Environmental Application of Biogenic Magnetite Nanoparticles to Remediate Chromium(III/VI)-Contaminated Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Magnetite Nanoparticles and Characterization
2.2. Chromium (III/VI) Removal
2.3. Analytical Methods
3. Results
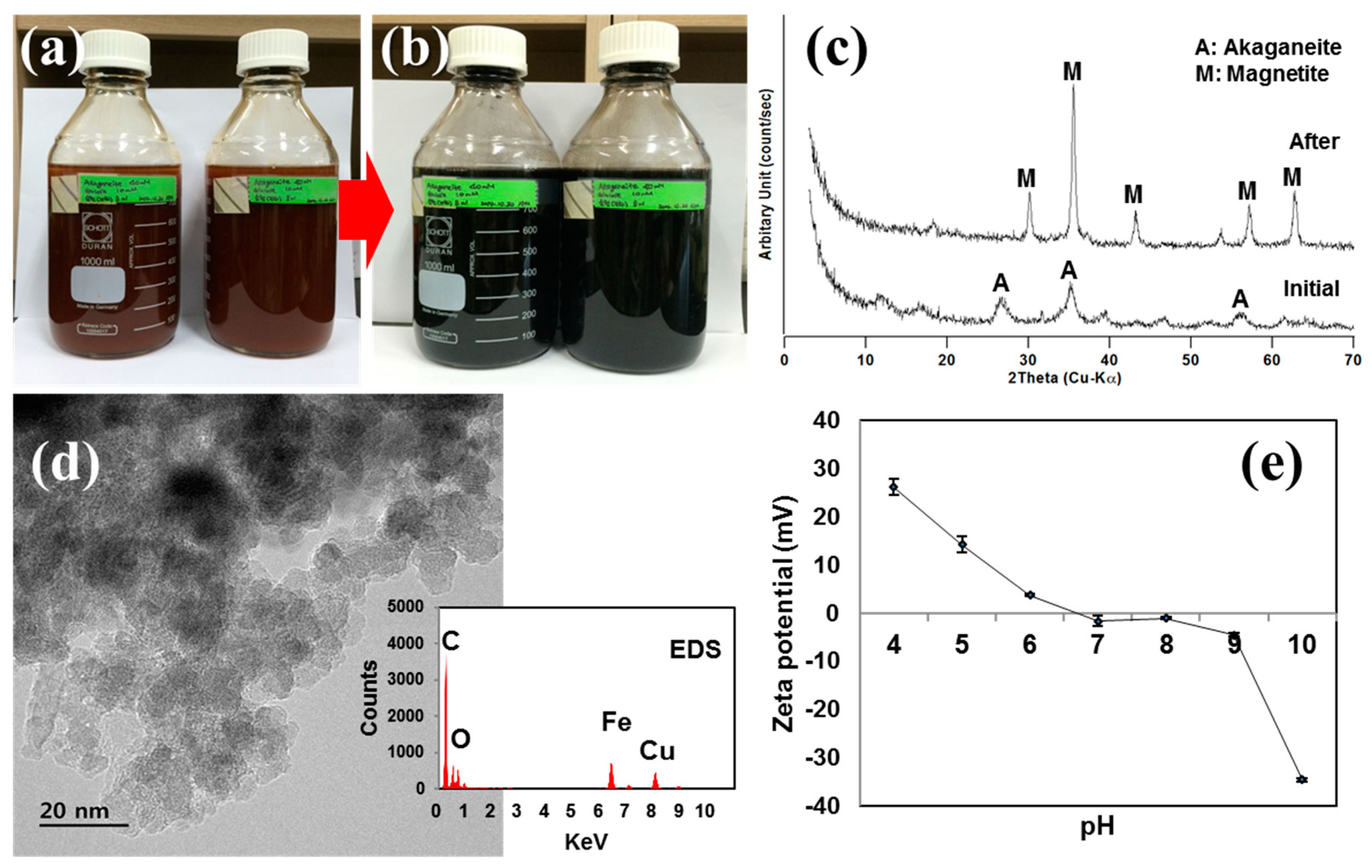
3.1. Biogenic Magnetite Nanoparticles
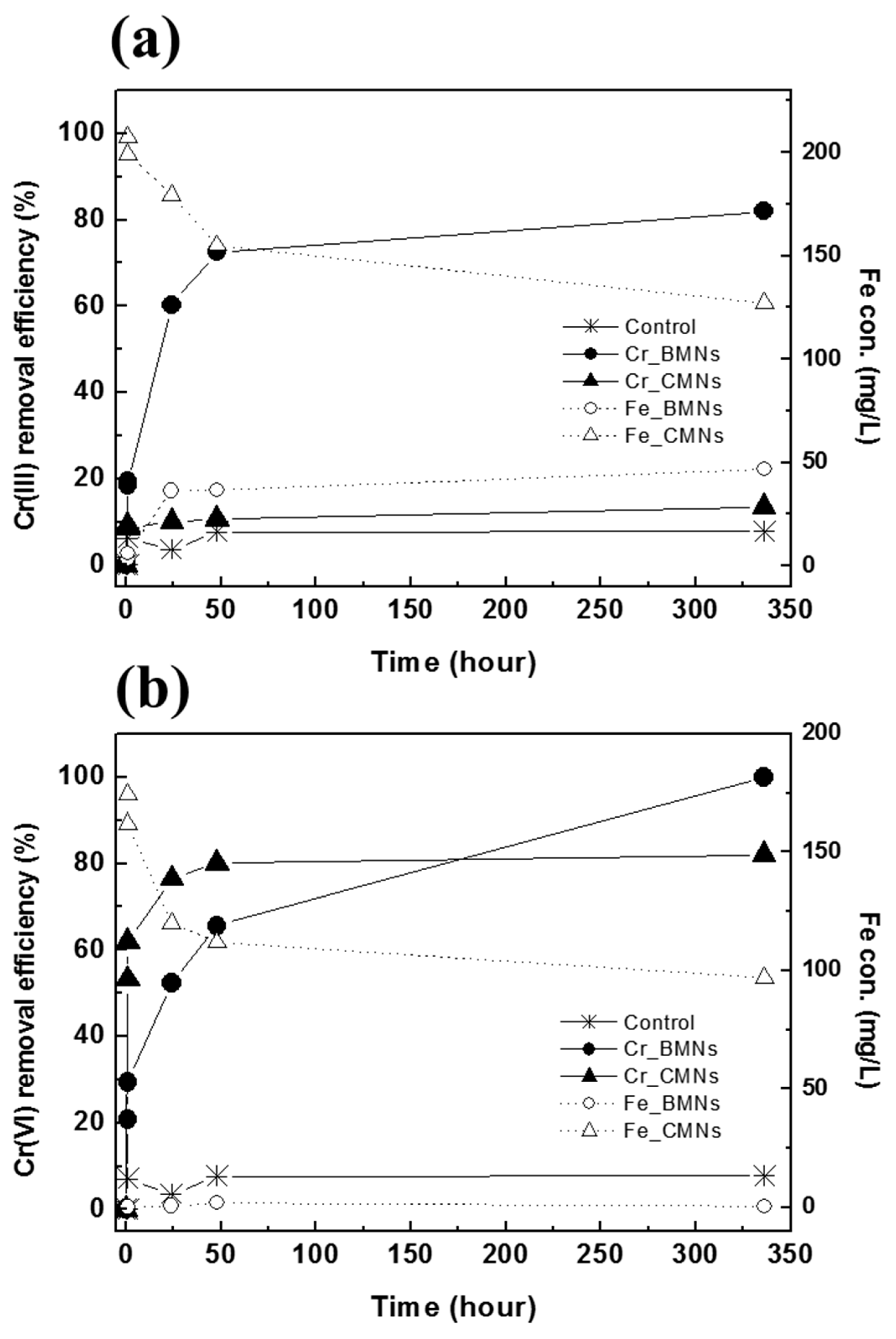
3.2. Cr(III) and Cr(VI) Removal
3.3. Effect of pH for Cr(VI) Removal
3.4. Effect of Common Ions on Cr(VI) Removal
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guertin, J.; Jacobs, J.A.; Avakian, C.A. Chromium (VI) Handbook; CRC Press: Boca Raton, FL, USA, 2005; p. 800. ISBN 978-0203487969. [Google Scholar]
- Baig, S.A.; Wang, Q.; Wang, Z.; Zhu, J.; Lou, Z.; Sheng, T.; Xu, X. Hexavalent chromium removal from solutions: Surface efficacy and characterizations of three iron containing minerals. CLEAN–Soil Air Water 2014, 42, 1409–1414. [Google Scholar] [CrossRef]
- Erdem, M.; Gur, F.; Tumen, F. Cr(VI) reduction in aqueous solutions by siderite. J. Hazard Mater. 2004, 113, 219–224. [Google Scholar] [CrossRef]
- Mullet, M.; Boursiquot, S.; Ehrhardt, J.J. Removal of hexavalent chromium from solutions by mackinawite, tetragonal FeS. Colloids Surf. A Physicochem. Eng. Asp. 2004, 244, 77–85. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M.C. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K. Arsenic and chromium removal by mixed magnetite–maghemite nanoparticles and the effect of phosphate on removal. J. Environ. Manag. 2010, 91, 2238–2247. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Liu, Z.; Gao, Y.; Wu, B.; Xu, H. Fe3O4 nanoparticle-coated mushroom source biomaterial for Cr(VI) polluted liquid treatment and mechanism research. R. Soc. Open Sci. 2018, 5, 171776. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y.; Tian, X.; Yang, Z.; Jiang, Y.; Zhao, F. Interactions between iron mineral-humic complexes and hexavalent chromium and the corresponding bio-effects. Environ. Pollut. 2018, 241, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Cai, Q.; Xu, W.; Yang, M.; Cai, Y.; Dionysiou, D.D.; O’Shea, K.E. Cr(VI) adsorption and reduction by humic acid coated on magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef] [PubMed]
