Abstract
Red mud (RM) is a by-product of extracting of alumina from bauxite. Red mud contains high quantities of alkali-generating minerals and metal ions, which can cause significant environmental damage. Many valuable components such as rare-earth elements, Al, and Fe, in RM are difficult to be utilized owing to their particle size and alkalinity. Thus, developing an economical and efficient technology to consume a large amount of RM can efficiently solve RM disposal issues. This paper systematically reviews the comprehensive utilization methods for reducing RM environmental pollution and divides the comprehensive utilization of RM into three aspects: the effective extraction of valuable components, resource transformation, and environmental application. Based on resource, economic, and environmental benefits, the development of new technologies and new processes with market competitiveness, environmental protection, and ecological balance should be the prerequisite for the low-energy, low-pollution, low-cost, and high-efficiency comprehensive utilization of RM. The direction of future research to solve RM disposal issues is also suggested.
1. Introduction
Bauxite residue is a strong alkaline solid waste discharged during the industrial production of alumina [1]. The color of bauxite residue is red because of its rich iron (III) oxides, comprising approximately 20–40% of its mass [2]. This property explains its common name, red mud (RM) [3]. For 1 t of alumina produced, approximately 1.5 t of RM is discharged [4]. According to the statistics, the global stock of RM has reached nearly 4 billion tons by 2015 [5], and also with the annual production increasing by approximately 150 million tons [6,7]. In China, the amount of RM discharged is 88 million tons per year, and the total stock of RM is over 0.6 billion tons [8]. Table 1 shows the annual output of RM in different countries, and Table 2 shows the production of RM in different provinces of China.

Table 1.
Annual production of red mud (RM) in some countries [9]. Reproduced with permission from Mukiza et al., Resources, Conservation and Recycling; published by Elsevier, 2019 [9].

Table 2.
The production of RM in different provinces of China in 2016 [9]. Reproduced with permission from Mukiza et al., Resources, Conservation and Recycling; published by Elsevier, 2019 [9].
Given its high alkalinity and contents of trace radioactive elements, and potentially harmful elements (PHEs), RM causes serious environmental problems, such as surface water and groundwater pollution [9,10,11,12]. RM also causes air pollution, because its fine-grained particles are easily dispersed by the wind [13,14]. The large storage of RM requires money to build and manage bauxite residues dams, as well as leads to substantial environmental problems, such as the leaking of bauxite residue slurry caused by damage to pipelines, failure of retaining dams, of leaching of alkaline solution from the containing barrier [15].
The key to solve environmental problems caused by RM is to develop technologies which can convert RM into secondary resource and consume a large amount of RM or designed depositories for long term storage [6,16]. Belviso et al. successfully synthesized magnetic zeolite at low temperature with fly ash and RM which was used as aluminum source [17,18]. At present, the treatment and utilization of RM are concentrated on metal recovery and the manufacture of adsorbents, ceramics, bricks, high-performance concrete admixtures, and road base materials [19,20,21,22,23,24,25].
Considering the abundant valuable components, including Fe, Al, Ti, and rare earth elements, such as thorium (20–30 g/t), uranium (50–60 g/t), gallium (60–80 g/t), yttrium (60–150 g/t), and scandium (60–120 g/t), etc. [26], RM is a valuable secondary resource. In recent years, although much effort and various studies have been made to recycle these valuable components of RM through hydrometallurgical and pyrometallurgical methods, these methods are not effective for the large-scale utilization of RM until now [27,28,29,30].
In 2011, the output of RM in China was 42.6 million tons with a utilization rate of 5.2%; in 2013, it reached 73 million tons, and the utilization rate dropped to 4% [19]. Thus, a major proportion of RM was unutilized. The failure of the application of RM in large quantities can be attributed to its high alkalinity, economic cost, technical restrictions, the public consideration of the toxicity of RM leaching, and the market demand. Moreover, the mineral and chemical components of RM vary greatly with the source and processing conditions of bauxite; therefore, no unified method and standard are available for the treatment of RM [31]. With the increasing need for practices that minimise environmental and public health burdens, the utilization of RM is imperative.
This paper describes the basic characteristics and mineral compositions of RM. The research progress of the comprehensive recovery of valuable metals and large-scale utilization of RM in recent years is also summarized. The study aims to provide some suggest perspectives and valuable information for further investigation of RM.
2. Production and Characteristics of RM
2.1. Production of RM
Aluminum is the largest produced non-ferrous metal that is widely used in construction, air transport, automobile production, daily supplies, domestic appliances, and mechanical equipment. At present, 28 billion tons of bauxite reserves are identified in the world [32]. The base bauxite reserves in China are 3.7 billion tons, ranking the fifth in the world, followed by Guinea, Australia, Brazil, and Jamaica [33]. The distribution of bauxite resources in China is relatively concentrated. Approximately 98% of the resources are concentrated in Guizhou, Guangxi, Henan, Shanxi, Yunnan, and Shandong provinces [28].
Around the world, approximately 95% of bauxite is used to produce alumina [34]. Considering the different raw materials used for bauxite, the methods for alumina production by alkaline process also vary. Generally, the Bayer process, Bayer–sintering combination, and sintering process are applied, among which Bayer process is used in 95% of alumina production owning to the low energy consumption and simple production process [9]. Bauxite ores is milled first, pre-desilication using Ca(OH)2 at temperature 100 °C, and digested in high concentration of NaOH from 145–250 °C [35]. Under such circumstance, aluminum will be dissolved in solution, and the undissolved components of bauxite are called RM.
2.2. Characteristics of RM
The composition and properties of RM depend on the bauxite ore sources and production processes. Table 3 and Table 4 list the mineral and chemical constituents of RM by using different production methods. The main chemical constituents of RM are CaO, Al2O3, Fe2O3, SiO2, TiO2, and Na2O. RM produced from the Bayer method has much higher contents of Fe2O3 and Al2O3 than that by the two other methods. The properties of RM produced by the combination method are similar to those produced by sintering. The contents of CaO and SiO2 in RM produced by the combined process and sintering process are much higher than that produced by the Bayer process.
Table 4 shows that the RM produced by the combined process and sintering process is characterized by high calcium and silicon, with the main phase being 2CaO∙SiO2 and a mass ratio close to 50%. The main mineral constituents of the RM produced by the Bayer process are boehmite, anorthite, and sodium aluminosilicate.
Table 5 shows the main chemical constituents of RM in Australia, India, and in Shandong, Henan, and Shanxi provinces of China. Considering the different mineral compositions and grades, bauxite at different countries adopts different alumina production processes. Among which, the bauxites from Australia and India were found to be dealt with by Bayer process, while the RM samples from Shandong, Henan, and Shanxi provinces in China were identified to be produced by sintering process, sintering process, and Bayer process, respectively. The content of iron in RM found in China was lower than those in Australia and India.
Notably, although the content of the single elements is not very high, the total amount of resources is huge considering the various valuable components because of the abundance of RM in the word. For example, the RM produced in the Shandong Aluminum Plant, China contains approximately 30% ferric oxide, 18% alumina, and approximately 8% sodium oxide. The calculation based on 1 million tons of the annual output of alumina and 1.2 million tons of RM produced revealed that more than 360,000 tons of ferric oxide, 210,000 tons of alumina, and almost 100,000 tons of sodium oxide were found. Considering the growing shortage of mineral resources, increasing attention has been paid to study the resource recycling technology.
Particle size analyzers, scanning electron microscope, and other instruments were used to characterize and observe RM [36]. The RM was strongly alkaline, has a high salt content and electrical conductivity (EC) dominated by sodium (Na+) and has compacted particles (high bulk density (ρ)). Besides, RM exits by the aggregation of uniformly dispersed particles, with an average particle size of less than 10 µm. Among which, particles with size less than 75 µm account for nearly 90% of the total amount [36]. The RM particles have large specific surface areas. The specific surface areas of different RMs ranged from 15–58 m2/g, with properties similar to those of porous materials [37]. Few rare-earth and radioactive elements exist in RM, such as scandium (60–120 g/ton), gallium (60–80 g/ton), yttrium (60–150 g/ton), and uranium (50–60 g/ton) [38]. Table 6 shows some typical physical and chemical characteristics of the residues of RM.




Table 3.
The chemical constituents of RM using different production methods (%) [39].
Table 3.
The chemical constituents of RM using different production methods (%) [39].
| Chemical Constituents | CaO | Al2O3 | Fe2O3 | SiO2 | TiO2 | Na2O | K2O | MgO | Nb2O5 | Sc2O3 | TREO | Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sintering process | 38.09 | 9.18 | 6.66 | 18.1 | 6.72 | 4 | - | - | 0.0193 | 0.02 | 0.25 | 16.96 |
| Combined process | 40.78 | 7.68 | 10.97 | 22.67 | 3.26 | 2.93 | 0.38 | 1.77 | - | - | - | 11.77 |
| Bayer process | 20.88 | 17.67 | 28.3 | 8.34 | 7.34 | 2.29 | 0.059 | 0.65 | - | - | - | 13.88 |

Table 4.
The mineral constituents of RM (%, ω) [40].
Table 4.
The mineral constituents of RM (%, ω) [40].
| Mineral Constituents | Bayer Process | Combined Process | Sintering Process |
|---|---|---|---|
| Boehmite Al2O3·H2O | 21 | 143 | - |
| Anorthite 3CaO∙Al2O3∙3Si2O2 or 3CaO∙Al2O3∙xSiO2∙(6 − 2x)H2O | 20 | 2 | 5 |
| Sodium aluminosilicate (Na2O∙Al2O3∙1.7SiO2∙nH2O)·NaX or Na2X | 20 | 4 | 4 |
| 2CaO∙SiO2 | - | 43 | 46 |
| Calcite CaCO3 | 19 | 10 | 14 |
| Na2O∙Al2O3∙2SiO2 | - | 8 | 7 |
| CaO∙TiO2 | 15 | 12 | 4 |
| Fe2O3∙H2O | 4 | 4 | 7 |
| 4CaO∙Al2O3∙Fe2O3 | - | 12 | 6 |
| FeS2 | - | - | 1 |
| Others | 1 | - | 1 |
| Total | 100 | 96 | 95 |

Table 5.
