Exceptional Multi Stage Mineralization of Secondary Minerals in Cavities of Flood Basalts from the Deccan Volcanic Province, India
Abstract
:1. Introduction
2. Geological Setting and Secondary Mineralization
2.1. Geological Setting
2.2. Distribution and Mineralization Sequence of Secondary Minerals
3. Materials and Methods
3.1. Sample Material
3.2. Optical Microscopy, Scanning Electron Microscopy (SEM) and Electron Microprobe Analysis (EMP)
3.3. Cathodoluminescence (CL)
3.4. X-Ray Diffractometry
3.5. Fluid Inclusions
3.6. C and O Isotope Analyses
3.7. Geochronology
| Mineral | Stilbite-Ca | Mordenite | Heulandite-Ca | Plagioclase |
|---|---|---|---|---|
| at [%] | at [%] | at [%] | at [%] | |
| O | 73,94 | 50,06 | 71,16 | 61,58 |
| Si | 16,63 | 40,09 | 19,39 | 26,93 |
| Al | 5,09 | 6,91 | 6,15 | 6,99 |
| Ca | 2,99 | 1,61 | 2,42 | 3,62 |
| Na | 1,34 | 1,24 | 0,68 | 0,66 |
| K | 0,01 | 0,08 | 0,20 | 0,22 |
| Total | 100,00 | 100,00 | 100,00 | 100,00 |
| Sample | Material | Rb | Sr | 87Rb/86Sr | 87Sr/86Sr | +/-2sm | Age | Calculated | 87Sr/86Sr |
|---|---|---|---|---|---|---|---|---|---|
| [ppm] | [ppm] | [Ma] (±2s) | with | Initial | |||||
| JAL-1 | Cal III | 0,002 | 11,04 | 0,0005 | 0,706549 | 0,000004 | 28.0 ± 0.3 | Cal-App | 0.706549 ± 0.000004 |
| JAL-1 | Stb | 0,206 | 4,490 | 0,1331 | 0,707733 | 0,000005 | 27.8 ± 0.3 | Stb-App | 0.707680 ± 0.000004 |
| JAL-1 | Apo | 99,87 | 0,732 | 401,21 | 0,866312 | 0,000144 | 27.9 ± 2.8 | Cal-Stb-App | 0.7071 ± 0.0072 |
| JAL-2 | Stb | 0,059 | 5,291 | 0,0323 | 0,707788 | 0,000004 | |||
| JAL-2 | Apo | 102,5 | 1,892 | 158,18 | 0,805454 | 0,000017 | 44.3 ± 0.4 | Stb-App | 0.707768 ± 0.000004 |
| JAL-12A | Apo | 106,8 | 2,429 | 128,31 | 0,791158 | 0,000017 | 46.1 ± 0.5 | WR-App | 0.707193 ± 0.000005 |
| JAL-13A | Cel | 293,4 | 1525 | 0,5568 | 0,708432 | 0,000004 | |||
| JAL-13B | WR | 17,65 | 259,0 | 0,1971 | 0,707322 | 0,000004 | |||
| JAL-13C | Apo-core | 97,26 | 3,157 | 89,688 | 0,769401 | 0,000008 | 48.8 ± 0.4 | WR-App | 0.707185 ± 0.000002 |
| JAL-13C | Apo-rim | 102,7 | 2,500 | 119,84 | 0,787254 | 0,000023 | 47.0 ± 0.6 | WR-App | 0.707190 ± 0.000002 |
| JAL-16B | Cal III | 0,202 | 10,611 | 0,0550 | 0,707653 | 0,000007 | |||
| JAL-16B | Apo1 | 95,84 | 0,759 | 370,07 | 0,840116 | 0,000026 | 25.2 ± 0.3 | WR-App1 | 0.707633 ± 0.000007 |
| JAL-16B | Apo2 | 95,88 | 0,907 | 309,20 | 0,821699 | 0,000015 | 26.0 ± 0.3 | WR-App2 | 0.707632 ± 0.000007 |
| NAS-6 | Stb | 0,065 | 3,112 | 0,0601 | 0,716487 | 0,000005 | |||
| NAS-6 | Apo | 161,6 | 0,584 | 857,07 | 1,417889 | 0,002596 | 58.7 ± 0.6 | Stb-App | 0.716437 ± 0.000005 |
| NAS-9 | Stb | 0,178 | 26,55 | 0,0194 | 0,710789 | 0,000005 | |||
| NAS-9 | Apo | 113,8 | 3,373 | 98,371 | 0,783354 | 0,000014 | 55.8 ± 0.6 | Stb-App | 0.710775 ± 0.000005 |
| NAS-10 | Stb | 0,070 | 12,69 | 0,0160 | 0,712251 | 0,000008 | |||
| NAS-10 | Apo | 175,5 | 1,826 | 280,66 | 0,800312 | 0,000010 | 22.1 ± 0.2 | Stb-App | 0.712246 ± 0.000008 |
| NAS-11A | Stb | 0,042 | 20,75 | 0,0058 | 0,708837 | 0,000003 | |||
| NAS-11A | Apo | 93,21 | 2,198 | 123,19 | 0,746279 | 0,000014 | 21.4 ± 0.2 | Stb-App | 0.708835 ± 0.000004 |
4. Results
4.1. Observations in the Outcrops and Host Rock Characteristics
4.2. Secondary Minerals (According to Their Precipitation Sequence)
4.2.1. Clay Minerals
4.2.2. Subsurface Filamentous Fabrics (SFFs)
4.2.3. Calcite
4.2.4. Silica Phases
4.2.5. Feldspar, Zeolites, and Powellite
4.2.6. Apophyllite
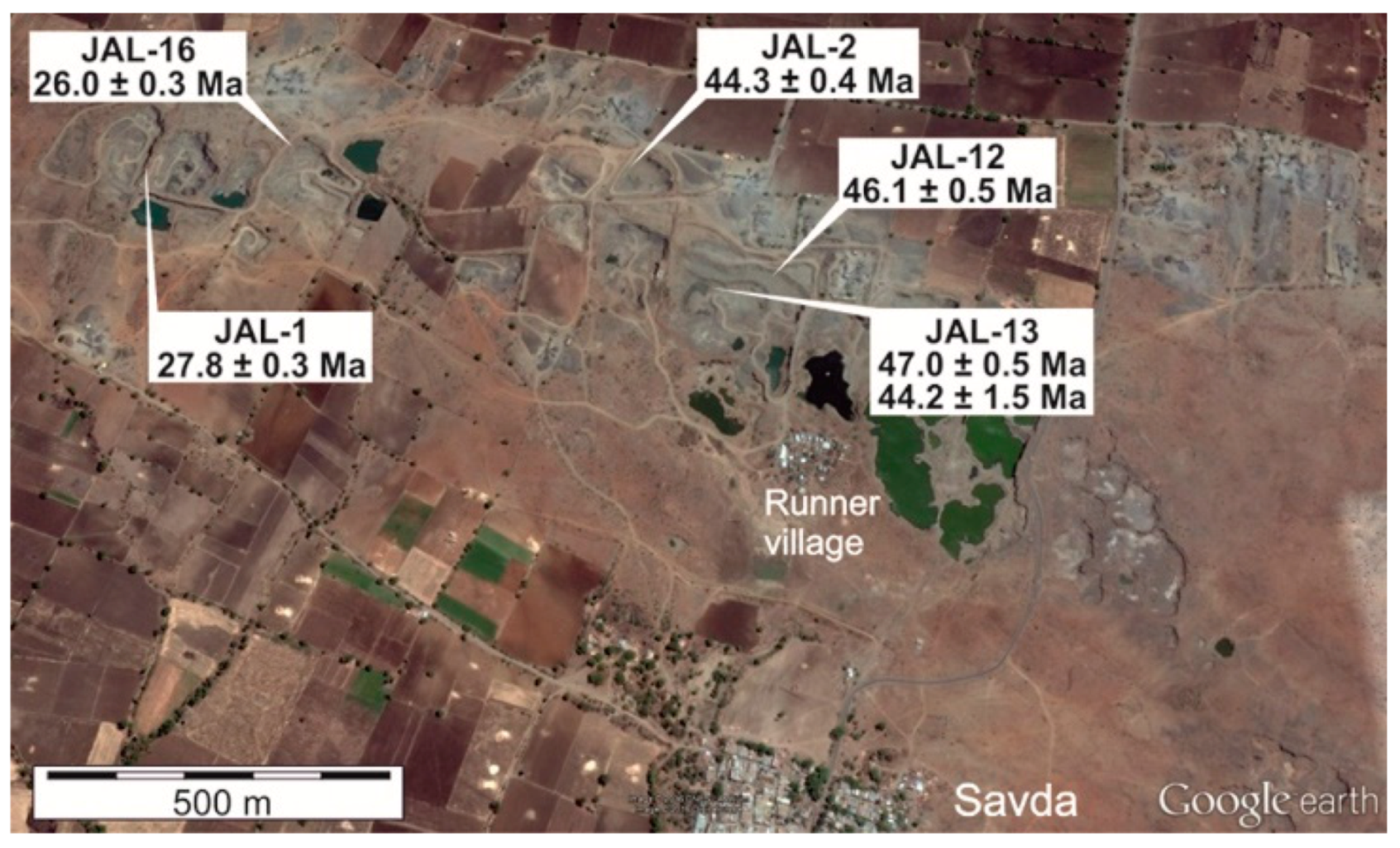
Rb-Sr and K-Ar Geochronology
Rb-Sr data
K-Ar Data
4.2.7. Mineralization Sequence
5. Discussion
5.1. Alteration of Primary Minerals
5.2. Clay Minerals
5.3. Origin of Subsurface Filamentous Fabrics (SFFs)
5.4. Calcite
5.5. Feldspar, Zeolites, and Powellite
5.6. Apophyllite
5.7. Multistage Mineralization Model of Secondary Minerals in Savda
5.8. General Relevance of the Multistage Mineralization Model for the Deccan Volcanic Province (DVP)
5.9. Comparison with Mineralization Models of Other Volcanic Provinces