- Hennebel, T.; Gusseme, B.D.; Boon, N.; Verstraete, W. Biogenic metals in advanced water treatment. Trends Biotechnol. 2009, 27, 90–98. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Telling, N.D.; Coker, V.S.; Cutting, R.S.; van der Laan, G.; Pearce, C.I.; Pattrick, R.A.D.; Arenholz, E.; Lloyd, J.R. Remediation of Cr(VI) by biogenic magnetic nanoparticles: An X-ray magnetic circular dichroism study. Appl. Phys. Lett. 2009, 95, 163701. [Google Scholar] [CrossRef]
- Crean, D.E.; Coker, V.S.; van der Laan, G.; Lloyd, J.R. Engineering biogenic magnetite for sustained Cr(VI) remediation in flow-through systems. Environ. Sci. Technol. 2012, 46, 3352–3359. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, H.; Suh, Y.; Roh, Y. Characterization of magnetite-organic complex nanoparticles by metal-reducing bacteria. J. Nanosci. Nanotechnol. 2011, 11, 7242–7245. [Google Scholar] [CrossRef]
- Roh, Y.; Lauf, R.J.; McMillanm, A.D.; Zhang, C.; Rawn, C.J.; Bai, J.; Phelps, T.J. Microbial synthesis and the characterization of metal substituted magnetites. Solid State Commun. 2001, 118, 529–534. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases magnetites. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, K.; Watanabe, J.; Tani, Y.; Seyama, H.; Miyata, N. Removal of heavy metal cations by biogenic magnetite nanoparticles produced in Fe(III)-reducing microbial enrichment cultures. J. Biosci. Bioeng. 2014, 117, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxide: Structure, Properties, Reactions, Occurrences and Uses; Wiley-VCH: Weinheim, Germany, 2003; pp. 703. ISBN 978-3527606443. [Google Scholar]