Major chemical constituents of RM in different areas of the world (mass fraction %) [41].
Table 5.
Major chemical constituents of RM in different areas of the world (mass fraction %) [41].
| Area | Al2O3 | Fe2O3 | SiO2 | CaO | Na2O | TiO2 |
|---|---|---|---|---|---|---|
| Australia (Bayer process) | 27.70 | 40.50 | 19.90 | - | 1~2 | 3.50 |
| India (Bayer process) | 21.9 | 28.1 | 7.5 | 10.2 | 4.5 | 15.8 |
| Shandong (Sintering process) | 8.32 | 5.7 | 32.5 | 41.62 | 2.33 | - |
| Henan (Sintering process) | 25.48 | 11.77 | 20.58 | 13.97 | 6.55 | 4.14 |
| Shanxi (Bayer process) | 10.5 | 6.75 | 22.2 | 42.25 | 3.00 | 2.55 |

Table 6.
The physical and chemical characteristics (average and data size) of RM [37]. Reproduced with permission from Gräfe et al., Hydrometallurgy; published by Elsevier, 2011 [37].
3. Environmental Problems from RM
The ecological disposal and resource utilization of RM are restricted by high alkalinity (pH > 13) and large amount of PHEs [42]. Many environmental risks are incurred in the process of stockpiling. The chemical properties of RM, such as high alkalinity, high salinity, lack of plant nutrients, and low content of organic matter, inhibit the growth of plants in the yard, which is extremely unfavorable to revegetation [42,43,44]. The RM particles are fine with high Na content. Efflorescence occurs on the surface of yards, and the alkaline dust formed pollutes the air (RM stored by dry process), thus endangering the surrounding vegetation and the staff of the yard. RM particles have large volume-to-weight ratio, low hydraulic conductivity, and waterlogging, which are common in RM dams. The migration ability of Al, As, and V in RM is high in strong alkali environment with high concentrations of Na+ and Al3+ [45].
RM stacked from sewage outfall causes serious pollution [4]. The mixing of RM and sediment on the seafloor leads to the decrease in biological distribution and community diversity. It directly threatens sea animals with profound influence. The RM stacked by wet process contains a large amount of alkaline solution (pH > 13), which easily leaks [14]. A large amount of PHEs in the attached solution can further pollute underground and surface water, and the attached solution with strong alkalinity can lead to soil degradation around the yard (alkalization). The leaching toxicity (PHEs) and radioactivity (Ra and Th) of the building materials prepared from RM (cement, brick, and ceramic material) pose certain environmental risks [46,47].
At present, the main treatment methods for RM include stockpiling by damming, direct sea-fill, and sea-fill after neutralization [48]. The alumina plants in Shandong, Henan, Guizhou, Shanxi, and Guangxi, China are mostly located in the inland area, and the RM is stacked by using high platform, valley dam, and concave filling. Therefore, high construction and maintenance costs are needed for RM yard [29]. A new yard for RM must adopt expensive impervious membrane materials. The construction cost of the yard for RM in Shandong aluminum plant is approximately $15 million, and the service life is only five years [49]. The annual cost of the construction is over $2 million. Moreover, the annual maintenance and management cost of RM dam exceeds $2 million. In recent years, considering the increasing alumina production and displacement of RM, dams for RM are built in the second yard. In 2004, China Aluminum Corporation initiated a project to solve the problem of fast stacking of RM. Under the existing conditions, the cost of landfill per ton of RM is conservatively estimated at $10 and above, even more than $20. Table 7 shows the general cost of properly handing RM in some countries, which is 15 US$/ton. The amount of funds spent by the government and enterprises in the RM will increase substantially with the amount treatment capacity of bauxite that is growing substantially every year. The yard for RM should occupy a large amount of land. Since the construction of Shandong Aluminum Corporation in 1954, more than 16 million tons of RM was stacked by the end of 1998, except for the use of 5,800,000 tons for making cement. The first yard was approximately 72 m high, reaching the stacking limit.

Table 7.
General cost of properly handing RM in some countries.
RM depositories have brought huge economic burden to enterprises and caused serious environmental safety risks [4]. Not even a blade of grass grows on a heap of RM. Dust and landslides are easily generated, and the ecological environment around the plant is destroyed [11]. In recent years, RM pollution incidents have occurred continuously. The dam break of a RM tailing pond in Hungary on 4 October, 2010 caused the most serious ecological disaster [11]. Approximately 600,000 to 700,000 m3 of RM spilled into the surrounding villages, thus causing serious ecological environmental pollution [11]. Dealing with large-scale RM has become one of the urgent problems to be solved in the word.
4. Present Situation of the Comprehensive Utilization of RM
RM has strong alkalinity and complex composition and properties. It is rich in metallic oxides, particularly iron. It also has good particle dispersion, large specific surface area, other characteristics similar to those of porous materials, and good stability in solution. Herein, they have made many achievements in building materials, metallurgy, environmental protection, and other fields [40]. The utilization of RM is of great economic and environmental importance.
The comprehensive utilization of RM must be combined with different characteristics of raw materials. The main components of RM discharged by sintering process and combination method are approximately the same, containing a large amount of 2CaO∙SiO2 and other active mineral components, which can be applied in building materials, ceramics, and other industries. The Bayer process for smelting alumina involves dissolving high-content aluminum, iron, boehmite, and gibbsite bauxite, and the produced Bayer RM contains almost no active components. Considering its high iron content and poor corrosion resistance, it is very difficult to use directly in building materials.
Generally, three ways are used to deal with RM. These methods includes extracting the valuable metals, the full use of RM as mineral material (cement, brick, and ceramic materials), and the application of RM for environmental protection (wastewater, waste gas, and PHE-contaminated soil restoration).
4.1. Recovery of Valuable Components from RM
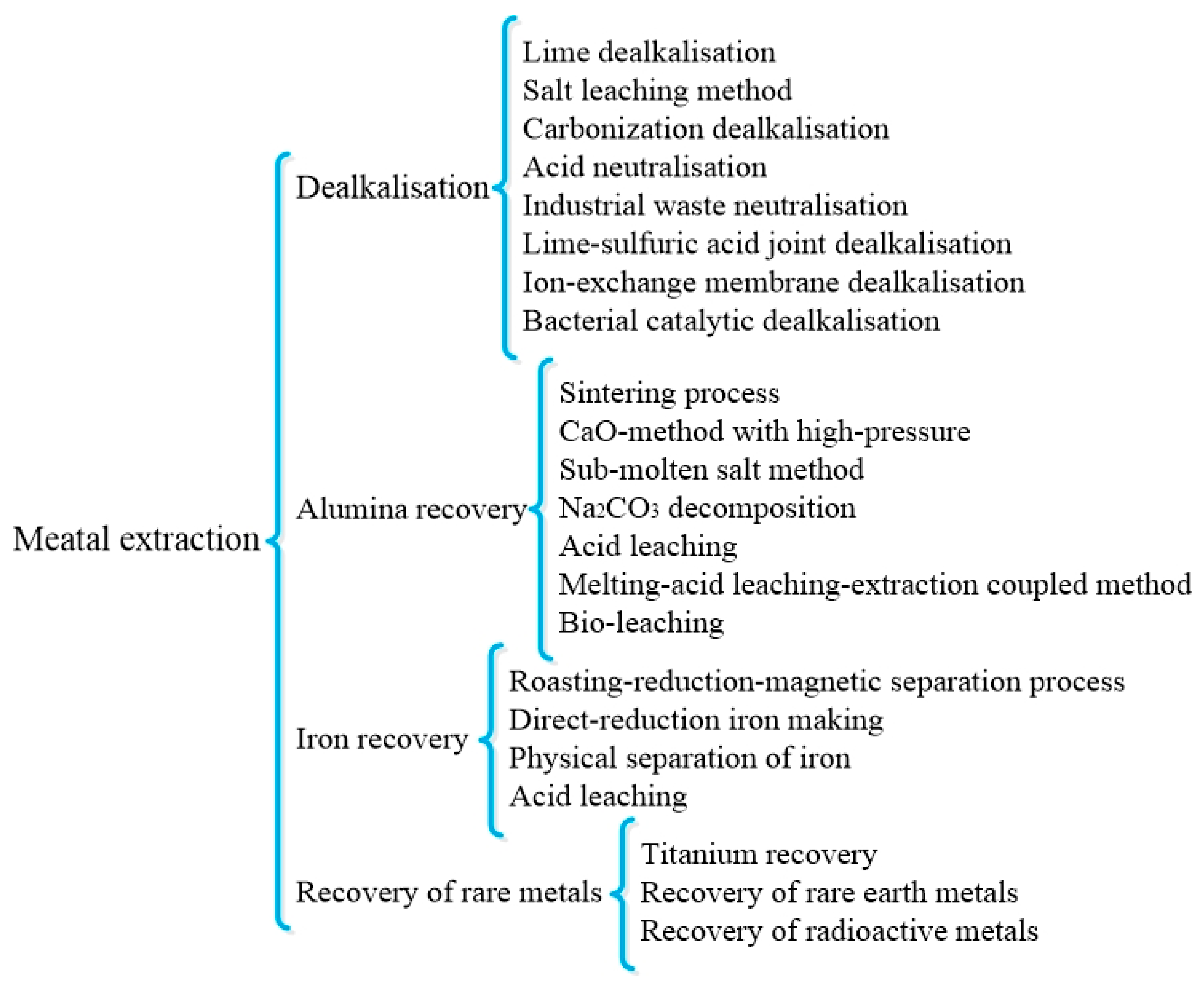
Recovery of metals such as aluminum, iron, titanium, and sodium from RM is very important because despite their useful values, some of them are harmful elements for RM in the application for the construction industry or other fields, such as sodium. The application of RM from different sources has been reported for the recovery of aluminum, iron, and rare earth metals. The main metal extraction methods from RM are listed in Figure 1.