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pande, K. Age and duration of the Deccan Traps, India: A review of radiometric and palaeomagnetic constraints. Proc. Indian Acad. Sci. Earth Planet Sci. 2002, 111, 115–123. [Google Scholar] [CrossRef]
- Sheth, H.C. Deccan volcanism: Recent Indian research. In Glimpses of Geoscience Research in India: The Indian Report to IUGS 1999-2004; Singhvi, A.K., Bhattacharya, A., Eds.; The Indian National Science Academy (INSA): New Delhi, India, 2004; pp. 19–25. [Google Scholar]
- Sheth, H.C. The Deccan beyond the Plume Hypothesis. Geophys. Res. Abstracts 2006, 8, 2. [Google Scholar]
- Sen, G.; Chandrasekharam, D. Deccan Traps Flood Basalt Province: An Evaluation of the Thermochemical Plume Model. In Topics in Igneous Petrology; Springer: Dortrecht, The Netherlands, 2011; pp. 29–53. [Google Scholar]
- Hooper, P.R. The Deccan Traps. In Deccan Volcanic Province; Subbarao, K.V., Ed.; Geological Society of India: Bangalore, India, 1999; pp. 153–165. [Google Scholar]
- Subbarao, K.V. Deccan Volcanic Province; Geological Society of India: Bangalore, India, 1999; West Volume 1, 548p. [Google Scholar]
- Sabale, A.B.; Vishwakarama, L.L. Zeolites and associated secondary minerals in Deccan volcanics: Study of their distribution, genesis and economic importance. In Proceedings of the National Symposium on Deccan Flood Basalts, Nagpur, India, 10–11 March 1996; pp. 511–518. [Google Scholar]
- Walker, G.P.L. Some Observations and Interpretations on the Deccan Traps. Mem. Geol. Soc. India 1969, 43, 367–395. [Google Scholar]
- Neuhoff, P.S.; Fridriksson, T.; Arnorsson, S. Porosity evolution and mineral paragenesis during low-grade metamorphism of basaltic lavas at Teigarhorn, Eastern Iceland. Am. J. Sci. 1999, 299, 467–501. [Google Scholar] [CrossRef]
- Neuhoff, P.S.; Fridriksson, T.; Bird, D.K. Zeolite paragenesis in the North Atlantic Igneous Province: Implications for geotectonics and groundwater quality of basaltic crust. Int. Geol. Rev. 2000, 42, 15–44. [Google Scholar] [CrossRef]
- Weisenberger, T.; Selbekk, R.S. Multi-stage zeolite facies mineralization in the Hvalfjördur area, Iceland. Int. J. Earth Sci. 2009, 98, 985–999. [Google Scholar] [CrossRef]
- Pe-Piper, G. Mode of Occurrence, Chemical Variation and Genesis of Mordenite and Associated Zeolites from the Morden Area, Nova Scotia, Canada. Can. Mineral. 2000, 38, 1215–1232. [Google Scholar] [CrossRef]
- Mattioli, M.; Cenni, M.; Passaglia, E. Secondary mineral assemblages as indicators of multistage alteration processes in basaltic lava flows: Evidence from the Lessini Mountains, Veneto Volcanic Province, Northern Italy. Periodico di Mineralogia 2016, 85, 1–24. [Google Scholar]
- Sukheswala, R.N.; Avasia, R.K.; Gangopadhyay, M. Zeolites and associated secondary minerals in the Deccan Traps of western India. Mineral. Mag. 1974, 39, 658–671. [Google Scholar] [CrossRef]
- Jeffery, K.L.; Henderson, P.; Subbarao, K.V.; Walsh, J.N. The zeolites of Deccan Basalts—A study of their distribution. In Deccan Flood Basalts; Subbarao, K.V., Ed.; Geological Society of India: Bangalore, India, 1988; pp. 151–162. [Google Scholar]
- Srikantappa, C.; Mookherjee, A. Water, Aqueous, H2O-CO2 and Gaseous Inclusions in Cavity Minerals in the Basaltic Lava flows around Pune, India: Evidence for Boiling. In Proceedings of the Second Meeting of the Asian Current Research on Fluid Inclusions (ACROFI–2), Kharagpur, West Bengal, India, 12–14 Noveber 2008; p. 176. [Google Scholar]
- James, S.; Walsh, J.N. Zeolites from the Deccan Basalts: Chemistry and Formation. Mem. Geol. Soc. India 1999, 43, 803–817. [Google Scholar]
- Ottens, B. Indien, Mineralien und Fundstellen; Weise Verlag: München, Germany, 2011; 384p, ISBN 978-3-921656-76-1. [Google Scholar]
- Walker, G.P.L. Zeolite zones and dike distribution in relation to the structure of basalts in eastern Icelands. J. Geol. 1960, 68, 515–528. [Google Scholar] [CrossRef]
