Spodumene: The Lithium Market, Resources and Processes
Abstract
:1. Introduction
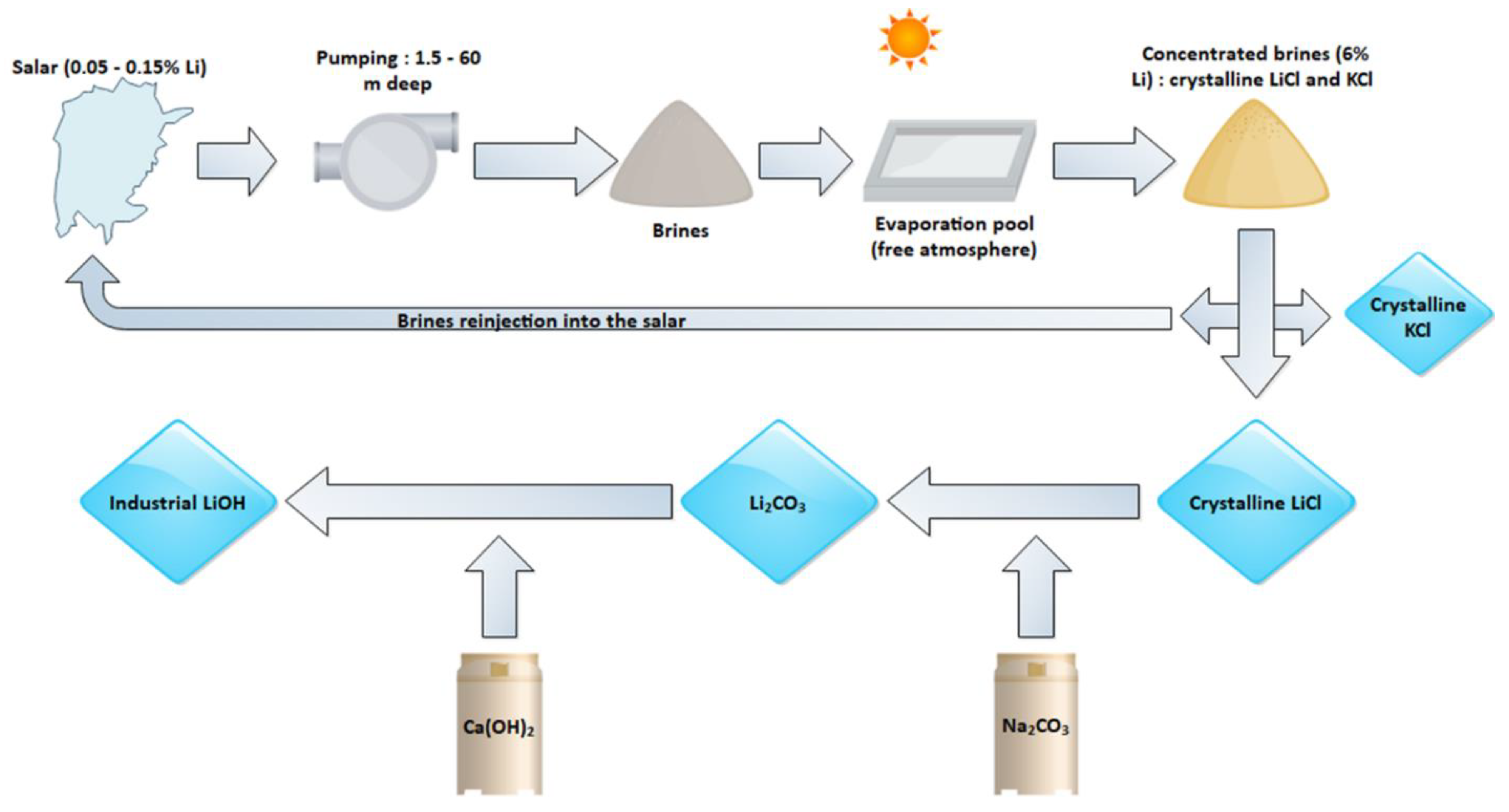
2. Lithium Usage and Resources
2.1. Usage
2.2. Resources
3. The Different Spodumene Phases
3.1. The Pegmatite Formation
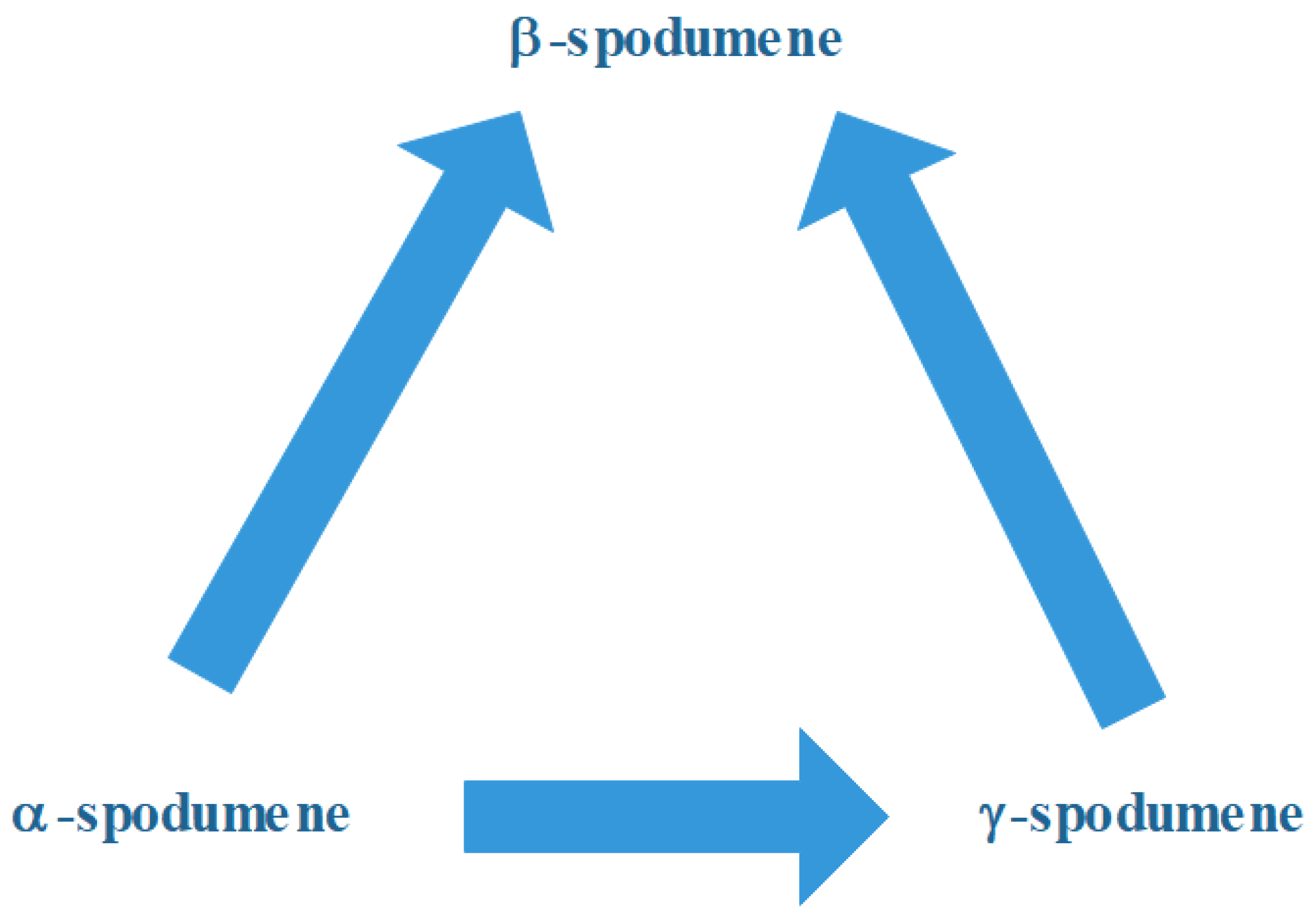
3.2. The α, β, γ System
- α-spodumene:
- β-spodumene and γ-spodumene:
3.3. General Flow Sheets
4. Production of Lithium from Spodumene
4.1. The Traditional Process
- α-spodumene is almost completely resilient toward acid roasting contrary to β-spodumene
- β-spodumene density is significantly lower than that of α-spodumene.