Notably, the composition of RM greatly varies because of the difference in bauxite composition and production process. For example, the iron content in RM varies in the range of 7–24%, SiO2 in the range of 8–23%, Al2O3 in the range of 7–19%, and the variation range of trace elements is also large [50]. It is impossible to have a general process to recover a certain valuable component from RM. Although the studies described in the following subsections focus mostly on a specific RM, their research guidance on RM with different properties is significant.

Figure 1.
Classification of main metal disposal methods from RM [51]. Reproduced with permission from Wang et al., Journal of Cleaner Production; published by Elsevier, 2018 [51].
Figure 1.
Classification of main metal disposal methods from RM [51]. Reproduced with permission from Wang et al., Journal of Cleaner Production; published by Elsevier, 2018 [51].
4.1.1. Recovery of Iron from RM
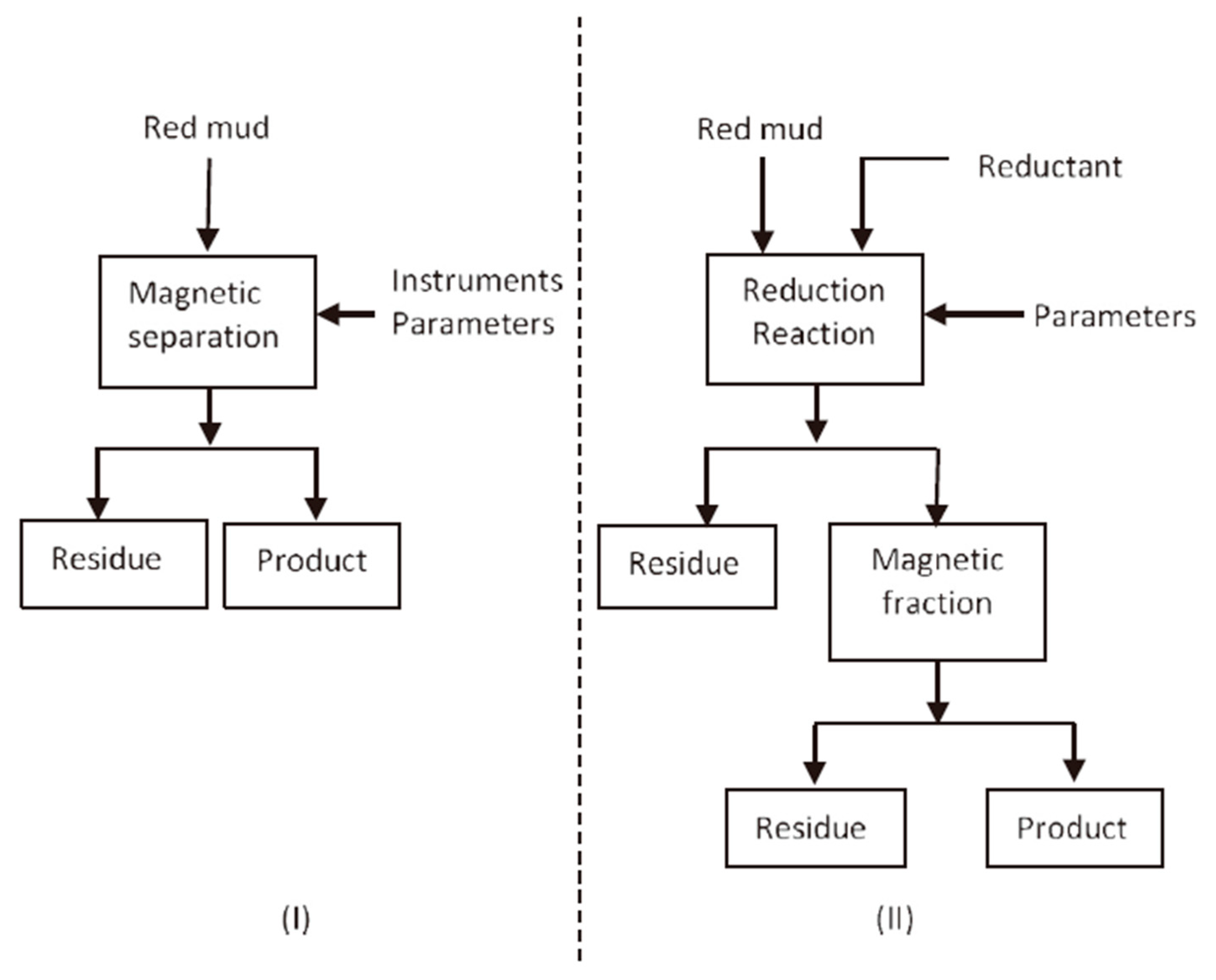
The methods of extracting iron from RM can be divided into pyrometallurgical recovery and direct magnetic separation. Figure 2 shows some technical schemes.
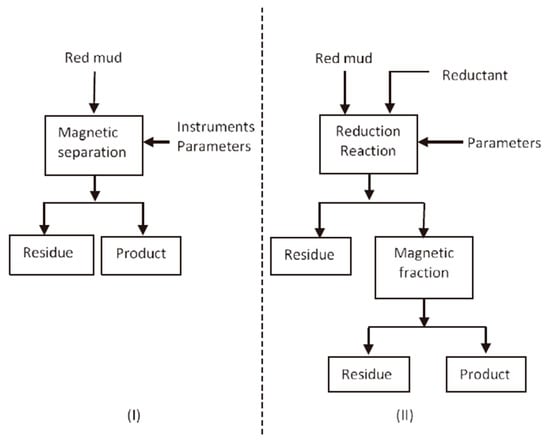
Figure 2.
Simplified schemes for recovering iron from RM-direct magnetic separation (I) and magnetic separation after reduction roasting (II) [38]. Reproduced with permission from Liu et al., Waste Management; published by Elsevier, 2014 [38].
Considering that the iron in RM is hematite and goethite with weak magnetic properties, direct magnetic process is applied as a basic technology for iron recovery from RM by using high-gradient magnetic separator. In comparison with pyrometallurgical recovery, recovering iron from RM by direct magnetic separation reduces energy costs, while maintaining Ti and other metals that are easily leachable [38]. Hammond et al. used a high-intensity magnet to separate the iron from RM (in slurry form). The resulting non-magnetic parts can be used in building materials or added back to Bayer processes, while magnetic products can be used as raw materials for iron production or as pigments for pottery [52]. The separation scheme is shown in Figure 2I. However, owing to the dissemination of fine iron ore, direct magnetic separation is inefficient.
One of the most widely studied methods to recover iron from RM is pyrometallurgy. The conversion of weakly magnetic hematite to strongly magnetic magnetite or metallic iron can improve the recovery efficiency of iron [53,54,55]. Figure 2II shows the whole scheme of the process. The process requires energy and a reductant in the solid phase or gaseous phase. Li et al. [56] used the vertical resistance furnace to recover Fe from RM by reduction roasting followed by magnetic separation. Sodium salts played an important role in facilitating the growth of metallic iron particles during reduction roasting. The leaching ratios of Fe increased from 51.9% to 94.7% in the presence of sodium salts. After reductive roasting under a reduction temperature of 1050 °C, 60 min of reduction time, 6% quantity of Na2SO4 and 6% quantity of Na2CO3, and magnetic concentrate containing 90.2% iron was extracted from roasted materials by magnetic separation method, and the iron recovery was 95.0% [56].
Liu et al. studied the recovery of iron from RM by magnetic separation after co-roasting with pyrite [57]. Experimental results showed that by adding pyrite to RM for reducing roasting, its iron oxide was transformed into magnetite. Based on the thermogravimetric and differential thermal analysis of roasting pyrite, the pyrite under N2 atmosphere had four weight-loss stages at 120 °C (dehydration stage) and in the range of 500–750 °C. Upon testing, they pointed out that the added pyrite produced thermo-decomposed products (element sulfur, iron monosulfide, and pyrrhotite) during roasting, which all had the reduction ability to react with hematite. Their research provided a new method to remove hematite from RM.
4.1.2. Technology of Removing Sodium and Extracting Aluminum from RM
Aluminum can be recovered from RM by alkali roasting with sodium carbonate and high-temperature hydrometallurgy [53,58]. In high-temperature hydrometallurgy, the aluminum in RM can be leached at high alkalinity (molar ratio of Na2O to Al2O3 > 10) and high temperatures (>260 °C) in autoclaves. However, this process has not been industrialized because of low alumina concentration in the leach solution and equipment-corrosion problems at high temperatures.
Therefore, the most common process of extracting aluminum is alkali roasting. In this process, the alumina can be converted to sodium aluminate (soluble) by thoroughly mixing RM with sodium carbonate (Na2CO3) and calcining it from 800–1200 °C, followed by leaching with water. The main reactions occurring in the roasting system are as follows:
Na2CO3 ⇆ Na2O + CO2
Al2O3 + Na2O ⇆ 2NaAlO2
SiO2 + Na2O ⇆ Na2SiO3
SiO2 + CaO ⇆ CaSiO3
Fe2O3 + Na2O ⇆ 2NaFeO2
CaO/Ca(OH)2 is used to replace Na2O Equations (6) and (7) in sodalite and cancrinite. Then, CO32− and Al(OH)4− in RM are cemented out in the form of CaCO3 and hydrocalumite. When the added CaO reaches the saturation state, the CaO–Na2O–Al2O3–SiO2–H2O system becomes balanced. In addition to proper temperature, CaO/RM, and stirring strength, Ca2+ in lime can partly replace Na+ in sodalite and cancrinite. The NaOH produced in the replacement reaction enters the solution and increases the pH of the solution. Through washing and separating, the recovered NaOH returns to the alumina extraction process for reuse.