- Asami, K.; Hashimoto, K. The X-ray photo-electron spectra of several oxides of iron and chromium. Corros. Sci. 1977, 17, 559–570. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Manjanna, J.; Venkateswaran, G. Effect of oxidative pretreatment for the dissolution of Cr-substituted hematites/magnetites. Ind. Eng. Chem. Res. 2002, 41, 3053–3063. [Google Scholar] [CrossRef]
- Aronniemi, M.; Sainio, J.; Lahtinen, J. Chemical state quantification of iron and chromium oxides using XPS: The effect of the background subtraction method. J. New Mat. Electrochem. 2004, 7, 173–177. [Google Scholar] [CrossRef]
- Palloukis, F.; Zafeiratos, S.; Jaksic, M.M.; Neophytides, S.G. The chemical state of electrodeposited thin Cr films on a polycrystaline Ni foil. Surf. Sci. 2005, 578, 108–123. [Google Scholar]
- Paterson, M.L.; White, A.F.; Brown, G.E.; Parks, G.A. Surface passivation of magnetite by reaction with aqueous Cr(VI): XAFS and TEM results. Environ. Sci. Technol. 1997, 31, 1573–1576. [Google Scholar] [CrossRef]
- Manuel, P.C.; Jose, M.M.; Rosa, T.M. Chromium removal with activated carbons. Water Res. 1995, 29, 2174–2180. [Google Scholar]
- Dimitri, M.; Vladimir, G.; Abraham, W. Ion Exchange; Marcel Dekker: New York, NY, USA, 2000; p. 905. ISBN 0824703251. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Roh, Y. Environmental Application of Biogenic Magnetite Nanoparticles to Remediate Chromium(III/VI)-Contaminated Water. Minerals 2019, 9, 260. https://doi.org/10.3390/min9050260
Kim Y, Roh Y. Environmental Application of Biogenic Magnetite Nanoparticles to Remediate Chromium(III/VI)-Contaminated Water. Minerals. 2019; 9(5):260. https://doi.org/10.3390/min9050260
Chicago/Turabian StyleKim, Yumi, and Yul Roh. 2019. "Environmental Application of Biogenic Magnetite Nanoparticles to Remediate Chromium(III/VI)-Contaminated Water" Minerals 9, no. 5: 260. https://doi.org/10.3390/min9050260
APA StyleKim, Y., & Roh, Y. (2019). Environmental Application of Biogenic Magnetite Nanoparticles to Remediate Chromium(III/VI)-Contaminated Water. Minerals, 9(5), 260. https://doi.org/10.3390/min9050260