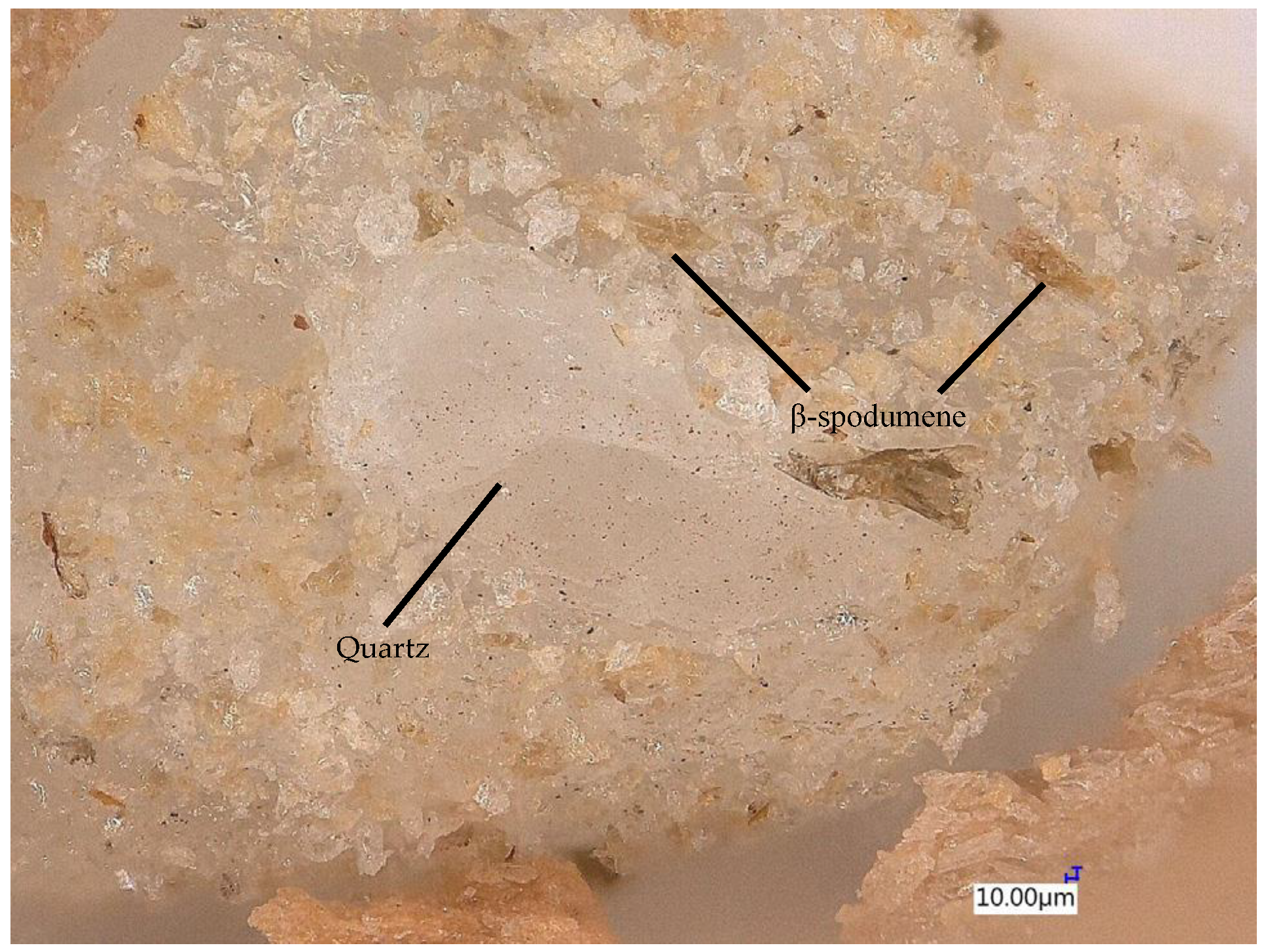
- The structure of the leached β-spodumene is very similar to that of β-spodumene.
4.2. Other Processes of Extracting Lithium from Spodumene
4.3. Conversion Processes of Spodumene
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Christmann, P.; Gloaguen, E.; Labbé, J.F.; Melleton, J.; Piantone, P. Global lithium resources and sustainability issues. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–40. [Google Scholar]
- Coplen, B.; Böhlke, J.K.; De Bièvre, P.; Ding, T.; Holden, N.E.; Hopple, J.A.; Krouse, H.R.; Lamberty, A.; Peiser, H.S.; Revesz, K.; et al. Isotope-abundance variations of selected elements (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 1987–2017. [Google Scholar] [CrossRef]
- Tomascak, P.B. Developments in the Understanding and Application of Lithium Isotopes in the Earth and Planetary Sciences. Rev. Mineral. Geochem. 2004, 55, 153–195. [Google Scholar] [CrossRef]
- Audi, G.; Kondev, F.G.; Wang, M.; Pfeiffer, B.; Sun, X.; Blachot, J.; MacCormick, M. The NUBASE2012 evaluation of nuclear properties. Chin. Phys. C 2012, 36, 1157–1286. [Google Scholar] [CrossRef]
- Audi, G.; Bersillon, O.; Blachot, J.; Wapstra, A.H. The Nubase evaluation of nuclear and decay properties. Nuc. Phys. A 2003, 729, 3–128. [Google Scholar] [CrossRef] [Green Version]
- Cyburt, R.H.; Fields, B.D.; Olive, K.A. Primordial nucleosynthesis in light of WMAP. Phys. Lett. B 2003, 567, 227–237. [Google Scholar] [CrossRef]
- Coc, A.; Vangioni-Flam, E.; Descouvemont, P.; Adahchour, A.; Angulo, C. Updated big bang nucleosynthesis compared with wilkinson microwave anisotropic probe observations and the abundance of light elements. Am. Astron. Soc. 2004, 600, 544–552. [Google Scholar]
- Asplund, M.; Lambert, D.L.; Nissen, P.E.; Primas, F.; Smith, V.V. Lithium isotopic abundances in metal-poor halo stars. Am. Astron. Soc. 2006, 644, 229–259. [Google Scholar] [CrossRef]
- Hou, S.Q.; He, J.J.; Parikh, A.; Kahl, D.; Bertulani, C.A.; Kajino, T.; Mathews, G.J.; Zhao, G. Non-extensive statistics to the cosmological lithium problem. Astrophys. J. 2017, 834, 165–170. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral commodity summaries 2016: U.S. Geological Survey Lithium; USGS: Reston, VA, USA, 2016; p. 202.
- Labbé, J.F.; Daw, G. Panorama 2011 du marché du lithium; Rapport public 2012, BRGM/RP-61340-FR; BRGM: Orléans, France, 2012; p. 94. [Google Scholar]
- Grosjean, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 2012, 166, 1735–1744. [Google Scholar] [CrossRef]
- Jaskula, B.W. Lithium. In Mineral Commodity Summaries 2019; USGS: Reston, VA, USA, 2019; pp. 98–99. [Google Scholar]
- Kesler, S.E.; Gruber., P.W.; Medina., P.A.; Keoleian., G.A.; Everson., M.P.; Wallington., T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Sociedad Quimica y Minera de Chile, 2003 to 2013, Annual Reports; SQM: Santiago, Chile, 2003–2013.