[Na6Al6Si6O24]∙[2NaOH] + 6CaO + 6H2O→[Ca3Al6Si6O24]∙[Ca(OH)2] + 8NaOH + 2Ca(OH)2
[Na6Al6Si6O24]∙2[CaCO3] + 6CaO + 6H2O→[Ca3Al6Si6O24]∙2[CaCO3] + 6NaOH + 3Ca(OH)2
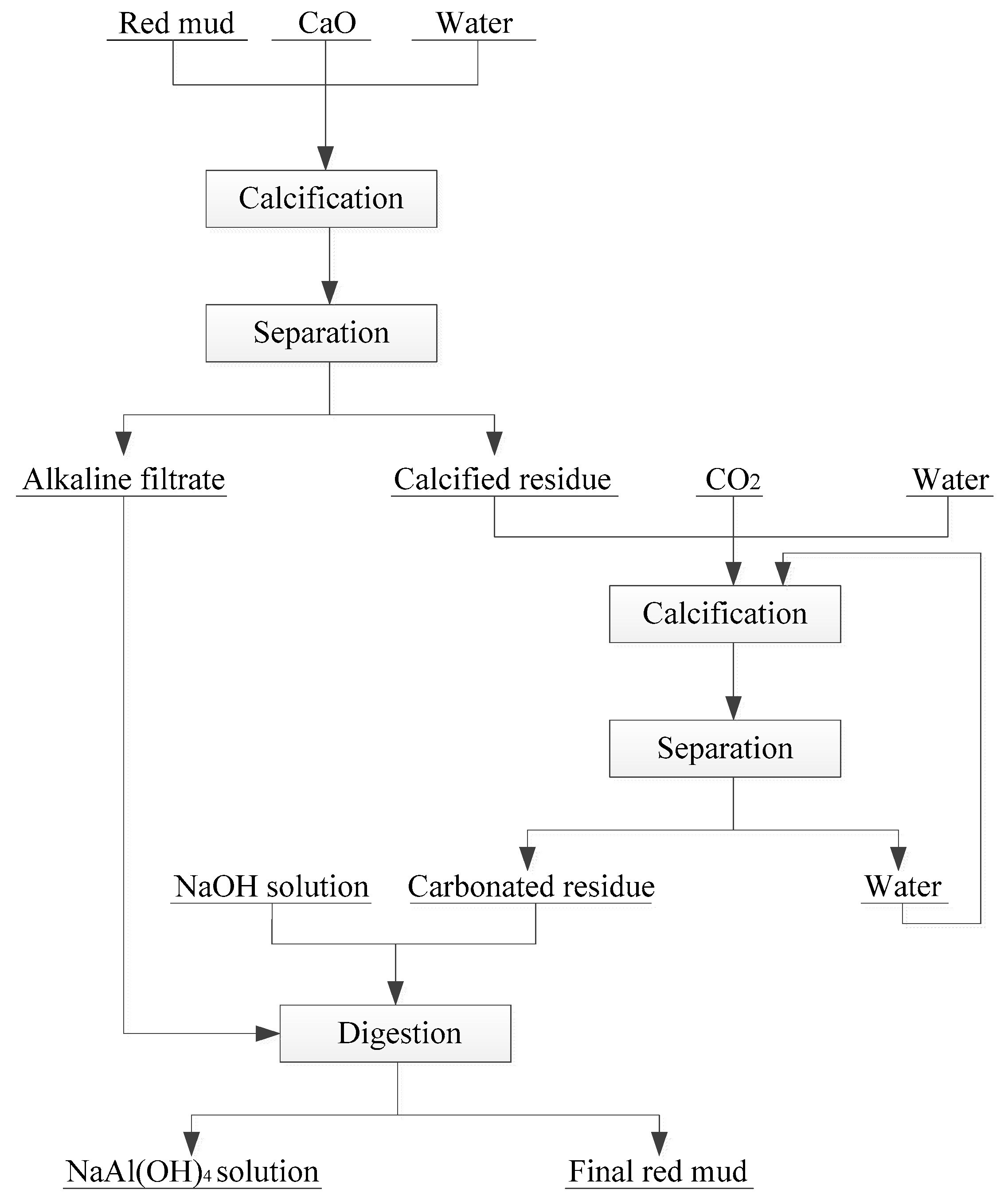
In recent years, some new technologies have been formed to treat RM, such as hydrothermal lime method, normal pressure lime method, lime–soda sintering method, and combined calcification–carbonization method [59]. These methods have achieved good results in controlling the alkalinity of RM in sintering process and combination method. Among which, combined calcification–carbonization method can regulate the alkaline and recover Al2O3 in RM. Zhang et al. proposed a novel combined calcification–carbonization method to recover aluminum and sodium from Bayer RM [51]. The novel process consists of two basic steps: calcification and carbonization (Figure 3). First, lime is added into the RM used to transform the main mineral phase Na2O∙Al2O3·xSiO2·(6 − 2x)H2O into hydrogarnet 3CaO·Al2O3·xSiO2·(6 − 2x)H2O and the Na2O dissolved in the solution. Then, the above calcified solid product was carbonized by high-pressure CO2, which decomposes it into calcium carbonate, calcium silicate, and aluminum hydroxide. Finally, under the low concentration of NaOH solution, aluminum hydroxide in the carbonized product can be easily extracted. The RM treated by this method can result in an alumina leaching rate of 46.5%, and the Na2O content in the leaching residue drops to 0.3% or less. These two steps turn RM into a nonhazardous and near-neutral residue that can be used for building material production.
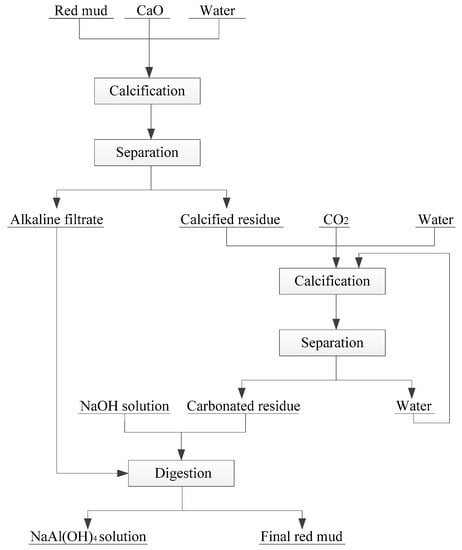
Figure 3.
Schematic of a novel calcification-carbonization for RM treatment [51]. Reproduced with permission from Wang et al., Journal of Cleaner Production; published by Elsevier, 2018 [51].
In addition to the lime method, acid leaching [60], three-waste neutralization [61], membrane desodium [62], suspension carbonization [63], selective flocculation [64], and other domestic processes, salt leaching [65], bacterial leaching [66], and foreign processes are available for alkali removal in RM.
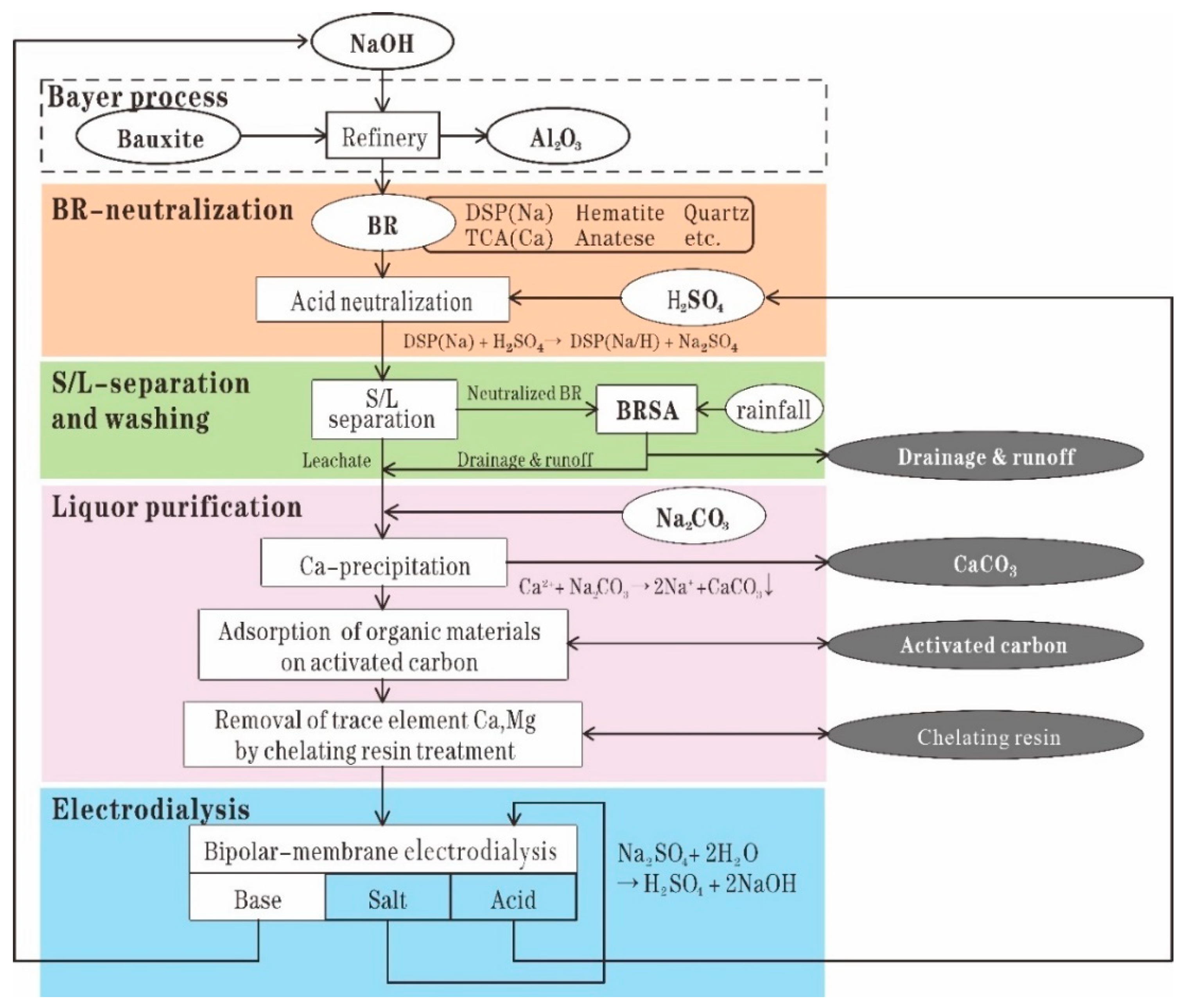
Sulfuric acid is the most commonly used regent used in RM neutralization owing to its low cost [67]. Recently, CSIRO developed a process to recover sodium from RM, which consists of the following steps: leaching RM by sulfuric acid to extract sodium as sodium sulfate; splitting the sodium sulfate through bipolar-membrane electrodialysis into sulfuric acid and sodium hydroxide, which can be returned to the alumina refinery; and neutralizing RM by using sulfuric acid [67]. The leached residues after sodium recovery show a low and stable pH and low soda. This property can provide great benefit to long-term residue management [68]. Figure 4 shows the process of in situ remediation of RM after treatment by CSIRO’s soda recovery process.
