P.; Stergiopoulos, C.; Serifi, O.; Tsopelas, F. Efficiency of sulfuric acid on selective scandium leachability from bauxite residue. Metals 2018, 8, 915. [Google Scholar] [CrossRef]
- Lymperopoulou, T.; Tsakanika, L.A.; Ochsenkühn, K.M.; Ochsenkühn-Petropoulou, M. Optimization of mineral acids leaching process for the recovery of rare earth elements from Greek red mud. In Proceedings of the 2nd conference on European Rare Earth Resources (ERES2017), Santorini Island, Greece, 28–31 May 2017; pp. 182–184. [Google Scholar]
- Phadke, M.S. Quality Engineering Using Robust Design; Prentice Hall: Upper Saddle River, NJ, USA, 1989. [Google Scholar]
- Rai, S.; Wasewar, K.L.; Lataye, D.H.; Mishra, R.S.; Puttewar, S.P.; Chaddha, M.J.; Mahindiran, P.; Mukhopadhyay, J. Neutralization of red mud with pickling waste liquor using taguchi’s design of experimental methodology. Waste Manag. Res. 2012, 30, 922–930. [Google Scholar] [CrossRef]
- Peace, G.S. Taguchi Methods: A Hands-on Approach; Addison-Wesley: Boston, MA, USA, 1993. [Google Scholar]
- De, A. Design for Reliability and Quality; Indian Institute of Technology: Bombay, India, 2012; pp. 1–25. [Google Scholar]
- Rai, S.; Wasewar, K.L.; Lataye, D.H.; Mukhopadhyay, J.; Yoo, C.K. Feasibility of red mud neutralization with seawater using taguchi’s methodology. Int. J. Environ. Sci. Technol. 2013, 10, 305–314. [Google Scholar] [CrossRef]
- Copur, M.; Özmetin, C.; Özmetin, E.; Kocakerim, M.M. Optimization study of the leaching of roasted zinc sulphide concentrate with sulphuric acid solutions. Chem. Eng. Process. Process Intensif. 2004, 43, 1007–1014. [Google Scholar] [CrossRef]
- Bayca, S.U.; Kisik, H. Optimization of leaching parameters of aluminum hydroxide extraction from bauxite waste using the taguchi method. Environ. Prog. Sustain. Energy 2018, 37, 196–202. [Google Scholar] [CrossRef]
- Taguchi, G.; Chowdhury, S.; Wu, Y. Taguchi’s Quality Engineering Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Phadke, M.S.; Kackar, R.N.; Speeney, D.V.; Grieco, M.J. Off-line quality control in integrated circuit fabrication using experimental design. Bell Syst. Tech. J. 1983, 62, 1273–1309. [Google Scholar] [CrossRef]
- EL Bar, D.; Barket, D. Leaching of metals from hydrometallurgical residue by sulfuric acid. Asp. Min. Miner. Sci. 2018, 1. [Google Scholar] [CrossRef]
- Liu, Z.-R.; Zeng, K.; Zhao, W.; Li, Y. Effect of temperature on iron leaching from bauxite residue by sulfuric acid. Bull. Environ. Contam. Toxicol. 2009, 82, 55–58. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Bonomi, C.; Paiste, P.; Sajó, I.E.; Blanpain, B.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Modes of occurrences of scandium in greek bauxite and bauxite residue. Miner. Eng. 2018, 123, 35–48. [Google Scholar] [CrossRef]
- Roy, R.K. A Primer on the Taguchi Method, 2nd ed.; Society of Manufacturing Engineers: Dearborn, MI, USA, 2010. [Google Scholar]



| Factor | Parameter | Unit | Level | ||
|---|---|---|---|---|---|
| 1 (Minimum) | 2 (Middle) | 3 (Maximum) | |||
| A | H2SO4 molarity | M | 2 | 4 | 6 |
| B | Temperature | °C | 25 | 55 | 85 |
| C | Solid-to-Liquid ratio | % | 10 | 20 | 30 |
| D | Leaching time | h | 1 | 4 | 7 |
| Experiment No. | Factors/Parameters | |||
|---|---|---|---|---|