- Yaksic, A.; Tilton, J.E. Using the cumulative availability curve to assess the threat of mineral depletion: The case of lithium. Resour. Policy 2009, 34, 185–194. [Google Scholar] [CrossRef]
- SignumBOX. Analysis: Lithium, Batteries and Vehicles/Perspectives and Trends; SignumBOX: Santiago, Chile, 2014; p. 8. [Google Scholar]
- Jaskula, B.W. Lithium, 2016 Mineral Yearbook 2018; USGS: Reston, VA, USA, 2018; p. 44.1.
- Jaskula, B.W. Lithium, Mineral Commodity Summaries 2018; USGS: Reston, VA, USA, 2018; p. 1.
- Laznicka, P. Giant Metallic Deposits; Springer: Berlin, Germany, 2006. [Google Scholar]
- Lithium-ion Batteries Market Development & Raw Materials; Roskill: London, England, 2018.
- Jahns, R.H.; Tuttle, O.F. Layered Pegmatite-Aplite Intrusives; Mineralogical Society of America: Chantilly, VA, USA, 1963; pp. 78–92. [Google Scholar]
- Swanson, S.E. Mineralogy of Spodumene Pegmatites and Related Rocks in the Tin-Spodumene Belt of North Carolina and South Carolina, USA. Can. Mineral. 2012, 50, 1589–1608. [Google Scholar] [CrossRef]
- Stewart, D.B. Petrogenesis of lithium-rich pegmatites. Am. Mineral. 1978, 63, 970–980. [Google Scholar]
- Barros, R.; Menuge, J.F. The Origin of Spodumene Pegmatites Associated with the Leinster Granite in Southeast Ireland. Can. Mineral. 2016, 54, 847–862. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- London, D. Pegmatites. Can. Mineral. 2008, 10, 18. [Google Scholar]
- Trueman, D.L.; Černý, P. Exploration for rare-element granitic pegmatites. In Short Course Granitic Pegmatites in Science and Industry; Mineralogical Association of Canada: Québec, QC, Canada, 1982; Volume 8, pp. 463–494. [Google Scholar]
- French, B.M.; Jezek, P.A.; Appleman, D.E. Virgilite, a new lithium aluminum silicate mineral from the Macusani glass, Peru. Am. Mineral. 1978, 63, 461–465. [Google Scholar]
- London, D. Experimental phase equilibria in the system LiAlSiO4-SiO2-H2O: A petrogenetic grid for lithium-rich pegmatites. Am. Mineral. 1984, 69, 995–1004. [Google Scholar]
- d’Andrada, J.B. Der eigenschaften und kennzeichen einiger neuen fossilien aus Schweden und Norwegen nebst einigen chemischen bemerkungen ueber dieselben. Allgemeines J. Chem. 1800, 4, 28–39. [Google Scholar]
- Sthulman, O. The thermophosphorescent radiations of Hidennite and Kunzite. J. Opt. Soc. Am. Rev. Sci. Instrum. 1929, 18, 365–369. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Spodumene. In Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2015; Available online: http://www.handbookofmineralogy.org/ (accessed on 12 March 2019).