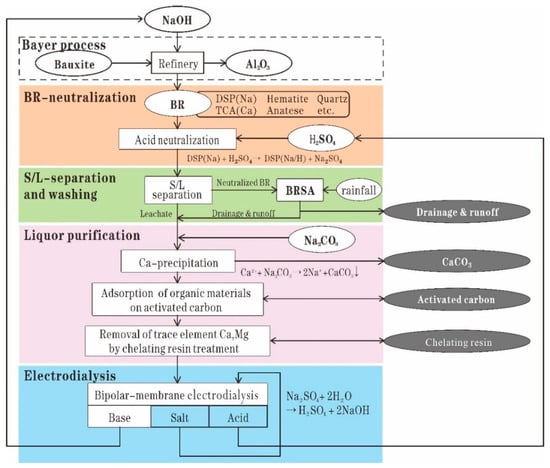
Figure 4.
Process of in situ remediation of RM by sulfuric acid neutralization [67]. Reproduced with permission from Kishida et al., Hydrometallurgy; published by Elsevier, 2017 [67].
4.1.3. Recovery of Titanium, Vanadium, and Rare-Earth Elements (REEs) from RM
Titanium is a rare metal in nature, because its distribution is too dispersed, and its extraction is difficult. However, many titanium resources are found in RM, especially TiO2 in Indian RM, which accounts for more than 20% [53]. RM is also rich in vanadium, which is typically mobile in RM leachate, and the concentrations of V ranges from 1.2–15.6 mg L−1 [45]. Vanadium exists in pentavalent form in RM leachate, is toxic, and is a possible human carcinogen [69,70,71]. Among the REEs in RM, scandium attracts the attention of many researchers because scandium accounts for more than 90% of REEs with economic value present in RM [72,73]. According to the properties and processing technology of bauxite, the content of Sc2O3 in RM ranges from 0.004–0.01 wt % [74].
For recent years, direct acid leaching has been the main technology used to dissolve titanium and scandium from RM in acid medium, followed by precipitation, adsorption, ion exchange, and solvent extraction [26,75,76,77]. In leaching technology, sulfuric acid, nitric acid, and hydrochloric acid are often used in leaching scandium from RM. Sulfuric acid (H2SO4) is the best choice to improve the extraction efficiency of titanium in inorganic acids [78]. Alkan et al. suggested a leaching process for recovering titanium from RM with sulfuric acid. They reported that the TiO2 recovery reached approximately 67.3% [79].
Helena et al. has tested the efficiency of ion exchange resins in recovering vanadium (V) from RM leachates at an alkaline pH of 11.5 and 13 [80]. Results showed that anion exchange resin can be used to recover vanadium from RM leachate with high alkaline pH value (up to 13). In the subsequent treatment, the vanadium in the resin is easily eluted out by NaOH solution. Results showed that it can use suitable NaOH solution to extract V with the recoveries of 42% and 76% from resins treated with RM leachate at pH 13 and 11.5, respectively.
The direct acid leaching process of RM avoids the high energy consumption of roasting pretreatment and simplifies the technological process. However, direct leaching process shows low selectivity towards other valuable components, such as Al, Fe, and Sc. Therefore, these components commonly enter the leaching solution during acid leaching, which makes the following separation process more complex and consumes a large amount of chemicals during subsequent processes such as solvent extraction [81]. To reduce the effect of impurity elements, many researchers have focused on developing novel acid leaching systems and organic extraction systems to improve the selectivity of scandium leaching.
Wang et al. first by used dilute sulfuric acid to leach scandium from an Australian RM [5]. For the following extraction process, a comparative experiment was carried out by using three organic acid extractants, and the result showed that di (2-ethylhexyl) phosphoric acid (D2EHP) exhibited the highest performance. The recovery rate of scandium reached 99%, and no iron and aluminum impurities were formed with the synthetic organic extractant consisting of 0.05 M D2EHPA and 0.05 M tri-butyl-phosphate in Shellsol D70 (100% aliphatic diluent) under the organic solution, and the synthetic leaching solution was mixed in a 5:1 ratio at pH 0.25 and 40 °C.
For reducing the influence of impurity elements on the leaching process, smelting is often carried out before leaching step to remove iron and aluminum from RM. Borra et al. removed iron from RM by smelting reduction, followed by recovering REEs from the slag [82]. By using 20 wt % wollastonite as flux and 5 wt % graphite as reducing agent, roasting experiments were carried out at 1500–1600 °C. More than 95% of the iron can be extracted from the RM. Under the leaching temperature of 90 °C and by using HCl and HNO3, more than 70% of titanium, 95% of scandium, and approximately >70% of REEs can be leached.
Another challenge for extract REEs from RM using acid leaching method is the formation of silica gel, because it decreases the filtration efficiency of RM dramatically [83]. Additionally, the silica gel may hinder the further dissolution of scandium and remarkably reduce the leaching kinetics. Recently, Rivera et al. extracted scandium from RM by dry digestion method with H2SO4 or HCl, followed by water leaching [84]. During the novel process, the water consumption is reduced by up to 60% and silicon dissolution can be limited to less than 5 wt %. Most importantly, silica gel does not form in the process, which significantly improves the filterability of the leach liquor.
Alkan et al. reported a novel scandium leaching process from RM based on oxidative leaching conditions. In this study, the combination of hydrogen peroxide (H2O2) and sulfuric acid (H2SO4) was utilized as the leaching solution. Results showed that the addition of 2.5 M H2SO4 and 2.5 M H2O2 at 90 °C and leaching time of 30 min are the best leaching conditions from the inhibition of silica gel formation; the extraction efficiencies of Ti and Sc was the highest, which were 91% and 68%, respectively [85]. Studies showed that the use of H2SO4 alone not only can form silicic acid and have the low selectivity to Ti, Sc and REEs, but also cause the co-precipitation of Sc and the rhomboclase phase, which greatly reduces the leaching rate of Sc. However, when H2SO4 and H2O2 are added together, it is observed that H2O2 provides an oxidizing atmosphere to precipitate the dissolved Si out in quartz phase, thereby preventing the formation of Si gel, and also forms titanium–peroxo–sulfate complex, which provides Ti with a high leaching efficiency. The medium formed in the leaching process also reduces the formation of rhomboclase phase, which entraps Sc during precipitation and is conducive to the formation of hematite [85]. Consequently, the combined use of hydrogen peroxide in the leaching process can improve the leaching efficiencies of Ti, Sc, and REEs. Furthermore, the liquid–solid ratio, temperature, and other factors must be well controlled in the treatment process because of the acid concentration. The cost must also be considered in the process of recovering Ti, Sc, and REEs from RM.
In the following process, much effort has been made to extract REEs from base elements. Among them, supported ionic liquid phase is proven effective in up-taking REEs from the acid solution because of its high adsorption kinetics and high selectivity for REEs. This novel chromatographic method also exhibits high reusability and stability without decreasing the recovery efficiencies [86].
4.2. Resource Transformation
The chemical composition and activity index of RM indicate that it is suitable in building material industries [87]. RM is an excellent binder material, and the application of RM in cement-based materials and concrete has been extensively studied. Its mechanical properties and moisture absorption meet the requirements of mechanical building materials. For example, Tang et al. proposed the use of RM to replace fly ash to produce environmentally friendly clear self-compacting concrete [88]. The alkaline components in RM can be used as chemical curing agents to activate the activity of fly ash and slag. They found that with increasing RM content, the mechanical strength of concrete slightly increases. The maximum compressive strength and elastic modulus was obtained for self-compacting concrete samples consisting 50% RM. Shetty et al. found the similar results that self-compacting concrete with 10% used foundry sand and 2% RM presented the highest compressive strength. When the content of RM exceeded 2%, the compressive strength started to decrease [89].
Pei et al. studied the Na+-solidification behavior of SiO2–Al2O3–CaO–MgO (10 wt %) ceramics prepared from RM [90]. They added 20 wt %, 35 wt %, and 50 wt % RM into the raw materials with high CaO and Na2O content and prepared batches of new SiO2–Al2O3–CaO–MgO (10 wt %) ceramics. After the physical properties and Na+-leaching of these ceramics were tested, they found regardless of the variation in the content of Na2O in the ceramics from 2.15–4.21 wt %, the leaching rates of Na+ in fired ceramics remained stable, which were approximately six times lower than those in green samples. The main crystal components include diopside augite, anorthite, and ferroan, which can solidify Na+. Moreover, with the increasing temperature, their solidifying abilities increased.
They further used Scanning Electron Microscope and Energy Dispersion Spectroscopy to test the Na+-solidification in pyroxene and anorthite with sample sintered at 1100 °C and 1130 °C. They found that as the temperature rose, the amount of Na+ solidified in augite, anorthite, and diopside ferroan increased, which was consistent with the Na+ leaching rates. Moreover, anorthite has the greatest ability to solidify Na+ because the atomic radius, ionic radius, coordination number of Na and Ca are basically the same, and sodium can replace calcium in anorthite. Anorthite (CaAl2Si2O8) and albite (NaAlSi3O8) have the same structure, i.e., both their framework comprise silicate minerals, and an infinite dissolution occurs between them. The process of dissolution is as follows: Si4+ + Na+ = Al3+ + Ca2+. Based on the XRD analysis, at 1100 °C, the chemical composition of anorthite is (Ca0.86Na0.14)(Al1.86Si0.14)Si2O8, which verified the dissolution process and the Na+-solidification behavior in anorthite.