- Neuhoff, P.S.; Watt, W.S.; Bird, D.K.; Pedersen, A.K. Timing and structural relations of regional zeolite zones in basalts of the East Greenland continental margin. Geology 1997, 25, 803–806. [Google Scholar] [CrossRef]
- Neuhoff, P.S.; Rogers, K.L.; Stannius, L.S.; Bird, D.K.; Pedersen, A.K. Regional very low-grade metamorphism of basaltic lavas, Disko-Nuussuaq Region, West Greenland. Lithos 2006, 92, 33–54. [Google Scholar] [CrossRef]
- Jørgensen, O. The regional distribution of zeolites in the basalts of the Faroe Islands and the significance of zeolites as palaeotemperature indicators. In Scientific Results from the Deepened Lopra-1 Borehole, Faroe Island; Chalmers, J.A., Waagstein, R., Eds.; GEUS: Copenhagen, Denmark, 2006; pp. 123–156. [Google Scholar]
- Triana, J.M.; Herrera, J.F.; Ríos, C.A.; Castellanos, O.M.; Henao, J.A.; Williams, C.D.; Roberts, C.L. Natural zeolites filling amygdales and veins in basalts from the British Tertiary Igneous Province on the Isle of Skye, Scotland. Earth Sci. Res. J. 2012, 16, 41–53. [Google Scholar]
- Robert, C. Hydrothermal alteration process of the Tertiary lavas of Northern Ireland. Mineral. Mag. 2001, 65, 543–554. [Google Scholar] [CrossRef]
- Kousehlar, M.; Weisenberger, T.B.; Tutti, F.; Mirnejad, H. Fluid control on low-temperature mineral formation in volcanic rocks of Kahrizak, Iran. Geofluids 2012, 12, 295–311. [Google Scholar] [CrossRef]
- Bazargani-Guilani, K.; Rabbani, M.S. Amygdaloidal and other Cavity Filling Zeolites of Kuh-e-Aradeh, Central Iran. J. Sci. Islamic Repub. Iran 2004, 15, 149–157. [Google Scholar]
- Giret, A.; Verdier, O.; Nativel, P. The zeolitization model of Kerguelen Islands, southern Indian Ocean. In Recent Progress in Antarctic Earth Science; Yoshida, K.K., Shiraishi, K., Eds.; Terrapub: Tokyo, Japan, 1992; pp. 457–463. [Google Scholar]
- Hooper, P.R.; Kleck, W.D.; Knowles, C.R.; Reidel, S.P.; Thiessen, R.L. Imnaha basalt, Columbia River Basalt Group. J. Petrol. 1983, 25, 473–500. [Google Scholar] [CrossRef]
- Murata, K.J.; Formoso, M.L.L.; Ari Roisenberg, A. Distribution of Zeolites in Lavas of Southeastern Parana Basin, State of Rio Grande Do Sul, Brazil. J. Geol. 1987, 95, 455–467. [Google Scholar] [CrossRef]
- Schenato, F.; Formoso, M.L.L.; Dudoignon, P.; Meunier, A.; Proust, D.; Mas, A. Alteration processes of a thick basaltic lava flow of the Paraná Basin, (Brazil): Petrographic and mineralogical studies. J. South Am. Earth Sci. 2003, 16, 423–444. [Google Scholar] [CrossRef]
- Kristmannsdottir, H. Alteration of Basaltic Rocks by Hydrothermal-Activity at 100–300 °C. Dev. Sedimentol. 1979, 27, 359–367. [Google Scholar]
- Gustavson, J.E. Analysis of Porosity Evolution during Low Temperature Metamorphism of Basaltic Lavas and Implications for Fluid Flow. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2006; 110p. [Google Scholar]
- Self, S.; Thordarsson, T.; Widdowson, M.; Jay, A. Volatile fluxes during flood basalt eruptions and potential effects on global environment: A Deccan perspective. Earth Planet. Sci. Lett. 2006, 248, 518–532. [Google Scholar] [CrossRef]
- Chenet, A.L.; Courtillot, V.; Fluteau, F.; Gérard, M.; Quidelleur, X.; Khadri, S.F.R.; Subbarao, K.V.; Thordarson, T. Determination of rapid Deccan eruptions across the Cretaceous-Tertiary boundary using paleomagnetic secular variation: 2. Constraints from analysis of eight new sections and synthesis for a 3500-m-thick composite section. J. Geophys. Res. Solid Earth 2009, 114, 1–38. [Google Scholar] [CrossRef]
- Keller, G.; Sahni, A.; Bajpai, S. Deccan volcanism, the KT mass extinction and dinosaurs. J. Biosci. 2009, 34, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Self, S.; Jay, A.E.; Widdowson, M.; Keszthelyi, L.P. Correlation of the Deccan and Rajahmundry Trap lavas: Are these the longest and largest lava flows on Earth? J. Volcanol. Geotherm. Res. 2008, 172, 2–19. [Google Scholar] [CrossRef]
- GSI Ajanta Quadrangle Map, Maharashtra; Geological Survey of India: Calcutta, India, 1994.