| A (H2SO4 Molarity) | B (Temperature) | C (Solid/Liquid) | D (Time) | |
| 1 | 2 | 25 | 10 | 1 |
| 2 | 2 | 55 | 20 | 4 |
| 3 | 2 | 85 | 30 | 7 |
| 4 | 4 | 25 | 20 | 7 |
| 5 | 4 | 55 | 30 | 1 |
| 6 | 4 | 85 | 10 | 4 |
| 7 | 6 | 25 | 30 | 4 |
| 8 | 6 | 55 | 10 | 7 |
| 9 | 6 | 85 | 20 | 1 |
| Exp. No. | Factors Combination | Concentration (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sc | Fe | ||||||||
| CSc1 | CSc2 | CSc3 | CScavg | CFe1 | CFe2 | CFe3 | CFeavg | ||
| 1 | A1B1C1D1 | 3.72 | 3.57 | 3.69 | 3.66 | 1263 | 1255 | 1294 | 1271 |
| 2 | A1B2C2D2 | 9.36 | 8.89 | 8.96 | 9.07 | 22806 | 21815 | 22698 | 22440 |
| 3 | A1B3C3D3 | 13.77 | 14.58 | 14.31 | 14.22 | 50260 | 51389 | 52072 | 51240 |
| 4 | A2B1C2D3 | 7.80 | 7.65 | 7.68 | 7.71 | 15075 | 15133 | 14855 | 15021 |
| 5 | A2B2C3D1 | 13.16 | 13.94 | 13.48 | 13.53 | 25684 | 25594 | 25459 | 25579 |
| 6 | A2B3C1D2 | 8.90 | 9.66 | 9.41 | 9.32 | 36225 | 39154 | 37358 | 37579 |
| 7 | A3B1C3D2 | 11.88 | 10.93 | 12.71 | 11.84 | 19588 | 19111 | 20814 | 19838 |
| 8 | A3B2C1D3 | 8.14 | 7.99 | 8.91 | 8.34 | 31388 | 32697 | 32685 | 32257 |
| 9 | A3B3C2D1 | 12.57 | 14.29 | 13.22 | 13.36 | 43741 | 41526 | 41370 | 42212 |
| Factor | Degree of Freedom | Sequential Sums of Squares | Contribution (%) | Adjusted Sums of Squares | Adjusted Mean Squares | F-Value | P-Value |
|---|---|---|---|---|---|---|---|
| A | 2 | 2.2742 | 7.71 | 7.2742 | 3.6371 | * | * |
| B | 2 | 31.4224 | 33.32 | 31.4224 | 15.7112 | * | * |
| C | 2 | 55.5981 | 58.95 | 55.5981 | 27.7991 | * | * |
| D | 2 | 0.0187 | 0.02 | 0.0187 | 0.0094 | * | * |
| Error | 0 | * | * | * | * | ||
| Total | 8 | 94.3135 | 100.00 |
| Factor | Degree of Freedom | Sequential Sums of Squares | Contribution (%) | Adjusted Sums of Squares | Adjusted Mean Squares | F-Value | P-Value |
|---|---|---|---|---|---|---|---|
| A | 2 | 7.169 × 107 | 3.90 | 7.169 × 107 | 3.584 × 107 | * | * |
| B | 2 | 150.350 × 107 | 81.89 | 150.350 × 107 | 75.175 × 107 | * | * |
| C | 2 | 11.274 × 107 | 6.14 | 11.274 × 107 | 5.637 × 107 | * | * |
| D | 2 | 14.805 × 107 | 8.06 | 14.805 × 107 | 7.403 × 107 | * | * |
| Error | 0 | * | * | * | * | ||
| Total | 8 | 183.597 × 107 | 100.00% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lymperopoulou, T.; Georgiou, P.; Tsakanika, L.-A.; Hatzilyberis, K.; Ochsenkuehn-Petropoulou, M. Optimizing Conditions for Scandium Extraction from Bauxite Residue Using Taguchi Methodology. Minerals 2019, 9, 236. https://doi.org/10.3390/min9040236
Lymperopoulou T, Georgiou P, Tsakanika L-A, Hatzilyberis K, Ochsenkuehn-Petropoulou M. Optimizing Conditions for Scandium Extraction from Bauxite Residue Using Taguchi Methodology. Minerals. 2019; 9(4):236. https://doi.org/10.3390/min9040236
Chicago/Turabian StyleLymperopoulou, Theopisti, Paraskevas Georgiou, Lamprini-Areti Tsakanika, Konstantinos Hatzilyberis, and Maria Ochsenkuehn-Petropoulou. 2019. "Optimizing Conditions for Scandium Extraction from Bauxite Residue Using Taguchi Methodology" Minerals 9, no. 4: 236. https://doi.org/10.3390/min9040236
APA StyleLymperopoulou, T., Georgiou, P., Tsakanika, L.-A., Hatzilyberis, K., & Ochsenkuehn-Petropoulou, M. (2019). Optimizing Conditions for Scandium Extraction from Bauxite Residue Using Taguchi Methodology. Minerals, 9(4), 236. https://doi.org/10.3390/min9040236