Wang et al. studied the recycling of RM to produce high-strength and lightweight ceramic floor tile [31]. They succeeded in producing low-cost ceramic floor tiles by adding kaolin to the recycled RM. Further, to alter the mullitization reaction pathway and promote the growth of mullite crystals, they added (NH4)6Mo7O24 as a catalyst. They mixed the starting materials in a series of designated proportions, added 6 wt % distilled water, and pressed it into a cylindrical sample with dimensions of 70 mm × 6 mm × 6 mm. After 24 h of drying, the samples were sintered at the sintering temperature ranging from 1150–1200 °C for 2 h. Finally, the samples were cooled to room temperature in an oven. By comparing the XRD patterns of the samples with the different catalyst contents, they pointed out that the presence of ammonium molybdate was beneficial to the mullite crystals change and the sintering process under a low sintering temperature.
4.3. Application in the Environmental Field
RM is characterized by small particle size, porous skeleton structure, large specific surface area, and good stability in water medium, which provides good adsorption properties and its utilization as a cheap adsorption material in the field of environmental management [19,23,91]. RM adsorbents can adsorb PHEs in wastewater and also remove F−, PO43−, organic pollutants (dyes), and radioactive ions (Cs-137, Sr-90, and U) in wastewater. Domestic and foreign scholars consider RM as the main body of adsorption and treat it to improve its adsorption performance and expand its applicability (Table 8).
Dharitri et al. studied the use of RM as a catalyst for the solar degradation of aquatic pollutants [92]. In the study, RM was successfully utilized as an effective photocatalyst for removing Cr (VI) and malachite green from wastewater. A series of RM/MCM-41 composite materials (RMCM) were prepared by simple sol-gel method in which the structural advantages of MCM-41 played a vital role in inhibiting e−/h+ recombination. RMCM (1:1) can reduce 85% of 20Cr(VI) solution and oxidize 97% of malachite green. The oxidation–reduction process provides a beneficial idea for the purification of wastewater and environmental remediation. They pointed out that acid-activated RM is a visible-light active substance, which can be used as an effective catalyst for the photocatalytic oxidation of malachite green and reduction of Cr (VI). In the photocatalytic process, the surface area of the material plays a crucial role, whereas the effective surface area of RM is only approximately 60 m2/g. Therefore, to increase the surface area of activated RM, a material with a large surface area should be used to make a composite material. They used mesoporous silica MCM-41, which is suitable for all types of materials.
5. Main problems Existing in the Comprehensive Utilization of RM
First, RM has complex components, low-grade valuable components, and difficult utilization. Therefore, key technology with industrial competitive power for the large-scale utilization of RM is lacking. For example, iron, aluminum, and sodium are the main components of Bayer RM, which account for 50–70% of the total mass of RM. If these components can be recycled, reduction, resource-based, and harmless RM may be achieved. However, SiO2 is one of the main components of RM, which accounts for approximately 20% of the mass of RM. It increases the cost and difficulty of recovering iron, aluminum, and sodium in RM. RM is characterized by strong alkalinity, large specific surface area, and the ability to wrap and embed various components. Thus, reference to mature technologies, processes, and equipment in other fields for comprehensive utilization is difficult. In China, no technical support system is available for efficient utilization and large-scale promotion.
Second, the process design is unreasonable, the economic benefits are poor, and industrial production is difficult to achieve. Thus, it cannot solve the problem thoroughly. Some technologies only consider the recovery and utilization of an element with poor economic value. For example, researches showed that direct reduction and recovery technology for iron is feasible. However, considering the large material flow and relatively low iron content, the economy is not good. The beneficiation technique for iron recovery has low energy consumption, but a large amount of residue remains to be transported to the yard. Some technologies have considered the combined recovery of various resources. However, defects in the process design and low comprehensive utilization level are observed. For example, acid leaching and rare-earth metal recovery technology generates many acid residues, thus easily causing secondary pollution. Some technical routes in high-temperature melting process incurs large energy consumption, which are difficult to be accepted by enterprises. Although bioleaching is generally considered to be a “green technology” with low energy consumption and low cost, it is almost impossible for naturally stacked RM piles to provide growth conditions suitable for the strains that are demanding on the growing environment [93]. In other words, bioleaching has the disadvantages of low efficiency and long cycle time in the treatment of RM.

Table 8.
Different treatment methods and their application of RM.
Table 8.
Different treatment methods and their application of RM.
| Serial Number | Contaminant | Processing Method | References |
|---|---|---|---|
| 1 | H2S | First, the magnetic stirrer was stirred to mix RM with distilled water. The H2S gas enters the suspensions as microbubbles through a rotameter. | [94] |
| 2 | Dyestuff | The best adsorption condition for the adsorption of methylene blue by sintering process RM was achieved at pH 10, and the maximum adsorption capacity of 51.7 mg/g was achieved for sintering RM. | [95] |
| 3 | Cu (II) | Compared with untreated RM, the thermally activated RM had a higher adsorption capacity for Cu2+, which was 2.08 mmol g−1. | [96] |
| 4 | Arsenic | Under 25 °C, the adsorption of As (III) and As (V) reached equilibrium in 45 and 90 min, respectively. The alkaline aqueous medium (pH 9.5) was beneficial to As (III) removal, while pH value between 1.1 and 3.2 was effective for As (V) removal. | [10] |
| 5 | PO43− | Under the stirring speed of 180 rpm, stirring time of 30 min, and precipitation time of 30 min, 8 g/L RM adsorbent can be used to remove PO43− in 60 mg/L concentration, and the dephosphorization rate reached 74.1%. | [97] |
| 6 | Zn (II) | RM was activated with CO2 sequestration and then used for the adsorption of Zn (II) ions from the aqueous solutions. The maximum adsorption capacity was 14.92 mg g−1. | [98] |
Third, attention to the comprehensive utilization of RM is improved. Comprehensive utilization of RM is not the main objective of alumina enterprise. Many enterprises use the stacking method to dispose RM. The environmental risks and hidden dangers of RM stacking are long-term and concealed. Thus, the attention of enterprises and related departments is insufficient. The comprehensive utilization of RM is an important embodiment of the implementation of scientific outlook on the development, transformation of economic development mode, development of circular economy, and the construction of a resource-saving and environment-friendly society. It is also the only way for the sustainable development of alumina industry in China. Relevant regions and enterprises must be highly aware of the urgency and importance of the comprehensive utilization of RM and the active and comprehensive utilization of RM.
6. Conclusions and the Outlook of the Technology
Comprehensively utilizing the RM discharged from the alumina production process is a worldwide problem. The most ideal target for the comprehensive utilization of RM is to extract valuable metals and REEs without causing secondary waste residue pollution, that is, zero emissions. Considering that the properties of RM vary from the place of origin, treatment process, and other factors, a general process to recover a certain valuable component from RM cannot be formulated. The recovery of valuable components in RM has been widely studied. However, although RM contains a certain amount of minerals such as iron, aluminum, sodium, titanium, and REEs, the current research on the comprehensive recovery of these valuable substances from RM is not feasible for industrial application from the perspective of economic and environmental benefits.
At present, the research on the use of RM in low value-added building materials, road building materials and other fields involves the use of a certain technology, is applied in practice, and achieves certain results. RM de-alkali, which directly determines the quality of the product, is an important technology that makes the application in building materials and road building materials feasible. RM can also be used as a restorative material in the environmental field to treat inorganic/organic contamination in waste liquid or soil. In comparison with the valuable elements of extracting high value-added products in RM, the RM treatment scheme introduced above has the advantages of low cost, simple process, and high industrial feasibility.
We should pay more attention in studying the application of RM in the field of environmental protection, the technology of RM dealkali, and the application after dealkali. Hence, we should develop and research new technologies and processes that have market competitiveness, offer environmental protection and ecological balance, and conform to the sustainable development strategy. This condition is necessary for the high efficiency, low cost and high feasibility of industrial applications.
Author Contributions
Conceptualization, L.W., N.S. and W.S.; Writing-Original Draft Preparation, L.W. and N.S.; Writing-Review & Editing, H.T.
Funding
This research was funded by the National Key R&D Program of China (2018YFC1901901); Key Laboratory of Hunan Province for Clean and Efficient Utilization of Strategic Calcium-containing Mineral Resources (No. 2018TP1002); Natural Science Foundation of China (51704329, 51705540); Innovation Driven Plan of Central South University (No. 2015CX005); the National 111 Project (No. B14034), and Collaborative Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the result.