- Bondre, N.R.; Duraiswami, R.A.; Dole, G. Morphology and emplacement of flows from the Deccan Volcanic Province. Bull. Volcanol. 2004, 66, 29–45. [Google Scholar] [CrossRef]
- Bondre, N.R.; Duraiswami, R.A.; Dole, G. A brief comparison of lava flows from the Deccan Volcanic Province and the Columbia-Oregon Plateau Flood Basalts: Implications for models of flood basalt emplacement. Proc. Indian Acad. Sci. (Earth Planet Sci.) 2004, 113, 809–817. [Google Scholar] [CrossRef] [Green Version]
- West, W.D. The petrography and Petrogenesis of forty-eight flows of Deccan Trap penetrated by borings in Western India. Mem. Geol. Soc. India 1999, 43, 641–704. [Google Scholar]
- Melluso, L.; Barbieri, M.; Beccaluva, L. Chemical evolution, petrogenesis, and regional chemical correlations of the flood basalt sequence in the central Deccan Traps, India. J. Earth Syst. Sci. 2004, 113, 587–603. [Google Scholar] [CrossRef]
- Sethna, S.F.; Sethna, B.S. Mineralogy and petrogenesis of Deccan Trap basalts. Mem. Geol. Soc. India 1988, 10, 69–90. [Google Scholar]
- Apte, A.S. Thermal Analysis of Zeolites and Associated Cavity Minerals from the Deccan Volcanic Province. Unpublished. Ph.D. Thesis, University of Pune, Pune, India, 1994; 218p. [Google Scholar]
- Melluso, L.; Sethna, S.F. Mineral compositions in the Deccan igneous 1 rocks 2 an overview. In Topics in Igneous Petrology; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Beane, J.E.; Turner, C.A.; Hooper, P.R.; Subbarao, K.V.; Walsh, J.N. Stratigraphy, composition and form of the Deccan Basalts, Western Ghats, India. Bull. Volcanol. 1986, 48, 61–83. [Google Scholar] [CrossRef]
- Beane, J.E.; Hopper, P.A. A note on the Picrite Basalts of the Western Ghats, Deccan Traps, India. Geol. Soc. India 1988, 10, 117–134. [Google Scholar]
- Ottens, B. Minerals of the Deccan Traps India. Mineral. Rec. 2003, 34, 1–82. [Google Scholar]
- Neuser, R.D.; Bruhn, F.; Götze, J.; Habermann, D.; Richter, D.K. Kathodolumineszenz: Methodik und Anwendung. Zentralblatt für Geologie und Paläontologie Teil I 1995, H1, 287–306. [Google Scholar]
- Bakker, R.J. Package FLUIDS 1 Computer programs for analysis of fluid inclusion data and for modelling bulk fluid properties. Chem. Geol. 2003, 194, 3–23. [Google Scholar] [CrossRef]
- Oakes, C.; Bodnar, R.; Simonson, J. The system NaCl-CaCl2-H2O: The ice liquidus at 1 atm total pressure. Geochimica et Cosmochimica Acta 1990, 54, 603–610. [Google Scholar] [CrossRef]
- Davis, D.W.; Lowenstein, T.K.; Spencer, R.J. Melting behavior of fluid inclusions in laboratory-grown halite crystals in the systems NaCl-H2O, NaCl-KCl-H2O, NaCl-MgCl2-H2O and NaCl-CaCl2-H2O. Geochim. Cosmochim. Acta 1990, 54, 591–601. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Systematics of Fluid Inclusions in Diagenetic Minerals. Short Course 31; Society of Economic Paleontologists and Mineralogists: Tulsa, OK, USA, 1994; 199p. [Google Scholar]
- Bodnar, R.-J. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Spötl, C.; Vennemann, T.W. Continuous-flow isotope ratio mass spectrometric analysis of carbonate minerals. Rapid Commun. Mass Spectrom. 2003, 17, 1004–1006. [Google Scholar] [CrossRef]
- Sölva, H.; Grasemann, B.; Thöni, M.; Thiede, R.; Habler, G. The Schneeberg Normal Fault Zone: Normal faulting associated with Cretaceous SE-directed extrusion in the Eastern Alps (Italy/Austria). Tectonophysics 2005, 401, 143–166. [Google Scholar] [CrossRef]
- Ludwig, K.R. Isoplot/Ex Version 3.0. A Geochronological Toolkit for Microsoft Excel; Berkeley Geochronological Centre Special Publication: Berkeley, CA, USA, 2003; 70p. [Google Scholar]
- Balogh, K. K/Ar dating of Neogene volcanic activity in Hungary: Experimental technique, experiences and methods of chronological studies. ATOMKI Koezlemenyek 1985, 27, 277–288. [Google Scholar]
- Nier, A. A Redetermination of the Relative Abundances of the Isotopes of Carbon, Nitrogen, Oxygen, Argon, and Potassium. Phys. Rev. 1950, 77, 789–793. [Google Scholar] [CrossRef]
- Steiger, R.; Jäger, E. Subcommission on geochronology: Convention on the use of decay constants in geo-and cosmochronology. Earth Planet. Sci. Lett. 1977, 36, 359–362. [Google Scholar] [CrossRef]
- IMA Masterlist. 2018. Available online: http://nrmima.nrm.se//IMA_Master_List_%282018-11%29.pdf (accessed on 1 November 2018).
- Baldermann, A.; Dohrmann, R.; Kaufhold, S.; Nicke, C. The Fe-Mg-saponite solid solution series—A hydrothermal synthesis study. Clay Miner. 2014, 49, 91–415. [Google Scholar] [CrossRef]
- Duplay, J.; Paquet, H.; Kossovskaya, A.; Tard, Y. Estimation de la température de formation des paragenèses saponite-céladonite et glauconite-nontronite dans les altérations sous-marines de basalte, par la méthode des corrélations entre éléments au sein de populations monominérales. CR Acad. Sci. Paris 1989, 309, 53–58. [Google Scholar]
- Hofmann, B.A.; Farmer, J.D. Filamentous fabrics in low-temperature mineral assemblages: Are they fossil biomarkers? Implications for the search for a subsurface fossil record on the early Earth and Mars. Planet Space Sci. 2000, 48, 1077–1086. [Google Scholar] [CrossRef]