- Li, C.T.; Peacor, D.R. The crystal structure of LiAlSi2O6-II (β-spodumene). Zeitschrift für Kristallographie 1967, 126, 46–65. [Google Scholar] [CrossRef]
- Keat, P.P. A new crystalline silica. Science 1954, 120, 328–330. [Google Scholar] [CrossRef]
- Lu, H. Formation of β-Eucryptite and β-spodumene from Topaz Mixture. Ph.D Thesis, University of New South Wales, Sydney, Australia, 2005. [Google Scholar]
- Peltosaari, O.; Tanskanen, P.A.; Heikkinen, E.P.; Fabritius, T. α→γ→β-phase transformation of spodumene with hybrid microwave and conventional furnaces. Miner. Eng. 2015, 82, 54–60. [Google Scholar] [CrossRef]
- Li, C.T. The crystal structure of LiAlSi2O6 III (high-quartz solid solution). Zeitschrift für Kristallographie 1967, 127, 327–334. [Google Scholar]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Virgilite. In Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2015; Available online: http://www.handbookofmineralogy.org/ (accessed on 12 March 2019).
- Fasshauer, D.W.; Chatterjee, N.D.; Cemic, L. A thermodynamic analysis of the system LiAlSiO4-NaAlSiO4-Al2O3-SiO2-H2O based on new heat capacity, thermal expansion, and compressibility data for selected phases. Contrib. Mineral. Petrol. 1998, 133, 186–198. [Google Scholar] [CrossRef]
- Robie, R.A.; Hemingway, B.S. Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar (105 Pascals) Pressure and at Higher Temperatures; USGS: Reston, VA, USA, 1995; Volume 2131, pp. 1–461.
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 1: Conventionnal heating. Miner. Eng. 2016, 98, 71–79. [Google Scholar] [CrossRef]
- Botto, I.L.; Arazi, S.C.; Krenkel, T.G. Aplicación de la teoría de Delmon al estudio des mecanismo de la transformación polimórfica espodumeno I en espodumeno II. Boletin Sociedad Española Cerámica Vidrio 1976, 15, 5–10. [Google Scholar]
- Brook, R.J. Concise Encyclopedia of Advanced Ceramic Materials; Pergamon Press: Oxford, England, 1991. [Google Scholar]
- Tran, T.; Luong, V.T. Lithium production processes. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 81–124. [Google Scholar]
- Seeley, F.G.; Baldwin, W.H. Extraction of lithium from neutral salt solutions with fluorinated β-diketones. J. Inorg. Nucl. Chem. 1976, 38, 1049–1052. [Google Scholar] [CrossRef]
