Study of the Adhesion Mechanism of Acidithiobacillus ferrooxidans to Pyrite in Fresh and Saline Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Preparation
2.2. Microorganisms Cultivation
2.3. Effect of Aqueous Medium on the Adhesion of Bacteria to Pyrite
2.4. Effect of pH on Streaming Potential Values
3. Results and Discussion
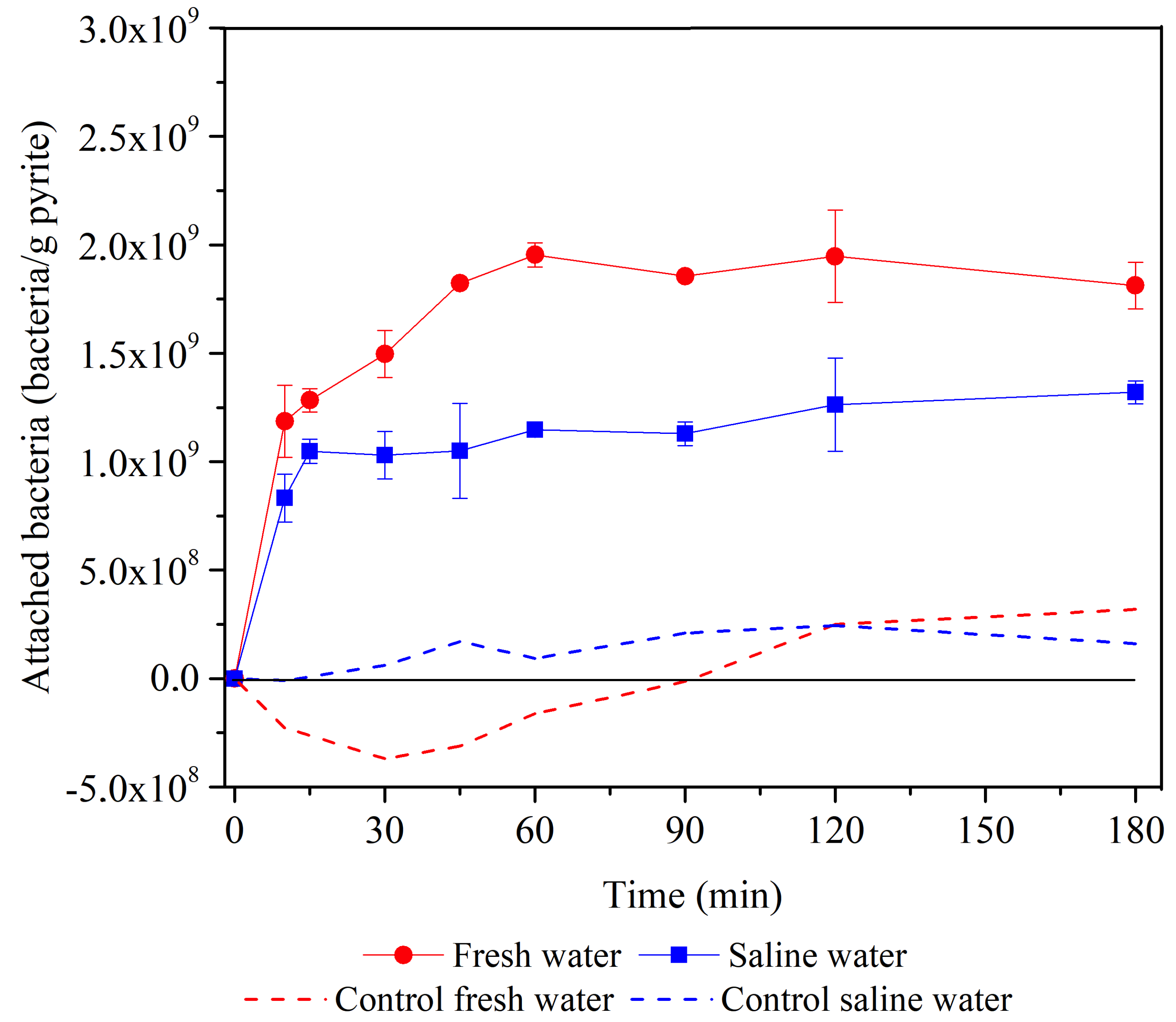
3.1. Effect of Aqueous Medium on the Adhesion of Bacteria to Pyrite
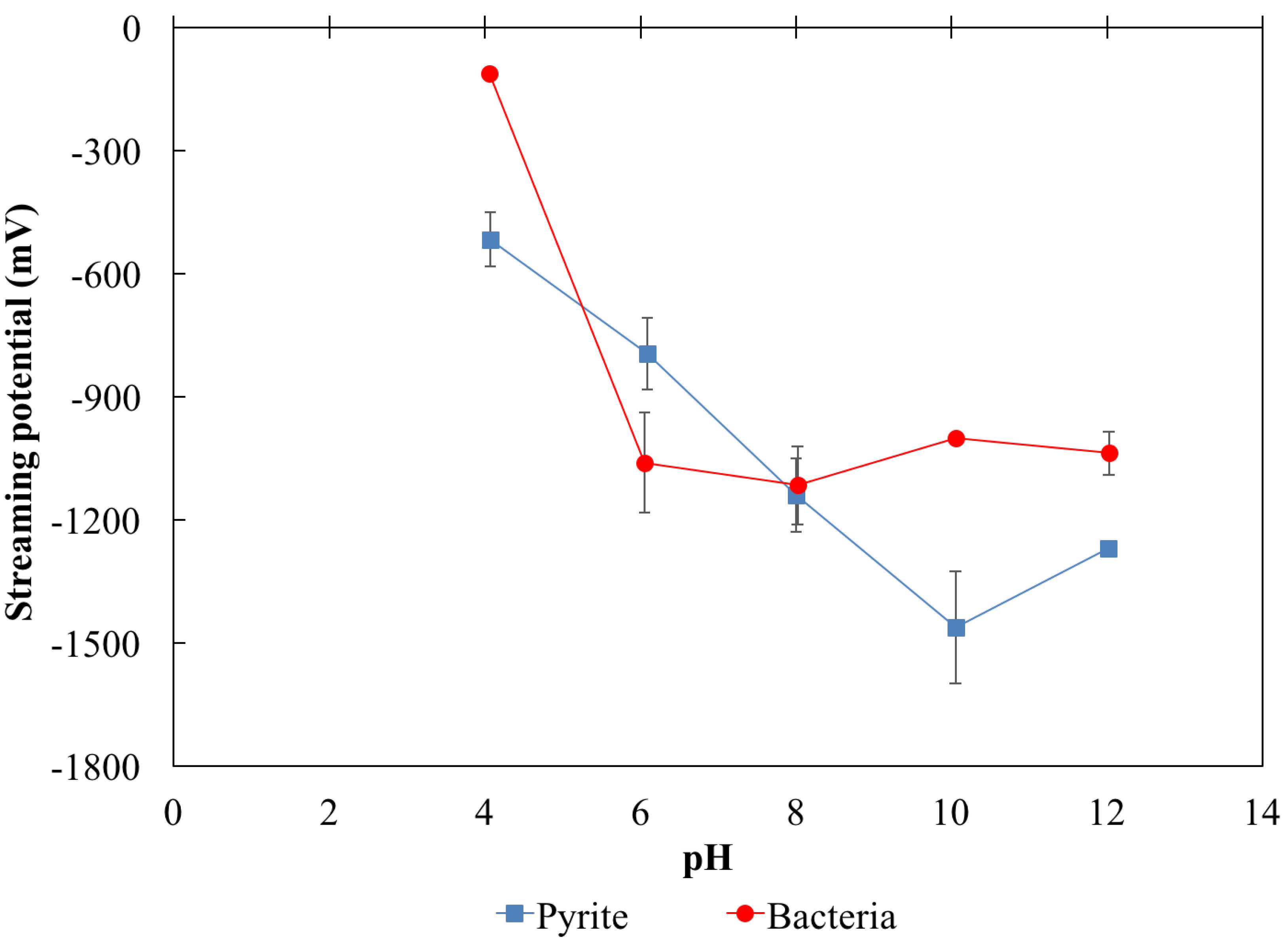
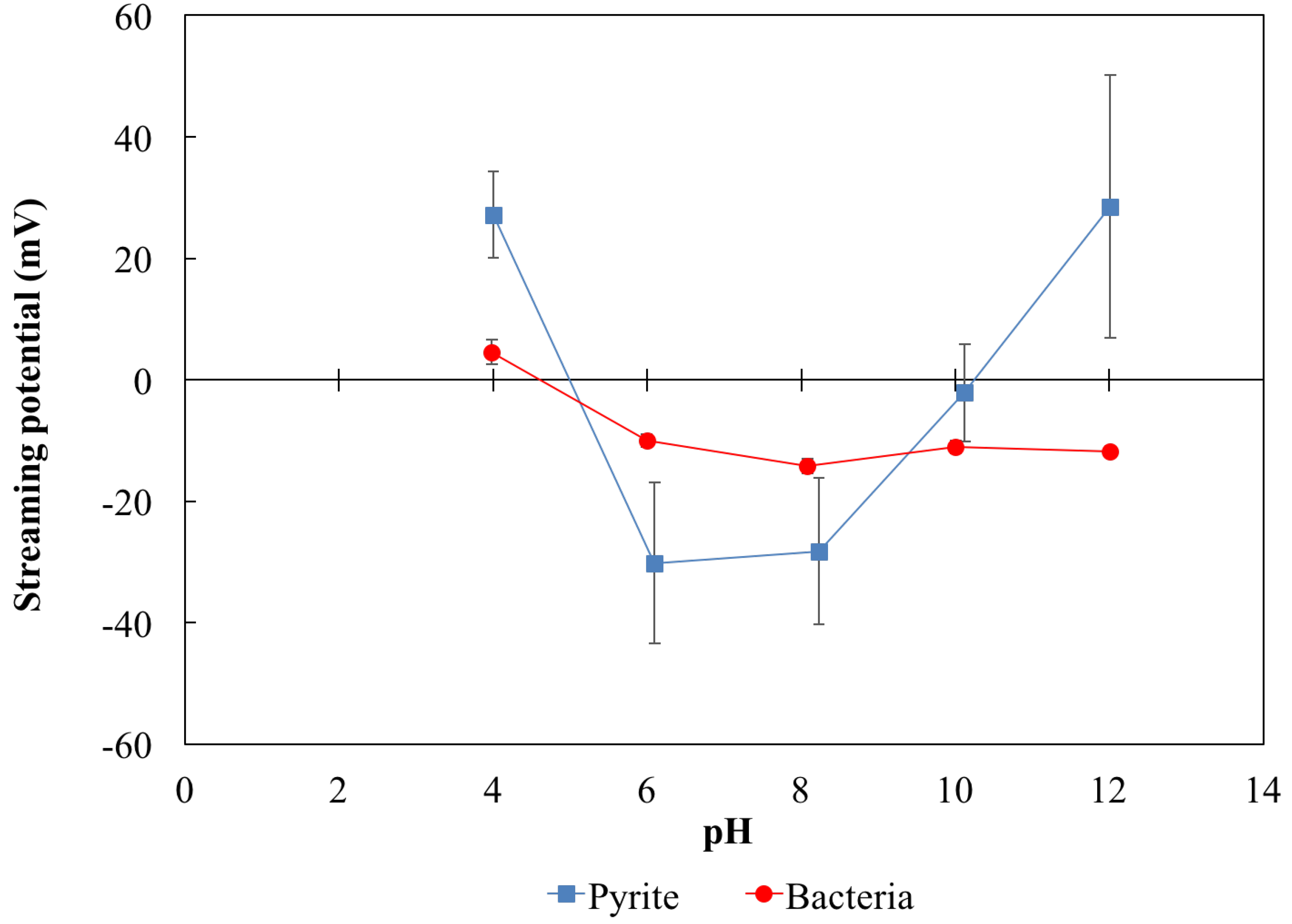
3.2. Effect of pH on Streaming Potential
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henry, J.G.; Heinke, G.W. Ingeniería Ambiental; Pearson Educación: London, UK, 1999. [Google Scholar]
- DGA. Balance Hídrico de Chile; Gobierno de Chile: Santiago, Chile, 1987.