- Hofmann, B.A.; Farmer, J.D.; von Blanckenburg, F.; Fallick, A.E. Subsurface filamentous fabrics: An evaluation of possible modes of origins based on morphological and geochemical criteria, with implications for exopalaeontology. Astrobiology 2008, 8, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Roedder, E. Fluid Inclusions. Rev. Mineral. 1984, 12, 646p. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry, 4th ed.; Springer: Berlin, Germany, 1997; 201p. [Google Scholar]
- Götze, J.; Möckel, R.; Vennemann, T.; Müller, A. Origin and geochemistry of agates from Permian volcanic rocks of the Sub-Erzgebirge basin (Saxony, Germany). Chem. Geol. 2016, 428, 77–91. [Google Scholar] [CrossRef]
- Hurlbut, C.S. Powellite from Nashik, India. Mineral. Rec. 1982, 13, 310. [Google Scholar]
- Phadke, A.V. Powellite a Rare Paragnesis with Cavity Minerals in Deccan Traps of Western Maharashtra, India, 14; IMA Poster Session I: Stanford, CA, USA, 1986. [Google Scholar]
- Fleming, T.H.H.; Foland, K.A.; Elliot, D.H. Apophyllite 40Ar/39Ar and Rb-Sr geochronology: Potential utility and application to the timing of secondary mineralization of the Kirkpatrick Basalt, Antarctica. J. Geophys. Res. 1999, 104, 20081–20095. [Google Scholar] [CrossRef]
- Rossman, G.R. Optical spectroscopy of green vanadium apophyllite from Poona, India. Am. Mineral. 1974, 59, 621–622. [Google Scholar]
- Peretyazhko, T.; Sutter, B.; Morris, R.V.; Agresti, D.G.; Le, L.; Ming, D.W. Smectite Formation from Basaltic Glass under Acidic Conditions on early Mars. Geochimica et Cosmochimica Acta 2016, 173, 37–49. [Google Scholar] [CrossRef]
- Polgári, M.; Hein, J.R.; Németh, T.; Pál-Molnár, E.; Vigh, T. Celadonite and smectite formation in the Úrkút Mn-carbonate ore deposit (Hungary). Sediment. Geol. 2013, 294, 157–163. [Google Scholar] [CrossRef]
- Seyfried, W.E.; Bischoff, J.L. Low temperature basalt alteration by seawater: An experimental study at 70 °C and 150 °C. Geochimica et Cosmochimica Acta 1979, 43, 1937–1947. [Google Scholar] [CrossRef]
- Treiman, A.H.; Morris, R.V.; Agresti, D.G.; Graff, T.G.; Achilles, C.N.; Rampe, E.B.; Bristow, T.F.; Ming, D.W.; Blake, D.F.; Vaniman, D.T.; et al. What Lurks in the Martian Rocks and soil? Investigations of Sulfates, Phosphates, and Perchlorates. Am. Mineral. 2014, 99, 2234–2250. [Google Scholar] [CrossRef]
- Pradeep, K.S.; Devdutt, V.U.; Vibhuti, W. Alteration of Volcanic Glass to well-crystallized Ferrosaponite in the Vesicles of the Deccan Trap Basalts at Bhuleshwar Ghat Section, Pune District, Maharashtra. J. Geol. Soc. India 2016, 88, 22–28. [Google Scholar]
- Self, C.A.; Hill, C.A. How Speleothems Grow: An Introduction to the Ontogeny of Cave Minerals. J. Cave Karst Stud. 2003, 65, 130–151. [Google Scholar]
- Self, C.A. The International Organization of Speleothems. Acta Carsol. 2004, 33, 245–255. [Google Scholar]
- Hill, C.; Forti, P. Cave Mineralogy and the NSS: Past, present, future. J. Caves Karst Stud. 1996, 69, 35–45. [Google Scholar]
- Morteani, G.; Kostitsyn, Y.; Preinfalk, C.; Gilg, H.A. The genesis of the amethyst geodes at Artigas (Uruguay) and the paleohydrology of the Guaraní aquifer: Structural, geochemical, oxygen, carbon, strontium isotope and fluid inclusion study. Int. J. Earth Sci. 2009. [Google Scholar] [CrossRef]
- Barge, L.M.; Cardoso, S.S.; Cartwright, J.H.; Cooper, G.J.; Cronin, L.; De Wit, A.; Doloboff, I.J.; Escribano, B.; Goldstein, R.E.; Haudin, F.; et al. From chemical gardens to chemobrionics. Chem. Rev. 2015, 115, 8652–8703. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J.H.E.; García-Ruiz, J.M.; Novella, M.L.; Otálora, F. Formation of chemical gardens. J. Colloid Interface Sci. 2002, 256, 351–359. [Google Scholar] [CrossRef]
- Thewalt, U.; Dörfner, G. Wie kommt das Moos in den Achat und wie nicht? Der Aufschluss 2012, 63, 1–16. [Google Scholar]
- Ruiz, J.M.; Nakouzi, E.; Kotopoulou, E.; Tamborrino, L.; Steinbock, O. Biomimetic mineral self-organization from silica-rich spring waters. Sci. Adv. 2017, 3, 1194–1197. [Google Scholar]
- Kellermeier, M.; Cölfen, H.; García-Ruiz, J.M. Silica Biomorphs: Complex Biomimetic Hybrid Materials from “Sand and Chalk”. Eur. J. Inorg. Chem. 2012, 32, 5123–5144. [Google Scholar] [CrossRef]
- Konhauser, K.O. Diversity of bacterial iron mineralization. Earth Sci. Rev. 1998, 43, 91–121. [Google Scholar] [CrossRef]
- Tazaki, K. Biomineralization of layer silicates and hydrated Fe/Mn Oxides in Microbial Mats: An Electron Microsopical Study. Clays Clay Miner. 1997, 45, 203–212. [Google Scholar] [CrossRef]
- Posth, N.R.; Canfield, D.E.; Kappler, A. Biogenic Fe(III) minerals: From formation to diagenesis and preservation in the rock record. Earth Sci. Rev. 2014, 135, 103–121. [Google Scholar] [CrossRef]
- Fortin, D.; Ferris, F.G.; Scott, D. Formation of Fe-silicates and Fe-oxides on bacterial surfaces in samples collected near hydrothermal vents on the Southern Explorer Ridge in the northeast Pacific Ocean. Am. Mineral. 1998, 83, 1399–1408. [Google Scholar] [CrossRef]
- Dublansky, Y.V.; Smirnov, S.Z. Cavity-based secondary mineralization in volcanic tuffs of Yucca Mountain, Nevada: A new type of the polymineral vadose speleothem, or a hydrothermal deposit? Int. J. Speleol. 2005, 34, 25–44. [Google Scholar] [CrossRef]