References
- Kirwan, L.J.; Hartshorn, A.; Mcmonagle, J.B.; Fleming, L.; Funnell, D. Chemistry of bauxite residue neutralisation and aspects to implementation. Int. J. Miner. Process. 2013, 119, 40–50. [Google Scholar] [CrossRef]
- Evans, K.; Nordheim, E.; Tsesmelis, K. Bauxite residue management. In Light Metals; Springer: Cham, Switzerland, 2012; pp. 61–66. [Google Scholar]
- Tsakiridis, P.E.; Agatzini-Leonardou, S.; Oustadakis, P. Red mud addition in the raw meal for the production of portland cement clinker. J. Hazard. Mater. 2004, 116, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Chu, Y.C. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Evans, K. The history, challenges, and new developments in the management and use of bauxite residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Cusack, P.B.; Healy, M.G.; Ryan, P.C.; Burke, I.T.; O’ Donoghue, L.M.T.; Ujaczki, É.; Courtney, R. Enhancement of bauxite residue as a low-cost adsorbent for phosphorus in aqueous solution, using seawater and gypsum treatments. J. Clean. Prod. 2018, 179, 217–224. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Xiao, B. Review on treatment and utilization of bauxite residues in china. Int. J. Miner. Process. 2009, 93, 220–231. [Google Scholar] [CrossRef]
- Mukiza, E.; Zhang, L.L.; Liu, X.; Zhang, N. Utilization of red mud in road base and subgrade materials: A review. Resour. Conserv. Recycl. 2019, 141, 187–199. [Google Scholar] [CrossRef]
- Altundoğan, H.S.; Altundoğan, S.; Tümen, F.; Bildik, M. Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag. 2000, 20, 761–767. [Google Scholar] [CrossRef]
- Mayes, W.M.; Burke, I.T.; Gomes, H.I.; Anton, Á.D.; Molnár, M.; Feigl, V.; Ujaczki, É. Advances in understanding environmental risks of red mud after the Ajka spill, Hungary. J. Sustain. Metall. 2016, 2, 332–343. [Google Scholar] [CrossRef]
- Lyu, F.; Gao, J.; Sun, N.; Liu, R.; Sun, X.; Cao, X.; Wang, L.; Sun, W. Utilisation of propyl gallate as a novel selective collector for diaspore flotation. Miner. Eng. 2019, 131, 66–72. [Google Scholar] [CrossRef]
- Balomenos, E.; Giannopoulou, I.; Panias, D.; Paspaliaris, I.; Perry, K.; Boufounos, D. Efficient and Complete Exploitation of the Bauxite Residue (Red Mud) Produced in the Bayer Process. In Proceedings of the European Metallurgical Conference, Düsseldorf, Germany, 23–26 June 2011. [Google Scholar]
- András, G.; Nóra, K.; Beatrix, T.; Ágnes, R.; András, H.; Kornélia, I.; Ilona, N.K.; Dorottya, C.M.; Ádám, T.; Aladár, C. The red mud accident in ajka (hungary): Characterization and potential health effects of fugitive dust. Environ. Sci. Technol. 2011, 45, 1608–1615. [Google Scholar]
- Liang, G.; Chen, W.; Nguyen, A.V.; Nguyen, T.A.H. Red mud carbonation using carbon dioxide: Effects of carbonate and calcium ions on goethite surface properties and settling. J. Colloid Interface Sci. 2018, 517, 230–238. [Google Scholar] [CrossRef] [PubMed]
- James, J.P.; Tobias, H.; Andrei, G.; Tkaczyk, A.H.; Yiannis, P.; Anna, B.R. Identifying hotspots of environmental impact in the development of novel inorganic polymer paving blocks from bauxite residue. Resour. Conserv. Recycl. 2018, 138, 87–98. [Google Scholar]
- Belviso, C.; Agostinelli, E.; Belviso, S.; Cavalcante, F.; Pascucci, S.; Peddis, D.; Varvaro, G.; Fiore, S. Synthesis of magnetic zeolite at low temperature using a waste material mixture: Fly ash and red mud. Microporous Mesoporous Mater. 2015, 202, 208–216. [Google Scholar] [CrossRef]
- Belviso, C.; Kharchenko, A.; Agostinelli, E.; Cavalcante, F.; Peddis, D.; Varvaro, G.; Yaacoub, N.; Mintova, S. Red mud as aluminium source for the synthesis of magnetic zeolite. Microporous Mesoporous Mater. 2018, 270, 24–29. [Google Scholar] [CrossRef]
- Hua, Y.; Heal, K.V.; Frieslhanl, W. The use of red mud as an immobiliser for metal/metalloid-contaminated soil: A review. J. Hazard. Mater. 2017, 325, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guan, X.; Zhang, S.; Xu, C.; Li, H.; Zhang, J. Sintering red mud based imitative ceramic bricks with CO2 emissions below zero. Mater. Lett. 2017, 191, 222–224. [Google Scholar] [CrossRef]
- Ujaczki, E.; Zimmermann, Y.; Gasser, C.; Molnár, M.; Feigl, V.; Lenz, M. Red mud as secondary source for critical raw materials–extraction study. J. Chem. Technol. Biotechnol. 2017, 92, 2835–2844. [Google Scholar] [CrossRef]
- Rai, S.; Wasewar, K.L.; Agnihotri, A. Treatment of alumina refinery waste (red mud) through neutralization techniques: A review. Waste Manag. Res. 2017, 35, 563–580. [Google Scholar] [CrossRef]
- Guo, T.; Yang, H.; Liu, Q.; Gu, H.; Wang, N.; Yu, W.; Dai, Y. Adsorptive removal of phosphate from aqueous solutions using different types of red mud. Water Sci. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Sun, W.; Hu, Y.; Yin, Z.; Zhang, C.; Guan, C.; Zhu, X.; Ahmed Khoso, S. Simultaneous control of particle size and morphology of α-CaSO4·1/2H2O with organic additives. J. Am. Ceram. Soc. 2019, 102, 2440–2450. [Google Scholar]
- Zhang, Y.; Hu, Y.; Sun, N.; Khoso, S.A.; Wang, L.; Sun, W. A novel precipitant for separating lithium from magnesium in high Mg/Li ratio brine. Hydrometallurgy 2019, 187, 125–133. [Google Scholar] [CrossRef]
- Deng, B.; Li, G.; Luo, J.; Ye, Q.; Liu, M.; Peng, Z.; Jiang, T. Enrichment of SC2O3 and TiO2 from bauxite ore residues. J. Hazard. Mater. 2017, 331, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, M.K.; Williams, F.S. Bacterial amelioration of bauxite residue waste of industrial alumina plants. J. Ind. Microbiol. Biotechnol. 2001, 27, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Hyeok-Jung, K.; Kang, S.-P.; Choe, G.-C. Effect of red mud content on strength and efflorescence in pavement using alkali-activated slag cement. Int. J. Concr. Struct. Mater. 2018, 12, 18. [Google Scholar] [CrossRef]
- Liu, D.Y.; Wu, C.S. Stockpiling and comprehensive utilization of red mud research progress. Materials 2012, 5, 1232–1246. [Google Scholar] [CrossRef]
- Liu, C.; Ma, S.; Zheng, S.; Luo, Y.; Ding, J.; Wang, X.; Zhang, Y. Combined treatment of red mud and coal fly ash by a hydro-chemical process. Hydrometallurgy 2018, 175, 224–231. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Liu, H.; Han, C. Recycling of waste red mud for production of ceramic floor tile with high strength and lightweight. J. Alloys Compd. 2018, 748, 876–881. [Google Scholar] [CrossRef]
- Plunkert, P.A. Bauxite and alumina. Min. Eng. 2000, 52, 28–29. [Google Scholar]
- Zeng, C.Y.; Huang, Z.H.; Fang, M.H.; Liu, Y.G. Synthesis of mullite using low-grade bauxite and coal gangue. Key Eng. Mater. 2014, 602–603, 331–334. [Google Scholar] [CrossRef]
- Barbosa, F.D.M.; Bergerman, M.G.; Horta, D.G. Removal of iron-bearing minerals from gibbsitic bauxite by direct froth flotation. Tecnol. Em Metal. Mater. E Min. 2016, 13, 106–112. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Rao, B.H.; Reddy, N.G. Zeta potential and particle size characteristics of red mud waste. In Geoenvironmental Practices and Sustainability; Springer: Singapore, 2017. [Google Scholar]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Guo, N. The current situation of recovery and utilization of red mud from alumina solid waste. China Resour. Compr. Util. 2011, 29, 21–24. (In Chinese) [Google Scholar]
- Nan, X.L.; Zhang, T.A.; Liu, Y.; Dou, Z.H. Analysis of comprehensive utilization of red mud in china. Chin. J. Process Eng. 2010, 10, 264–270. (In Chinese) [Google Scholar]
- Bin, L.; Baohua, Z.; Ping, N.; Liwei, H.; Xiaolin, Z. Present status and prospect of red mud resource utilization and safety treatment. Chem. Ind. Eng. Prog. 2018, 37, 714–723. (In Chinese) [Google Scholar]
- Lyu, F.; Sun, N.; Sun, W.; Khoso, S.A.; Tang, H.-H.; Wang, L. Preliminary assessment of revegetation potential through ryegrass growing on bauxite residue. J. Cent. South Univ. 2019, 26, 404–409. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Wang, Z.; Han, H.; Yang, Y.; Liu, R.; Hu, Y.; Tang, H.; Sun, W. Self-assembly of mixed dodecylamine–dodecanol molecules at the air/water interface based on large-scale molecular dynamics. J. Mol. Liq. 2019, 276, 867–874. [Google Scholar] [CrossRef]
- Di Carlo, E.; Boullemant, A.; Courtney, R. A field assessment of bauxite residue rehabilitation strategies. Sci. Total Environ. 2019, 663, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Burke, I.T.; Mayes, W.M.; Peacock, C.L.; Brown, A.P.; Jarvis, A.P.; Gruiz, K. Speciation of arsenic, chromium, and vanadium in red mud samples from the Ajka spill site, Hungary. Environ. Sci. Technol. 2012, 46, 3085–3092. [Google Scholar] [CrossRef]
- Hannian, G.; Ning, W.; Shirong, L. Radiological restrictions of using red mud as building material additive. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2012, 30, 961–965. [Google Scholar]
- János, S.; Viktor, J.; József, K.; Sándor, T.; Tibor, K. Radiological aspects of the usability of red mud as building material additive. J. Hazard. Mater. 2008, 150, 541–545. [Google Scholar]
- Paramguru, R.K.; Rath, P.C.; Misra, V.N. Trends in red mud utilization—A review. Miner. Process. Extr. Metall. Rev. 2004, 26, 1–29. [Google Scholar] [CrossRef]
- Hu, Y. Comprehensive benefit analysis of alumina plant wet red mud yard dry compatibilization. In Proceedings of the 15th Annual Conference of the Chinese Association of Science and Technology: National Seminar on Aluminum Metallurgy Technology, Guiyang, China, 25 May 2013. (In Chinese). [Google Scholar]
- Nan, X.L.; Zhang, T.A.; Liu, Y.; Dou, Z.H.; Zhao, Q.Y.; Jiang, X.L. Main categories of red mud and its environmental impacts. Chin. J. Process Eng. 2009, 9, 459–464. (In Chinese) [Google Scholar]