- Mundial, B. Diagnóstico de la Gestión de los Recursos Hídricos; Departamento de medio ambiente y Desarrollo Sostenible Región para América Latina y el Caribe: Santiago, Chile, 2011.
- DGA. Declaración de Agotamiento del Río Loa y sus Afluentes, Región de Antofagasta; Gobierno de Chile: Santiago, Chile, 2000.
- DGA. Declaración de Agotamiento de la Cuenca del Río Huasco y sus Afluentes, Provincia de Huasco, Región de Atacama; Gobierno de Chile: Santiago, Chile, 2016.
- DGA. Declaración de Agotamiento de la Cuenca del Río Vilama y sus Afluentes, Provincia del Loa, Región de Antofagasta; Gobierno de Chile: Santiago, Chile, 2017.
- COCHILCO. Proyección de la Porducción de Cobre en Chile 2017-2028; Gobierno de Chile: Santiago, Chile, 2017.
- COCHILCO. Consumo del Agua en la Minería del Cobre al año 2017; Gobierno de Chile: Santiago, Chile, 2018.
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment And Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Castro, S. Challenges in flotation of Cu-Mo sulfide ores in sea water. In Water in Mineral Processing; Drelich, J., Ed.; Society for Mining, Metallurgy and Exploration: Englewood, CO, USA, 2012; pp. 29–40. [Google Scholar]
- Chandraprabha, M.; Natarajan, K.; Modak, J.M. Selective separation of pyrite and chalcopyrite by biomodulation. Colloids Surf. B Biointerfaces 2004, 37, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chandraprabha, M.; Natarajan, K.; Somasundaran, P. Selective separation of pyrite from chalcopyrite and arsenopyrite by biomodulation using Acidithiobacillus ferrooxidans. Int. J. Min. Process. 2005, 75, 113–122. [Google Scholar] [CrossRef]
- Hosseini, T.; Kolahdoozan, M.; Tabatabaei, Y.; Oliazadeh, M.; Noaparast, M.; Eslami, A.; Manafi, Z.; Alfantazi, A. Bioflotation of Sarcheshmeh copper ore using Thiobacillus ferrooxidans bacteria. Min. Eng. 2005, 18, 371–374. [Google Scholar] [CrossRef]
- Mehrabani, J.; Mousavi, S.; Noaparast, M. Evaluation of the replacement of NaCN with Acidithiobacillus ferrooxidans in the flotation of high-pyrite, low-grade lead–zinc ore. Sep. Purif. Technol. 2011, 80, 202–208. [Google Scholar] [CrossRef]
- Misra, M.; Bukka, K.; Chen, S. The effect of growth medium of Thiobacillus ferrooxidans on pyrite flotation. Min. Eng. 1996, 9, 157–168. [Google Scholar] [CrossRef]
- Nagaoka, T.; Ohmura, N.; Saiki, H. A novel mineral flotation process using Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1999, 65, 3588–3593. [Google Scholar] [PubMed]
- Ohmura, N.; Kitamura, K.; Saiki, H. Mechanism of microbial flotation using Thiobacillus ferrooxidans for pyrite suppression. Biotechnol. Bioeng. 1993, 41, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Donati, E.R.; Sand, W. Microbial Processing of Metal Sulfides; Springer: Berlin/Heidelberg, Germany, 2007; Volume 130. [Google Scholar]
- Rawlings, D.E. Characteristics and adaptability of iron-and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Fact. 2005, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- San Martín, F.; Kracht, W.; Vargas, T. Biodepression of pyrite using Acidithiobacillus ferrooxidans in seawater. Miner. Eng. 2018, 117, 127–131. [Google Scholar] [CrossRef]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [PubMed]