- Schmitt-Riegraf, C. Magmenentwicklung und Spät-Bis Post-Magmatische Alterationsprozesse Permischer Vulkanite im Nordwesten der Nahe-Mulde; Münstersche Forschungen zur Geologie und Paläontologie: Münster, Germany, 1996; Volume 80, 251p. [Google Scholar]
- Götze, J.; Tichomirowa, M.; Fuchs, H.; Pilot, J.; Sharp, Z. Geochemistry of agates: A trace element and stable isotope study. Chem. Geol. 2001, 175, 523–541. [Google Scholar] [CrossRef]
- Blankenburg, H.-J. Achat; Deutscher Verlag für Grundstoffindustrie: Leipzig, Germany, 1988; 203p. [Google Scholar]
- Keller, J.; Hoefs, J. Stable isotope characteristics of recent natrocarbonatites from Oldoinyio Lengai. In Carbonatite Volcanism—Oldoinyio Lengai and the Petrogenesis of Natrocarbonatites; IAVCEI Proceedings of Volcanology; Bell, K., Keller, J., Eds.; Springer: Berlin, Germany, 1995; Volume 4, pp. 113–123. [Google Scholar]
- O’Neil, J.R.; Clayton, R.N.; Mayeda, T.K. Oxygen isotope fractionation in divalent metal carbonates. J. Chem. Phys. 1969, 51, 5547–5558. [Google Scholar] [CrossRef]
- Larson, S.A.; Tullborg, E.-L. Stable isotopes of fissure-filling calcite from Finnsjön, Uppland, Sweden. Lithos 1984, 17, 117–125. [Google Scholar] [CrossRef]
- Bottomley, D.J. The isotope geochemistry of fracture calcites from the Chalk River area, Ontario, Canada. Appl. Geochem. 1987, 2, 81–91. [Google Scholar] [CrossRef]
- Wallin, B.; Petermann, Z. Calcite fracture fillings as indicators of paleohydrology at Laxemar at the Äspö hard rock laboratory, southern Sweden. Appl. Geochem. 1999, 14, 953–962. [Google Scholar] [CrossRef]
- Kostov, I. Zeolitization processes in trap volcanics. In Deccan Volcanism and related Basalt Provinces in Other Parts of the World; Subbarao, K.V., Sukheswala, R.N., Eds.; Mem. 3, Geol. Soc. of India: Bangalore, India, 1981; pp. 428–433. [Google Scholar]
- Barth-Wirsching, U.; Holler, H. Experimental studies on zeolite formation conditions. Eur. J. Mineral. 1989, 1, 489–506. [Google Scholar] [CrossRef]
- Wirsching, U. Experiments on the hydrothermal formation of calcium zeolites. Clays Clay Miner. 1981, 29, 71–183. [Google Scholar] [CrossRef]
- Coombs, D.S. Recommended nomenclature for Zeolite minerals: Report of the subcommittee on Zeolites of the International Mineralogical Association, Commission on new minerals and mineral names. Can. Mineral. 1997, 35, 1571–1606. [Google Scholar]
- Embrey, P.G.; Couper, A.G. The Morphology of a Large Powellite Crystal from Nasik, India. Mineral. Rec. 1982, 5, 311–313. [Google Scholar]
- Liang, Y.H.; Halliday, A.N.; Siebert, C.; Fitton, J.G.; Burton, K.W.; Wang, K.L.; Harvey, J. Molybdenum isotope fractionation in the mantle. Geochim. Cosmochim. Acta 2017, 199, 91–111. [Google Scholar] [CrossRef] [Green Version]
- Mookherjee, A. Microthermometry and P-V-T-X Determinations of Fluid Inclusions in Some Cavity Minerals from the Western part, of the Deccan Volcanic Province, the Deccan Volcanological Society, Pune. Newsletter 2009, III, 3–8. [Google Scholar]
- Gottardi, G. The genesis of zeolites. Eur. J. Mineral. 1989, 1, 479–487. [Google Scholar] [CrossRef]
- Molzahn, M.; Wörner, G.; Hejes-Kunst, F.; Rocholl, A. Constraints on the Cretaceous thermal event in the Transantarctic Mountains from alteration processes in Ferrar flood basalts. Glob. Planet Chang. 1999, 23, 45–60. [Google Scholar] [CrossRef]
- Chukrov, F.V.; Yermilava, L.P.; Shanin, L.L. The age problem of apophyllite in late hydrothermal mineral associations. Int. Geol. Rev. 1973, 16, 417–426. [Google Scholar] [CrossRef]
- Purdy, J.W.; Jäger, E. K-Ar ages on rock-forming minerals from the Central Alps. Memorie degli Istituti di Geologia e Mineralogia dell’Università de Padova 1976, 30, 3–33. [Google Scholar]
- Jäger, E. Introduction to Geochronology. In Lectures in Isotopegeology; Jäger, E., Hunziger, J.C., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1979; pp. 1–12. [Google Scholar]
- Gilg, H.A.; Morteani, G.; Kostitsyn, Y.; Preinfalk, C.; Gatter, I.; Strieder, A.J. Genesis of amethyst geodes in basaltic rocks of the Serra Geral Formation (Ametista do Sul, Rio Grande do Sul, Brazil): A fluid inclusion, REE, oxygen, carbon, and Sr isotope study on basalt, quartz, and calcite. Miner. Depos. 2003, 38, 1009–1025. [Google Scholar] [CrossRef]
- Gilg, H.A.; Krüger, Y.; Taubald, H.; van den Kerkhof, A.M.; Frenz, M.; Morteani, G. Mineralisation of amethyst-bearing geodes in Ametista do Sul (Brazil) from low-temperature sedimentary brines: Evidence from monophase liquid inclusions and stable isotopes. Miner. Depos. 2014. [Google Scholar] [CrossRef]
- Faust, G.T. A Review and Interpretation of the Geologic Setting of the Watchung Basalt Flows, New Jersey; US Geological Survey Professional Paper 864-A; US Geological Survey Professional: Washington, DC, USA, 1975; 41p.
- Mason, B.H. Trap Rock Minerals of New Jersey. N. J. Geol. Surv. Bull. 1960, 64, 1–51. [Google Scholar]
- Kent, B.P.; Butkoswski, B. Minerals of the Millington quarry, Somerset County, New Jersey. Mineral. Rec. 2000, 31, 399–411. [Google Scholar]
- Laskowich, C.; Puffer, J.H. Prehnite and Zeolite distribution in the Orange Mountain Basalt Paterson, New Jersey. Mineral. Record 2016, 47, 479–490. [Google Scholar]
- Sahagian, D.L.; Proussevitch, A.A.; Carslon, W.D. Analysis of Visicular Basalts and Lava Empalacement processes for Application as a Palaobarometer/Paleoaltimeter. J. Geol. 2002, 110, 671–685. [Google Scholar] [CrossRef]




