- Wang, X.; Jing, Y.; Liu, H.; Yao, Y.; Shi, C.; Xiao, J.; Wang, S.; Jia, Y. Extraction of lithium from salt lake brines by bis[(trifluoromethyl)sulfonyl]imide-based ionic liquids. Chem. Phys. Lett. 2018, 707, 8–12. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, X.; Hu, S.; Xiang, X. Higly efficient extraction of lithium from salt lake brine by LiAl-layered double hydroxides as lithium-ion-selective capturing material. J. Energy Chem. 2019, 34, 80–87. [Google Scholar] [CrossRef]
- Ellestad, R.B.; Leute, K.M. Method of extracting lithium values from spodumene ores. U.S. Patent 2,516,109, 25 July 1950. [Google Scholar]
- Hader, R.N.; Nielsen, R.L.; Herre, M.G. Lithium and its compounds. Ind. Eng. Chem. 1951, 43, 2636–2646. [Google Scholar] [CrossRef]
- Skinner, B.J.; Evans, H.T. β-spodumene solid solutions and the join Li2O–Al2O3–SiO2. Am. J. Sci. 1960, 258-A, 312–324. [Google Scholar]
- Archambault, M.; Macewan, J.U.; Olivier, C.A. Method of producing lithium carbonate from spodumene. U.S. Patent 3,017,243, 16 January 1962. [Google Scholar]
- Xiao, M.; Wang, S.; Zhang, Q.; Zhang, J. Leaching mechanism of the spodumene sulphuric acid process. Rare Met. (Beijing) 1997, 16, 36–44. [Google Scholar]
- Lajoie-Leroux, F. Étude sur le Grillage Acide du β-spodumene: Comportements des impuretés, Maîtrise; Université de Sherbrooke: Sherbrooke, QC, Canada, 2018. [Google Scholar]
- Clifford, M.N. Production of lithium compounds. U.S. Patent 2,413,644, 31 December 1946. [Google Scholar]
- Hayes, E.T.; Sternberg, W.M.; Williams, F.P. Production of lithium chloride from spodumene. U.S. Patent 2,533,246, 12 December 1950. [Google Scholar]
- Adolphe, V.K. Method of recovering lithium compounds from lithium minerals. U.S. Patent 2,662,809, 15 December 1953. [Google Scholar]
- Cunningham, G.L. Preparation of lithium chloride from spodumene. U.S. Patent 2,627,452, 3 February 1953. [Google Scholar]
- Dwyer, T.E. Recovery of lithium from spodumene ores. U.S. Patent 2,801,153, 30 July 1957. [Google Scholar]
- Peterson, J.A.; Gloss, G.H. Lithium values recovery process. U.S. Patent 2,893,828, 7 July 1959. [Google Scholar]
- Peterson, J.A. Process for recovering lithium values. U.S. Patent 2,924,507, 9 February 1960. [Google Scholar]
- Chubb, P.A. Treatment of lithium ores. U.S. Patent 3,073,673, 15 January 1963. [Google Scholar]
- Lemay, H.P.; Archambault, M.; Savard, M.A.O.C. Sodium-ammonium compounds process for extracting lithium from spodumene. U.S. Patent 3,112,170, 23 November 1963. [Google Scholar]
- Archambault, M.; Olivier, C.A. Carbonatizing roast of lithium bearing ores. U.S. Patent 3,380,802, 30 April 1968. [Google Scholar]
- Dunn, W.E.; Van Jahnke, J. Cyclical Vacuum Chlorination Processes, Including Lithium Extraction. U.S. Patent 7,588,741, 15 September 2009. [Google Scholar]