- Yu, R.L.; Yang, O.; Tan, J.X.; Wu, F.D.; Jing, S.; Lei, M.; Zhong, D.L. Effect of EPS on adhesion of Acidithiobacillus ferrooxidans on chalcopyrite and pyrite mineral surfaces. Trans. Nonferrous Met. Soc. China 2011, 21, 407–412. [Google Scholar] [CrossRef]
- Harneit, K.; Göksel, A.; Kock, D.; Klock, J.H.; Gehrke, T.; Sand, W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254. [Google Scholar] [CrossRef]
- Chandraprabha, M.; Natarajan, K. Role of outer membrane exopolymers of Acidithiobacillus ferrooxidans in adsorption of cells onto pyrite and chalcopyrite. Int. J. Min. Process. 2013, 123, 152–157. [Google Scholar] [CrossRef]
- Sharma, P.; Das, A.; Rao, K.H.; Forssberg, K. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions. Hydrometallurgy 2003, 71, 285–292. [Google Scholar] [CrossRef]
- Chen, M.L.; Zhang, L.; Gu, G.H.; Hu, Y.H.; Su, L.J. Effects of microorganisms on surface properties of chalcopyrite and bioleaching. Trans. Nonferrous Met. Soc. China 2008, 18, 1421–1426. [Google Scholar] [CrossRef]
- Devasia, P.; Natarajan, K.; Sathyanarayana, D.; Rao, G.R. Surface chemistry of Thiobacillus ferrooxidans relevant to adhesion on mineral surfaces. Appl. Environ. Microbiol. 1993, 59, 4051–4055. [Google Scholar] [PubMed]
- Detector, F.G. Operation Manual; BTG Mutek GmbH: Herrsching, Germany, 2008. [Google Scholar]
- Devasia, P.; Natarajan, K. Adhesion of Acidithiobacillus ferrooxidans to mineral surfaces. Int. J. Min. Process. 2010, 94, 135–139. [Google Scholar] [CrossRef]
- Rao, K.H.; Vilinska, A. Surface thermodynamics and extended DLVO theory of Acidithiobacillus ferroxidans cells on pyrite and chalcopyrite. Open Colloid Sci. J. 2009, 2, 1–14. [Google Scholar]
- Tan, S.N.; Chen, M. Early stage adsorption behaviour of Acidithiobacillus ferrooxidans on minerals I: An experimental approach. Hydrometallurgy 2012, 119, 87–94. [Google Scholar] [CrossRef]
| Reagent | Chemical Formula | Concentration (g/L) | Molecular Weight (g/mol) |
|---|---|---|---|
| Ammonium sulfate | (NH4)2SO4 | 0.4 | 132.14 |
| Di-potassium hydrogen phosphate trihydrate | K2HPO4·3H2O | 0.056 | 228.23 |
| Magnesium sulfate heptahydrate | MgSO4·7H2O | 0.4 | 246.48 |
| Iron sulfate heptahydrate | FeSO4·7H2O | 14.85 | 278.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Martín, F.; Aguilar, C. Study of the Adhesion Mechanism of Acidithiobacillus ferrooxidans to Pyrite in Fresh and Saline Water. Minerals 2019, 9, 306. https://doi.org/10.3390/min9050306
San Martín F, Aguilar C. Study of the Adhesion Mechanism of Acidithiobacillus ferrooxidans to Pyrite in Fresh and Saline Water. Minerals. 2019; 9(5):306. https://doi.org/10.3390/min9050306
Chicago/Turabian StyleSan Martín, Francisca, and Claudio Aguilar. 2019. "Study of the Adhesion Mechanism of Acidithiobacillus ferrooxidans to Pyrite in Fresh and Saline Water" Minerals 9, no. 5: 306. https://doi.org/10.3390/min9050306