| Altitude asl [m] | Nashik 1 720-650 | Nashik 2 650-600 | Pune 680-590 | Savda 230-220 | Malad 40-30 |
|---|---|---|---|---|---|
| apophyllite | f | f | f | f | c |
| calcite | c | c | o | f | f |
| Ca-Fe minerals* | c | ||||
| cavansite | c | ||||
| chabazite | r | ||||
| clay minerals | f | f | f | f | f |
| epistilbite | r | r | |||
| fluorite | r | ||||
| goosecreekite | vr | ||||
| gyrolite-okenite | f | ||||
| heulandite | f | f | f | f | r |
| laumontite | c | c | |||
| mesolite | c | ||||
| mordenite | r | ||||
| natrolite | r | ||||
| powellite | vr | vr | |||
| prehnite | c | ||||
| quartz-chealcedony | f | f | f | c | |
| scolecite | c | r | |||
| stilbite-stellerite | f | f | f | f | r |
| yugawaralite | vr |
| Sample Number | Sample Material | Locality | Coordinates (WGS84) | |
|---|---|---|---|---|
| N | E | |||
| JAL-1 | Cal III, Stb and Apo crystals from single cavity | Jalgaon, Savda | 20° 59’ 12.1″ | 75°26’26.7″ |
| JAL-2 | Cal III, Stb and Apo crystals from single cavity | Jalgaon, Savda | 20° 59’ 12.2″ | 75°26’56.8″ |
| JAL-05 | complex filament, handspecimen | Jalgaon, Savda | 20° 59’ | 75°27’ |
| JAL-11 | single filament | Jalgaon, Savda | 20° 59’ | 75°27’ |
| JAL-12A | Apo crystals from single filament | Jalgaon, Savda | 20° 59’ 05.7″ | 75°27’04.1″ |
| JAL-13A | basalt with clay mineral, handspecimen | Jalgaon, Savda | 20° 59’ 05.5″ | 75°27’01.2″ |
| JAL-13B | basalt, handspecimen | Jalgaon, Savda | 20° 59’ 05.5″ | 75°27’01.2″ |
| JAL-13C | Apo crystal, 4 × 4 × 1 cm | Jalgaon, Savda | 20° 59’ 05.5″ | 75°27’01.2″ |
| JAL-13D | Apo crystal, 4 × 4 × 1 cm | Jalgaon, Savda | 20° 59’ 05.5″ | 75°27’01.2″ |
| JAL-16B | Qtz, Apo 1, Cal III, Apo 2 on handspecimen | Jalgaon, Savda | 20° 59’ 13.5″ | 75°27’01.2″ |
| JAL-24 | clay mineral on handspecimen | Jalgaon, Savda | 20° 59’ | 75°27’ |
| JAL-27 | clay mineral on handspecimen | Jalgaon, Savda | 20° 59’ | 75°27’ |
| JAL-31 | Cal I overgrown by Cha, 5 × 2 × 2 cm | Jalgaon, Savda | 20° 59’ | 75°27’ |
| JAL-32 | Cha, Cal I, Heu, Cal II on basalt, handspecimen | Jalgaon, Savda | 20° 59’ | 75°27’ |
| JAL-33 | Cha, Cal II, Apo on handspecimen | Jalgaon, Savda | 20° 59’ | 75°27’ |
| JAL-CHO | wallrock with clay mineral, Cha and Mor | Jalgaon, Savda | 20° 59’ | 75°27’ |
| JAL-SM-01 | filament without Cha overgrown | Jalgaon, Savda | 20° 59’ | 75°27’ |
| JAM-01 | section of single filament | Jamner | 20° 47’ | 75°44’ |
| JAM-10 | section of a complex filament | Jamner | 20° 47’ | 75°44’ |
| JLN-01 | section of a complex filament | Jalna | 19° 49.3’ | 75°51.1’ |
| NAS-6 | Stb and Apo on handspecimen | Nashik, Panduleni | 19° 56’ 24″ | 73°43’13″ |
| NAS-9 | Stb and Apo on handspecimen | Nashik, Dindori | 20° 08’ 39″ | 73°49’25″ |
| NAS-10 | Heu, Stb and Apo on handspecimen | Nashik, Mohu | 19° 54’ 24.8″ | 73°56’36.9″ |
| NAS-11 | Qtz, Stb and Apo on handspecimen | Nashik, Mahodari | 19° 52’ 51.2″ | 73°56’26.0″ |
| Sample | XRD, SEM/EDX (Table 4) | Fluid Incl. (Table 5) | C and O Isotope (Table 6) | Mineral Chemistry (Table 7 and Table 8) | Rb-Sr Dating (Table 9) | K-Ar Dating (Table 10) |
|---|---|---|---|---|---|---|
| JAL-1 | Cal III, Stb, Apo | |||||
| JAL-2 | Cal III | Stb, Apo | ||||
| JAL-05 | Clay min. | |||||
| JAL-11 | Zeo, Clay min. | |||||
| JAL-12A | Clay min. | Apo | Apo | |||
| JAL-13A | Clay min. | Cel | ||||
| JAL-13B | Apo | whole rock | ||||
| JAL-13C | Apo-core/rim | Apo-core/rim | ||||
| JAL-13D | Apo | Apo | ||||
| JAL-16B | Qtz, Cal III | Cal III, Apo 1. Apo 2 | Apo | |||
| JAL-24 | Clay min. | |||||
| JAL-27 | Clay min. | |||||
| JAL-31 | Cal I | Cal I | ||||
| JAL-32 | Cha, Cal I + II | Cal I +II | ||||
| JAL-33 | Cal II | Cal II | ||||
| JAL-CHO | Clay min. | |||||
| JAL-SM-01 | Clay min. | |||||
| JAM-01 | Clay min. | |||||
| JAM-10 | Zeo, Clay min. | |||||
| JLN-01 | Clay min. | |||||
| NAS-6 | Stb, Apo | Apo | ||||
| NAS-9 | Stb, Apo | |||||
| NAS-10 | Stb, Apo | Apo | ||||
| NAS-11 | Stb, Apo | Apo |
| Sample | Occurrence | Color | Method | Mineral | Fe [wt%] |
|---|---|---|---|---|---|
| JAL-SM-01 | filament | brown | SEM-EDX | saponite | 4.9–7.4 |
| JAL-05 | filament | black | SEM-EDX | saponite | 17.1–19.7 |
| JAL-11 | filament | brown | SEM-EDX | saponite | 2.5–5.6 |
| JAM-01 | filament | olive | SEM-EDX | glauconite | 12.7–16.7 |
| JAM-10 | filament | green | SEM-EDX | celadonite | 24.2–26.6 |
| JLN-01 | filament | green | SEM-EDX | celadonite | 16.6–22.5 |
| JAL-12A | wall lining | green | XRD | celadonite | - |
| JAL-13A | wall lining | green | XRD | celadonite | - |
| JAL-24 | wall lining | brown | XRD | saponite | - |
| JAL-27 | wall lining | brown | XRD | saponite | - |
| JAL-CHO | wall lining | green | XRD | celadonite | - |
| Sample | Host | n | System | Phase | Te(Ice) [°C] | Tm(Ice) [°C] | Th (L) [°C] | p [g/cm3] |
|---|---|---|---|---|---|---|---|---|
| JAL-16B | Qtz | 15 | H2O-NaCl ± KCl ± MgCl2 | L + V | ~ −34 | −0.5 to 0.0 | 140.0 to 142.5 | 0.93 |
| JAL-31 | Cal I | 15 | H2O-NaCl ± KCl ± MgCl2 | L + V | −37.9 to −40 | −1.5 to −0.5 | 101.2 to 157.6 | 0.96 to 0.92 |
| JAL-32 | Cal I | 15 | H2O-NaCl ± KCl ± MgCl2 | L + V | ~ −35 | −1.0 to 0.0 | 105.0 to 134.3 | 0.96 to 0.94 |