- Medina, L.F.; El-Naggar, M.M.A.A. An alternative method for the recovery of lithium from spodumene. Metall. Trans. B 1984, 15, 725–726. [Google Scholar] [CrossRef]
- Mast, E. Lithium Production from Spodumene. Master’s Thesis, McGill University, Montréal, QC, USA, 1989. [Google Scholar]
- Rezza, I.; Salinas, E.; Calvente, V.; Benuzzi, D.; De Tosetti, M.I.S. Extraction of lithium from spodumene by bioleaching. Lett. Appl. Microbiol. 1997, 25, 172–176. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Q.; Chen, B.; Shi, X.; Liao, T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process. Hydrometallurgy 2011, 109, 43–46. [Google Scholar] [CrossRef]
- Bieseki, L.; Melo, V.R.M.; Sobrinho, E.V.; Melo, D.M.A.; Pergher, S.B.C. Extração de lítio de amostras de β-espodumênio. Cerâmica 2013, 59, 557–562. [Google Scholar] [CrossRef]
- Rosales, G.D.; Ruiz, M.D.C.; Rodriguez, M.H. Novel process for the extraction of lithium from -spodumene by leaching with HF. Hydrometallurgy 2014, 147–148, 1–6. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Valente, G.; Oorosco, R.P.; González, J.A. Lithium extraction from β-spodumene through chlorination with chlorine gas. Miner. Eng. 2014, 56, 29–34. [Google Scholar] [CrossRef]
- Barbosa, L.I.; González, J.A.; Del Carmen Ruiz, M. Extraction of lithium from β-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar] [CrossRef]
- Kuang, G.; Liu, Y.; Li, H.; Xing, S.; Li, F.; Guo, H. Extraction of lithium from β-spodumene unsing sodium sulphate solution. Hydrometallurgy 2018, 177, 49–56. [Google Scholar] [CrossRef]
- Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 6th ed.; McGraw-Hill: New York, NY, USA, 1956; p. 966. [Google Scholar]
- Gupta, C.K. Chemical Metallurgy; Principles and Practice, Wiley-VCH: Weinheim, Germany, 2003; p. 133. [Google Scholar]
- Lajoie-Leroux, F.; Dessemond, C.; Soucy, G.; Laroche, N.; Magnan, J.F. Impact of the impurities on lithium extraction from β-spodumene in the sulphuric acid process. Miner. Eng. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Botto, I.L.; Arazi, S.C.; Krenkel, T.G. Estudio cinético de la transformación polimórfica espodumeno I en espodumeno II. Boletin dela Sociedad Española de Cerámica y Vidrio 1975, 14, 225–230. [Google Scholar]
- Mellor, J.W. A Comprehensive Treatise on Inorganic and Theoretical Chemistry, Volume VI, Part II, Si, Silicates, 1st ed.; Longmans, Green and Co. LTD: London, UK, 1930; 991p. [Google Scholar]