- Wang, Y.; Zhang, T.-A.; Lyu, G.; Guo, F.; Zhang, W.; Zhang, Y. Recovery of alkali and alumina from bauxite residue (red mud) and complete reuse of the treated residue. J. Clean. Prod. 2018, 188, 456–465. [Google Scholar] [CrossRef]
- Hammond, K.; Mishra, B.; Apelian, D.; Blanpain, B. CR3 communication: Red mud—A resource or a waste? JOM 2013, 65, 340–341. [Google Scholar]
- Li, X.-B.; Xiao, W.; Liu, W.; Liu, G.-H.; Peng, Z.H.; Zhou, Q.-S.; Qi, T.-G. Recovery of alumina and ferric oxide from bayer red mud rich in iron by reduction sintering. Trans. Nonferrous Met. Soc. China 2009, 19, 1342–1347. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Xiao, B. Application of bayer red mud for iron recovery and building material production from alumosilicate residues. J. Hazard. Mater. 2009, 161, 474–478. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Tsakiridis, P.E.; Potiriadis, K. Greek “red mud” residue: A study of microwave reductive roasting followed by magnetic separation for a metallic iron recovery process. J. Hazard. Mater. 2013, 254–255, 193. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, M.; Rao, M.; Jiang, T.; Zhuang, J.; Zhang, Y. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts. J. Hazard. Mater. 2014, 280, 774–780. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, B.; Yang, T.; Wan, P.; Chen, Y.; Lv, Z. Recycling of iron from red mud by magnetic separation after co-roasting with pyrite. Thermochim. Acta 2014, 588, 11–15. [Google Scholar] [CrossRef]
- Chun, T.; Zhu, D.; Pan, J.; He, Z. Recovery of alumina from magnetic separation tailings of red mud by Na2CO3 solution leaching. Metall. Mater. Trans. B 2014, 45, 827–832. [Google Scholar] [CrossRef]
- Xie, L.Q.; Zhang, T.A.; Lv, G.Z.; Zhu, X.F. Direct calcification–carbonation method for processing of bayer process red mud. Russ. J. Non-Ferr. Met. 2018, 59, 142–147. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liu, J.; Pan, H.; Ke, M.; Lv, F.; Tong, W.; Chu, P.K. Dealkalization of red mud by carbide slag and flue gas. Clean Soil Air Water 2017, 46, 1700634. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yang, G.; Zhang, J.P. Novel process for sodium elimination from red mud of alumina production. Nonferrous Met. 2010, 62, 61–64. (In Chinese) [Google Scholar]
- Wang, Q. Study on the dealkalization of red mud by suspension and carbonation. Chin. J. Environ. Eng. 2009, 3, 2275–2280. (In Chinese) [Google Scholar]
- Huang, Y.; Han, G.; Liu, J.; Wang, W. A facile disposal of bayer red mud based on selective flocculation desliming with organic humics. J. Hazard. Mater. 2016, 301, 46–55. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Xiao, Y.; Luo, Z.; Han, Y. Research on dealkalization of sintering process red mud by lime process at normal atmosphere and mechanism thereof. Inorg. Chem. Ind. 2012, 44, 40–42. (In Chinese) [Google Scholar]
- Williams, F.S.; Hamdy, M.K. Induction of biological activity in bauxite residue. In Essential Readings in Light Metals; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Kishida, M.; Harato, T.; Tokoro, C.; Owada, S. In situ remediation of bauxite residue by sulfuric acid leaching and bipolar-membrane electrodialysis. Hydrometallurgy 2017, 170, 58–67. [Google Scholar] [CrossRef]
- Harato, T.; Smith, P.; Oraby, E. Recovery of soda from bauxite residue by acid leaching and electrochemical processing. In Proceedings of the 9th International Alumina Quality Workshop, Perth, Australia, 18–32 March 2012; pp. 193–201. [Google Scholar]
- Burke, I.T.; Peacock, C.L.; Lockwood, C.L.; Stewart, D.I.; Mortimer, R.J.G.; Ward, M.B.; Philip, R.; Katalin, G.; Mayes, W.M. Behavior of aluminum, arsenic, and vanadium during the neutralization of red mud leachate by HCL, gypsum, or seawater. Environ. Sci. Technol. 2013, 47, 6527–6535. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.A. Iarc monographs on the evaluation of carcinogenic risks to humans. J. Clin. Pathol. 1995, 48, 691. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Sun, N.; Liu, R.; Wang, Z.; Wang, L.; Sun, W. Systematic review of feldspar beneficiation and its comprehensive application. Miner. Eng. 2018, 128, 141–152. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of rare earths and major metals from bauxite residue (red mud) by alkali roasting, smelting, and leaching. J. Sustain. Metall. 2017, 3, 393–404. [Google Scholar] [CrossRef]
- Kumari, A.; Jha, M.K.; Pathak, D.D. Review on the processes for the recovery of rare earth metals (rems) from secondary resources. In TMS Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Li, G.; Ye, Q.; Deng, B.; Luo, J.; Rao, M.; Peng, Z.; Jiang, T. Extraction of scandium from scandium-rich material derived from bauxite ore residues. Hydrometallurgy 2018, 176, 62–68. [Google Scholar] [CrossRef]
- Onghena, B.; Borra, C.R.; Gerven, T.V.; Binnemans, K. Recovery of scandium from sulfation-roasted leachates of bauxite residue by solvent extraction with the ionic liquid betainium bis(trifluoromethylsulfonyl)imide. Sep. Purif. Technol. 2017, 176, 208–219. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Jing, Q.; Zhang, M. Recovery of scandium from leachate of sulfation-roasted bayer red mud by liquid–liquid extraction. JOM 2017, 69, 2373–2378. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Gerven, T.V. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Agatzinileonardou, S.; Oustadakis, P.; Tsakiridis, P.E.; Markopoulos, C. Titanium leaching from red mud by diluted sulfuric acid at atmospheric pressure. J. Hazard. Mater. 2008, 157, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Alkan, G.; Schier, C.; Gronen, L.; Stopic, S.; Friedrich, B. A mineralogical assessment on residues after acidic leaching of bauxite residue (red mud) for titanium recovery. Met. Open Access Metall. J. 2017, 7, 458. [Google Scholar] [CrossRef]
- Gomes, H.I.; Jones, A.; Rogerson, M.; Burke, I.T.; Mayes, W.M. Vanadium removal and recovery from bauxite residue leachates by ion exchange. Environ. Sci. Pollut. Res. 2016, 23, 23034–23042. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, L.; Tang, S.; Zeng, M.; Bai, P.; Chen, L. Selective recovery of vanadium and scandium by ion exchange with D201 and solvent extraction using P507 from hydrochloric acid leaching solution of red mud. Chemosphere 2017, 175, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef]
- Abkhoshk, E.; Jorjani, E.; Al-Harahsheh, M.S.; Rashchi, F.; Naazeri, M. Review of the hydrometallurgical processing of non-sulfide zinc ores. Hydrometallurgy 2014, 149, 153–167. [Google Scholar] [CrossRef]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Van Gerven, T. Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel approach for enhanced scandium and titanium leaching efficiency from bauxite residue with suppressed silica gel formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef]
- Avdibegović, D.; Regadío, M.; Binnemans, K. Efficient separation of rare earths recovered by a supported ionic liquid from bauxite residue leachate. RSC Adv. 2018, 8, 11886–11893. [Google Scholar] [CrossRef]
- Lima, M.S.S.; Thives, L.P.; Haritonovs, V.; Bajars, K. Red mud application in construction industry: Review of benefits and possibilities. In IOP Conference Series: Materials Science and Engineerin; IOP Publishing: Bristol, UK, 2017; Volume 251, p. 012033. [Google Scholar]
- Tang, W.C.; Wang, Z.; Liu, Y.; Cui, H.Z. Influence of red mud on fresh and hardened properties of self-compacting concrete. Constr. Build. Mater. 2018, 178, 288–300. [Google Scholar] [CrossRef]
- Shetty, K.K. Self compacting concrete using red mud and used foundry sand. Int. J. Res. Eng. Technol. 2014, 3, 708–711. [Google Scholar]
- Pei, D.; Li, Y.; Cang, D.; Pei, D.; Li, Y.; Cang, D.; Pei, D.; Li, Y.; Cang, D. Na+-solidification behavior of SiO2-Al2O3-CaO-MgO (10 wt %) ceramics prepared from red mud. Ceram. Int. 2017, 43, 16936–16942. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, X.; Luo, L.; Zhou, Y.; Wei, J.; Chen, A.; Tang, L.; Wu, H.; Deng, Y.; Zhang, F. Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. Int. Biodeterior. Biodegrad. 2017, 118, 73–81. [Google Scholar] [CrossRef]
- Rath, D.; Nanda, B.; Parida, K. Sustainable nano composite of mesoporous silica supported red mud for solar powered degradation of aquatic pollutants. J. Environ. Chem. Eng. 2017, 5, 6137–6147. [Google Scholar] [CrossRef]
- Yang, Q.; Lian, B.; Mo, B.; Liu, C. Bioleaching of heavy metals from red mud using aspergillus niger. Hydrometallurgy 2013, 136, 71–77. [Google Scholar]
- Sahu, R.C.; Patel, R.; Ray, B.C. Removal of hydrogen sulfide using red mud at ambient conditions. Fuel Process. Technol. 2011, 92, 1587–1592. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Tian, Y.; Chen, Z.; Han, L. Adsorption of methylene blue from aqueous solutions onto sintering process red mud. Desalin. Water Treat. 2012, 47, 31–41. [Google Scholar] [CrossRef]
- Conceição, F.T.D.; Pichinelli, B.C.; Silva, M.S.G.; Moruzzi, R.B.; Menegário, A.A.; Antunes, M.L.P. Cu(ΙΙ) adsorption from aqueous solution using red mud activated by chemical and thermal treatment. Environ. Earth Sci. 2016, 75, 362. [Google Scholar] [CrossRef]
- Hu, Y.; Song, Y.; Qian, F.; Wang, B. Phosphorus recovery from wastewater by red mud-seeded crystallization of calcium phosphate. J. Environ. Eng. Technol. 2014, 4, 60–66. [Google Scholar]
- Sahu, R.C.; Patel, R.; Ray, B.C. Adsorption of Zn(ΙΙ) on activated red mud: Neutralized by CO2. Desalination 2011, 266, 93–97. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).