| JAL-32 | Cha | 5 | H2O-NaCl ± KCl ± MgCl2 | L + V | n.o. | −0.5 to 0.0 | n.r. | -------- |
| JAL-2 | Cal III | 12 | H2O-NaCl ± KCl ± MgCl2 | L + V | ~ −27 | −0.6 to −0.5 | 167.0 to 254.2 | 0.9 to 0.7 |
| JAL-32 | Cal II | 12 | H2O-NaCl ± KCl ± MgCl2 | L + V | −32.0 to −39.0 | −0.4 to 0.0 | 94.1 to 149.0 | 0.96 to 0.92 |
| JAL-33 | Cal II | 18 | H2O-NaCl ± KCl ± MgCl2 | L + V | ~ −30 | −0.1 to 0.0 | 116.7 to 173.7 | 0.95 to 0.89 |
| JAL-16B | Cal III | 25 | H2O-NaCl ± KCl ± MgCl2 | L + V | −35.0 to −40.0 | −0.8 to −0.2 | 144.2 to 246.4 | 0.93 to 0.80 |
| JAL-13D | Apo | 20 | H2O-NaCl ± KCl ± MgCl2 | L + V | −29.0 to −31.0 | −0.1 to 0.0 | 141.1 to 222.2 | 0.94 to 0.85 |
| Sample | Mineral | δ13C VPDB | δ13C VPDB | δ18O SMOW |
|---|---|---|---|---|
| JAL-31 | Cal I | −10.00 | −15.30 | +15.13 |
| JAL-31 | Cal I | −10,28 | −15.32 | +14.91 |
| JAL-32 | Cal I | −10,95 | −15.55 | +14.88 |
| JAL-32 | Cal I | −10,94 | −15.39 | +15.05 |
| JAL-32 | Cal II | −13.61 | −6.84 | +23.86 |
| JAL-32 | Cal II | −13.68 | −8.06 | +22.60 |
| JAL-33 | Cal II | −15.11 | −10.96 | +19.61 |
| JAL-33 | Cal II | −13.81 | −2.93 | +27.89 |
| Label | JAL-13D-8 | JAL-13D-9 | JAL-13D-10 | JAL-13D-11 | JAL-13D-12 | JAL-13D-13 | JAL-16B-6 | JAL-16B-7 | JAL-16B-8 | JAL-16B-13 | JAL-16B-14 | JAL-16B-16 | JAL-16B-17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 52,76 | 52,26 | 52,78 | 52,85 | 52,46 | 52,08 | 52,52 | 52,78 | 52,75 | 52,88 | 52,80 | 52,72 | 52,25 |
| Al2O3 | 0,18 | 0,33 | 0,38 | 0,34 | 0,32 | 0,28 | 0,23 | <0.1 | 0,19 | 0,25 | <0.1 | 0,24 | 0,39 |
| CaO | 23,95 | 23,70 | 23,83 | 23,75 | 23,78 | 23,80 | 23,82 | 23,76 | 23,96 | 24,05 | 23,90 | 23,81 | 23,73 |
| K2O | 4,88 | 4,99 | 4,89 | 4,82 | 4,76 | 4,77 | 5,19 | 4,99 | 5,09 | 4,97 | 5,15 | 4,96 | 4,97 |
| Na2O | 0,18 | 0,21 | 0,19 | 0,25 | 0,21 | 0,19 | 0,09 | 0,09 | 0,05 | 0,09 | 0,14 | 0,09 | 0,24 |
| F | 2,13 | 2,07 | 2,12 | 2,08 | 2,03 | 2,10 | 2,04 | 2,09 | 1,94 | 1,93 | 2,13 | 1,99 | 2,18 |
| H20* | 15,81 | 15,66 | 15,81 | 15,83 | 15,72 | 15,60 | 15,73 | 15,81 | 15,80 | 15,84 | 15,82 | 15,79 | 15,65 |
| Sum | 99,88 | 99,21 | 100,00 | 99,92 | 99,27 | 98,82 | 99,62 | 99,52 | 99,77 | 100,00 | 99,94 | 99,60 | 99,42 |
| H20* = calculated | |||||||||||||
| Si | 8,000 | 8,000 | 8,000 | 8,000 | 8,000 | 8,000 | 8,000 | 8,000 | 8,000 | 8,000 | 8,000 | 8,000 | 8,000 |
| Al | 0,032 | 0,059 | 0,068 | 0,060 | 0,057 | 0,051 | 0,042 | - | 0,034 | 0,044 | - | 0,043 | 0,071 |
| Ca | 3,891 | 3,886 | 3,869 | 3,851 | 3,885 | 3,918 | 3,887 | 3,859 | 3,894 | 3,898 | 3,879 | 3,872 | 3,893 |
| K | 0,944 | 0,975 | 0,945 | 0,931 | 0,926 | 0,935 | 1,008 | 0,964 | 0,984 | 0,959 | 0,996 | 0,959 | 0,970 |
| Na | 0,053 | 0,063 | 0,055 | 0,073 | 0,063 | 0,056 | 0,027 | 0,025 | 0,015 | 0,026 | 0,042 | 0,028 | 0,071 |
| F | 1,020 | 1,001 | 1,018 | 0,997 | 0,977 | 1,019 | 0,984 | 1,000 | 0,929 | 0,923 | 1,021 | 0,954 | 1,057 |
| Sum | 13,94 | 13,98 | 13,96 | 13,91 | 13,91 | 13,98 | 13,95 | 13,85 | 13,86 | 13,85 | 13,94 | 13,86 | 14,06 |
| K/Ca | 0,243 | 0,251 | 0,244 | 0,242 | 0,238 | 0,239 | 0,259 | 0,250 | 0,253 | 0,246 | 0,257 | 0,248 | 0,249 |
| Sample | Material | Labrat. | K | K20 | 40Ar rad | 40Ar rad | K-Ar Age | Rb-Sr Age | Diffenence |
|---|---|---|---|---|---|---|---|---|---|
| Number | [wt%] | [wt%] | [ccSTP/g] | [%] | [Ma] (± 1s) | [Ma] (± 2s) | K-Ar/Rb-Sr [%] | ||
| JAL-12A | Apo | 8780 | 4,036 | 4,862 | 5,6084E-06 | 35,3 | 35.4 ± 1.4 | 46.1 ± 0.5 | 23,2 |
| JAL-13C | Apo-core | 8737/2 | 3,854 | 4,643 | 6,8065E-06 | 42,5 | 44.9 ± 1.6 | 48.8 ± 0.4 | 8,0 |
| JAL-13C | Apo-rim | 8737/1 | 3,829 | 4,612 | 6,6535E-06 | 52,1 | 44.2 ± 1.4 | 47.0 ± 0.6 | 6,0 |
| JAL-16B | Apo | 8778 | 4,088 | 4,924 | 1,6500E+01 | 16,5 | 22.2 ± 1.8 | 25.2 ± 0.3 | 34,5 |
| NAS-6 | Apo | 8781 | 4,053 | 4,882 | 6,3391E-06 | 23,1 | 39.8 ± 2.4 | 58.7 ± 0.6 | 32,2 |
| NAS-10 | Apo | 8779 | 3,965 | 4,776 | 3,1475E-06 | 36,7 | 20.3 ± 0.8 | 22.1 ± 0.2 | 8,1 |
| NAS-11 | Apo | 8738 | 4,016 | 4,838 | 3,1597E-06 | 18,4 | 20.1 ± 1.5 | 21.4 ± 0.2 | 6,1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottens, B.; Götze, J.; Schuster, R.; Krenn, K.; Hauzenberger, C.; Zsolt, B.; Vennemann, T. Exceptional Multi Stage Mineralization of Secondary Minerals in Cavities of Flood Basalts from the Deccan Volcanic Province, India. Minerals 2019, 9, 351. https://doi.org/10.3390/min9060351
Ottens B, Götze J, Schuster R, Krenn K, Hauzenberger C, Zsolt B, Vennemann T. Exceptional Multi Stage Mineralization of Secondary Minerals in Cavities of Flood Basalts from the Deccan Volcanic Province, India. Minerals. 2019; 9(6):351. https://doi.org/10.3390/min9060351
Chicago/Turabian StyleOttens, Berthold, Jens Götze, Ralf Schuster, Kurt Krenn, Christoph Hauzenberger, Benkó Zsolt, and Torsten Vennemann. 2019. "Exceptional Multi Stage Mineralization of Secondary Minerals in Cavities of Flood Basalts from the Deccan Volcanic Province, India" Minerals 9, no. 6: 351. https://doi.org/10.3390/min9060351
APA StyleOttens, B., Götze, J., Schuster, R., Krenn, K., Hauzenberger, C., Zsolt, B., & Vennemann, T. (2019). Exceptional Multi Stage Mineralization of Secondary Minerals in Cavities of Flood Basalts from the Deccan Volcanic Province, India. Minerals, 9(6), 351. https://doi.org/10.3390/min9060351