- White, G.D.; McVoy, T.N. Some Aspects of the Recovery of Lithium from Spodumene; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1958; pp. 1–17.
- Kotsupalo, N.P.; Menzheres, L.T.; Ryabtsev, A.D.; Boldyrev, V.V. Mechanical Activation of α-Spodumene for Further Processing into Lithium Compounds. Theor. Found. Chem. Eng. 2010, 44, 503–507. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Nikoloski, A.N.; Singh, P. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 2: Microwave heating. Miner. Eng. 2017, 100, 191–199. [Google Scholar] [CrossRef]
- Hatch, R.A. Phase Equilibrium in the system: Li2O-Al2O3-SiO2. American Mineralogist 1943, 28, 471–496. [Google Scholar]
- Shoucri, A. Étude de la Conversion α vers β d’un Minerai de Spodumene, Maîtrise; Université de Sherbrooke: Sherbrooke, QC, Canada, 2015. [Google Scholar]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.F. Revisiting the Traditionnal Process of Spodumene Conversion and Impact on Lithium Extraction. In Proceedings of the First Global Conference on Extractive Metallurgy; The Minerals, Metals and Materials Society: Pittsburgh, PA, USA, 2018; pp. 2281–2291. [Google Scholar]
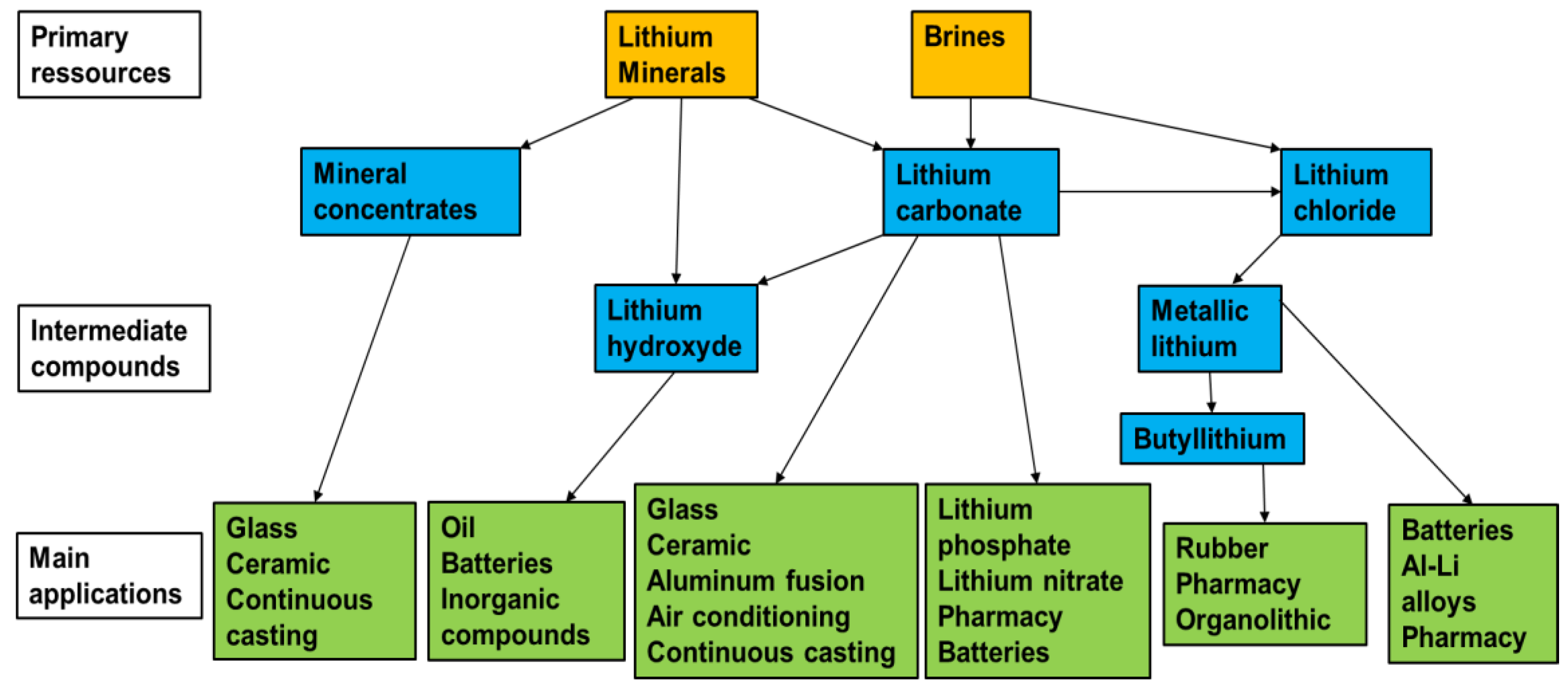
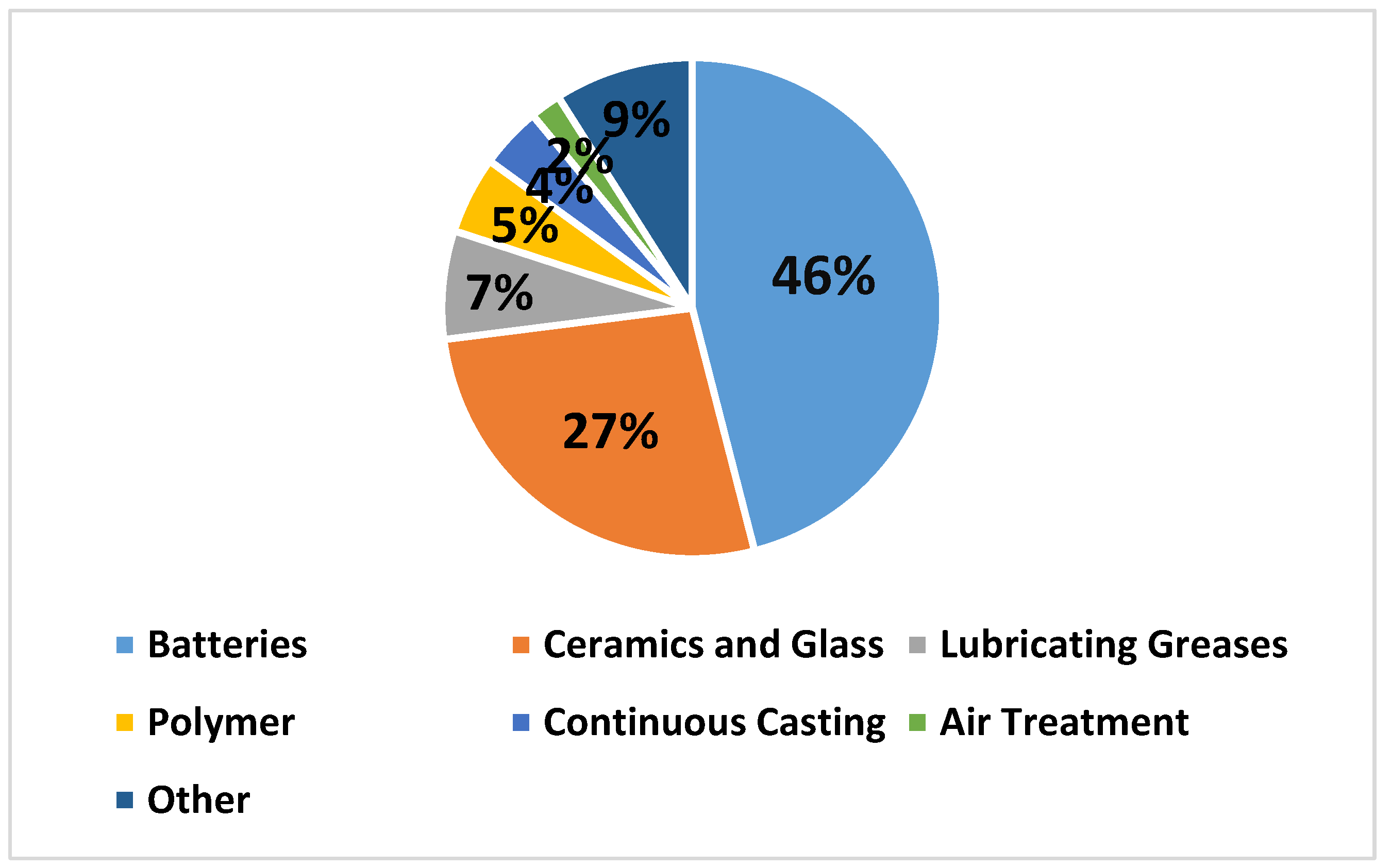
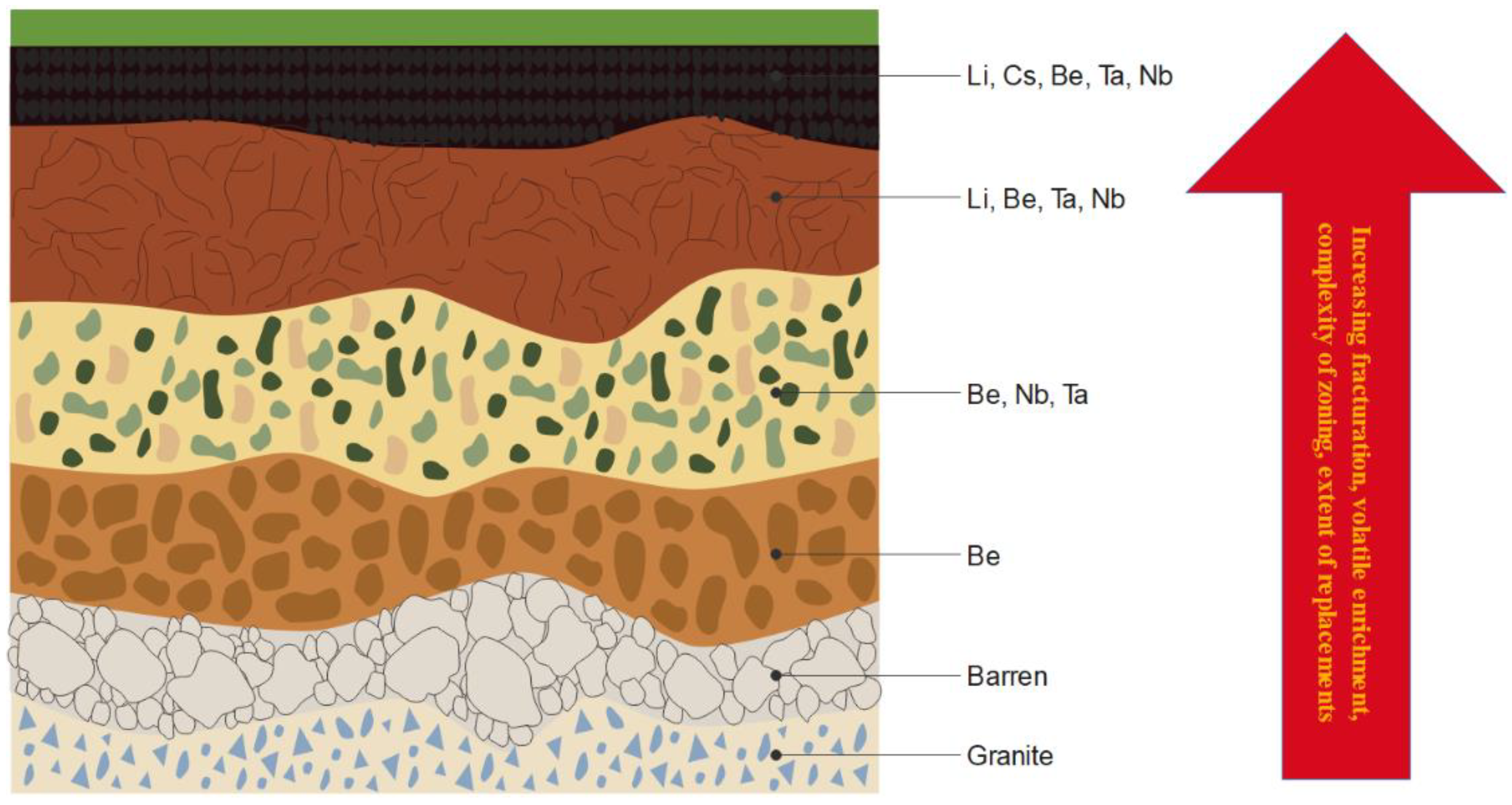
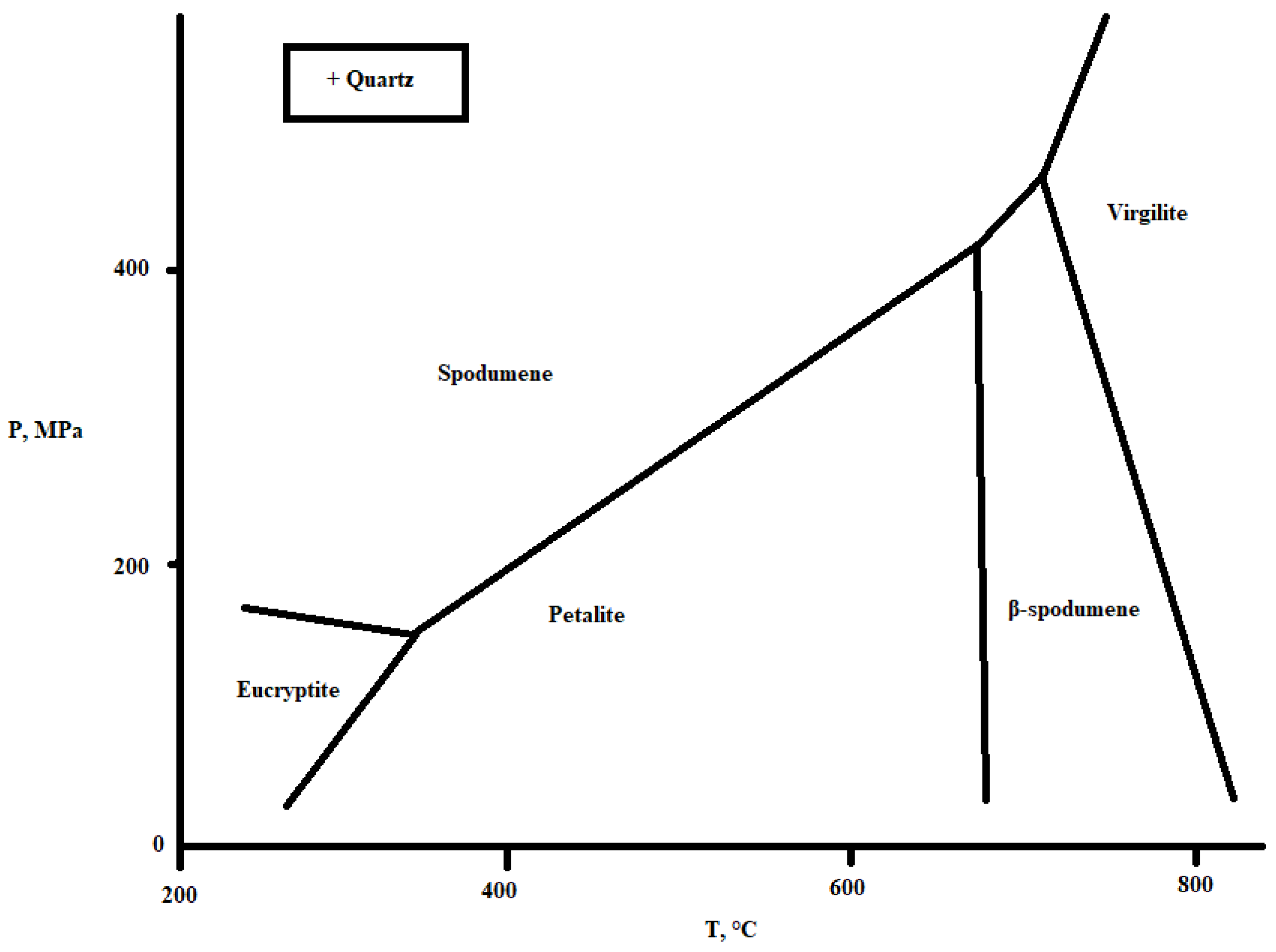
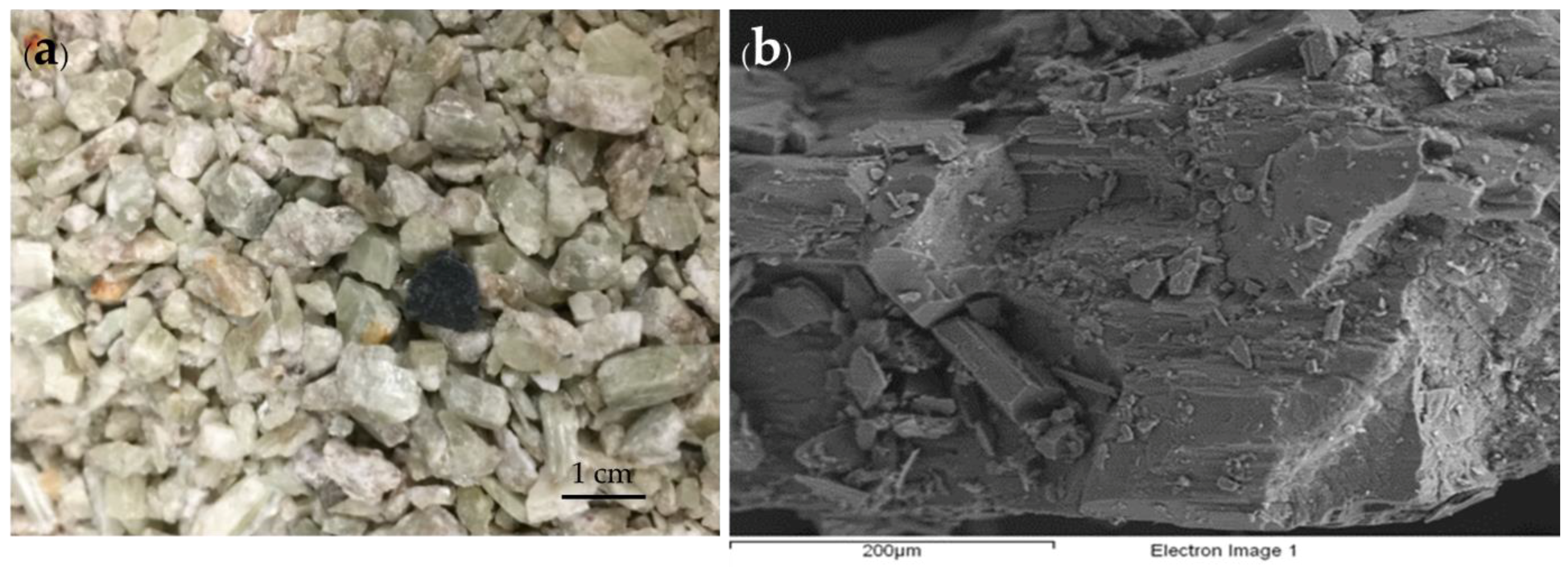
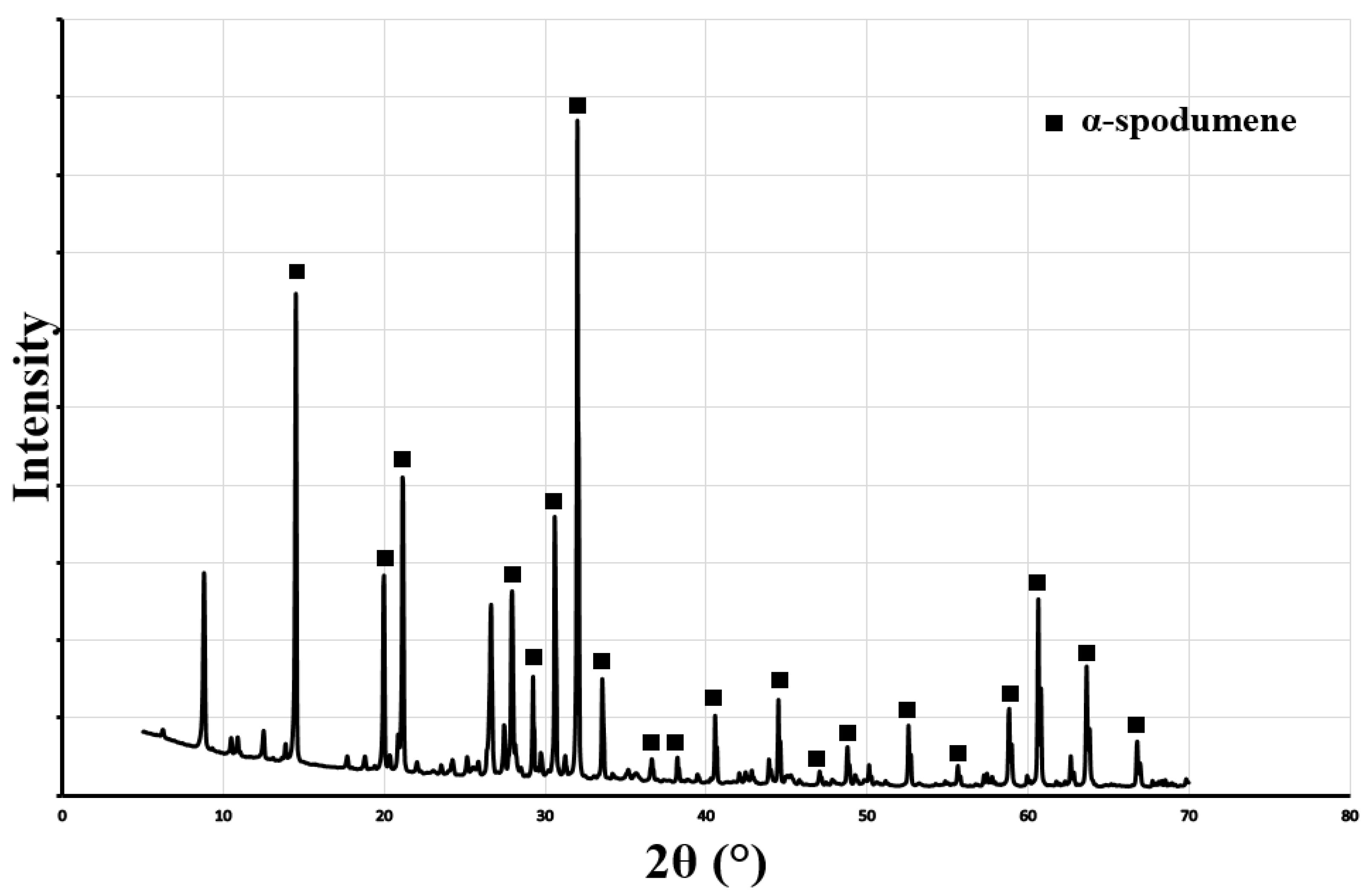

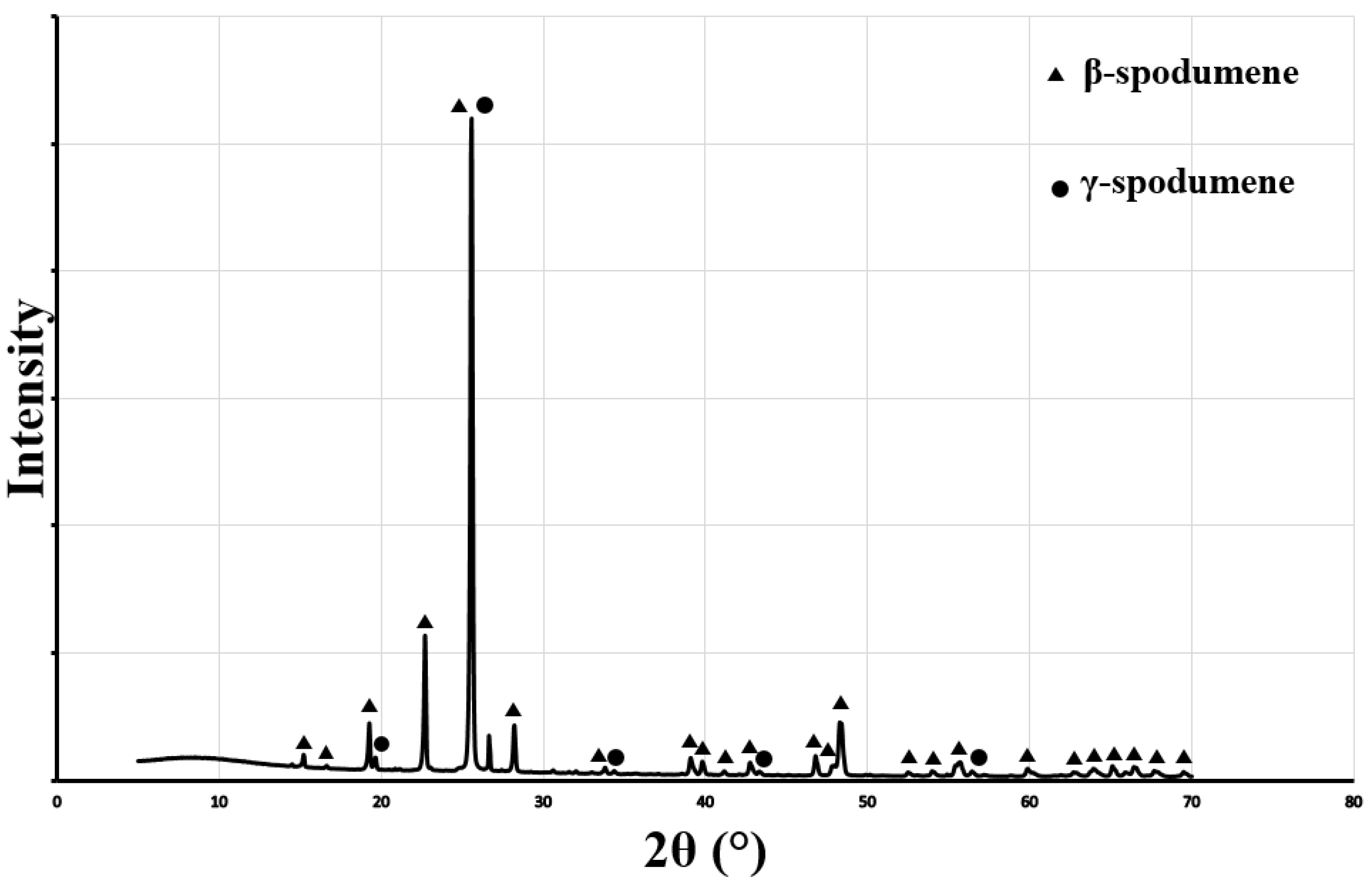
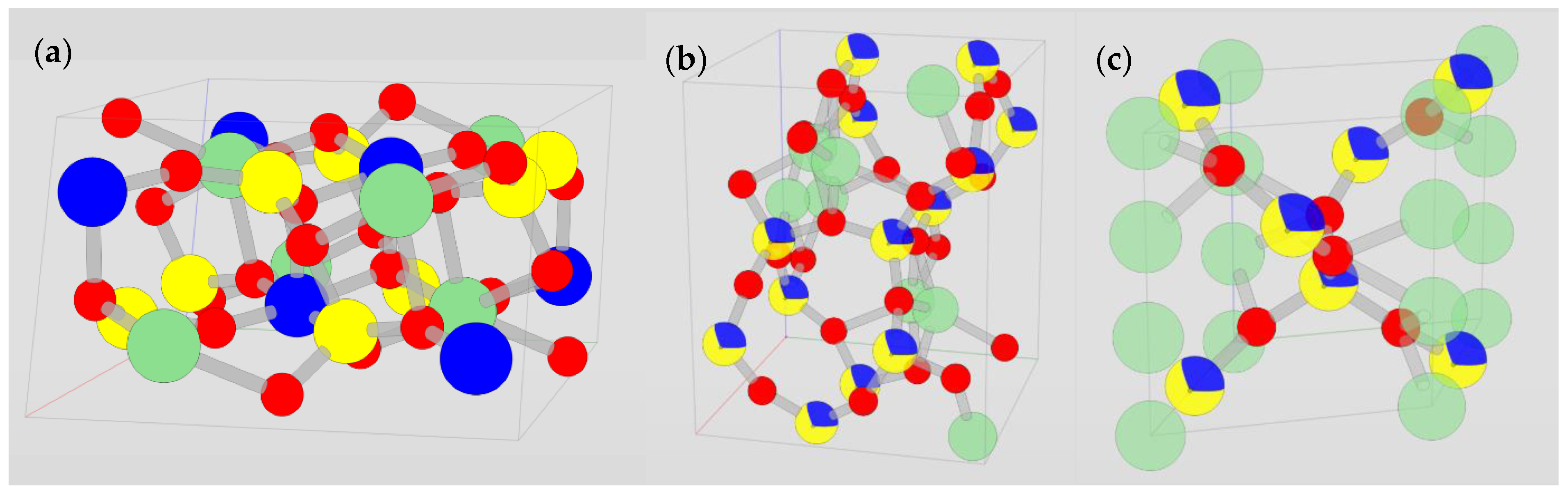

| Mineral | Formula | Theoretical Li Content (%) |
|---|---|---|
| Spodumene | LiAlSi2O6 | 3.73 |
| Petalite | LiAlSi4O10 | 2.27 |
| Eucryptite | LiAlSiO4 | 5.51 |
| Bikitaite | LiAlSi2O6.H2O | 3.40 |
| Lepidolite | KLi2AlSi3O10(OH,F)2 | ~3.84 |
| Zinnwaldite | KLiFeAl2Si3O10(F,OH)2 | 1.59 |
| Amblygonite | (Li,Na)AlPO4(OH,F) | 4.73 |
| Montebrasite | LiAl(PO4)(OH) | 1 to 4 |
| Lithiophylite | LiMnPO4 | 4.43 |
| Triphylite | LiFePO4 | 4.40 |
| Hectorite | Na0,3(Mg,Li)3Si4O10(OH)2 | ~1.93 |
| Jadarite | LiNaAlSiB2O7(OH) | 2.85 |
| Zabuyelite | Li2CO3 | 18.79 |
| Elbaite | Na(Li1,5Al1,5)Al6Si6B3O27(OH)4 | 1.11 |
| Form | Structure | Space Group | a (Å) | b (Å) | c (Å) | Angles (°) | Z |
|---|---|---|---|---|---|---|---|
| α-spodumene | Monoclinic | C2/c | 9.45 | 8.39 | 5.215 | β = 110 | 4 |
| β-spodumene | Tetragonal | P43212 | 7.541 | - | 9.156 | - | 4 |
| γ-spodumene | Hexagonal | P6222 | 5.217 | - | 5.464 | - | 1 |
| Form | Cp(298 K) (J∙K−1∙mol−1) | H0i (kJ∙mol−1) | S0i (J∙K−1∙mol−1) |
|---|---|---|---|
| α-spodumene | 158.93 | −3053.500 | 129.412 |
| β-spodumene | 162.77 | −3031.888 | 155.376 |
| γ-spodumene | 162.77 | −3032.128 | 162.038 |
| Reagent(s) | Size (µm) | Yield (%) | Decrepitation (°C) | Temperature (°C) | Duration | Reference |
|---|---|---|---|---|---|---|
| H2SO4 (l) | <600 | 90 | 1000 | 250 | « Short » | [49] |
| Ca(OH)2 (aq.) | <100 | 90 | 1100 | 100–205 | 2 h | [55] |
| CaCO3 (s) + CaSO4 (s) | <75 | 85–90 | - | During the decrepitation | [56] | |
| CaO (s) | - | 84–100 | - | 700 | - | [57] |
| CaCO3 (s) + CaCl2 (s) + SiO2 (s) | <175 | 90–95 | 900–1100 | 1100–1200 | - | [58] |
| (NH4)2SO4/NH4HSO4 (l) | <175 | - | 1030 | 150–370 | - | [59] |
| KCl (s) + KCl·NaCl (s) | <150 | 100 | 1050 | During the decrepitation | [60] | |
| NaCOOH + Na2CO3 | <300 | 98–100 | 100 | 290 | 30–90 min | [61] |
| SO3 (g) | <600 | 97 | 870 | 335–450 | 15 min | [52] |
| NaOH/Na2CO3 (aq.) + CaO/Ca(OH)2 (aq.) | - | - | 1010 | 100–200 | - | [62] |
| NaOH/Na2SiO3/2Na2O·B2O3/Na2S (aq.) | <150 | 93 | - | 70–130 | 1–48 h | [63] |
| Na2CO3 (s) | - | 85–97 | 1000 | 450–750 | 10–120 min | [64] |
| Cl2 (g) + CO (g) | <44 | 90 | 1040 | 1000 | - | [65] |
| Reagent(s) | Size (µm) | Yield (%) | Decrepitation (°C) | Temperature (°C) | Duration | Reference |
|---|---|---|---|---|---|---|
| CaMg2Cl6·12H2O (s) | <75 | 87 | 1100 | During the decrepitation | [66] | |
| Mg(l) | - | 100 | 1050 | 1500 | - | [67] |
| Bacteria (aq.) | - | <10 | No decrepitation | Room | 30 d | [68] |
| Na2CO3 (aq.) | - | 94 | 1050 | 225 | 1 h | [69] |
| Na2CO3 (s)/H2O/H2O + NH4HCO3 (aq.) | - | <10 | - | 600 then room | 30 min then 4 h | [70] |
| HF (aq) | - | 90 | 1100 | 75 | 20 min | [71] |
| Cl2 (g) | <50 | 99 | 1180 | 1000 | 3 h | [72] |
| CaCl2 (s) | <50 | 90 | 1180 | 900 | 2 h | [73] |
| Na2SO4 (aq)/NaOH or CaO (aq.) | <75 | 90 | 1100 | 230 | - | [74] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. https://doi.org/10.3390/min9060334
Dessemond C, Lajoie-Leroux F, Soucy G, Laroche N, Magnan J-F. Spodumene: The Lithium Market, Resources and Processes. Minerals. 2019; 9(6):334. https://doi.org/10.3390/min9060334
Chicago/Turabian StyleDessemond, Colin, Francis Lajoie-Leroux, Gervais Soucy, Nicolas Laroche, and Jean-François Magnan. 2019. "Spodumene: The Lithium Market, Resources and Processes" Minerals 9, no. 6: 334. https://doi.org/10.3390/min9060334
APA StyleDessemond, C., Lajoie-Leroux, F., Soucy, G., Laroche, N., & Magnan, J.-F. (2019). Spodumene: The Lithium Market, Resources and Processes. Minerals, 9(6), 334. https://doi.org/10.3390/min9060334





