Critical Metals Ga, Ge and In: Experimental Evidence for Smelter Recovery Improvements
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oguchi, M.; Murakami, S.; Sakanakura, H.; Kida, A.; Kameya, T. A preliminary categorization of end-of-life electrical and electronic equipment as secondary metal resources. Waste Manag. 2011, 31, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Ghosh, M.K.; Parhi, P.; Mukherjee, P.S.; Mishra, B.K. Waste printed circuit boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Holgersson, S.; Steenari, B.M.; Björkman, M.; Cullbrand, K. Analysis of the metal content of small-size Waste Electric and Electronic Equipment (WEEE) printed circuit boards—Part 1: Internet routers, mobile phones and smartphones. Resour. Conserv. Recycl. 2018, 133, 300–308. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.S.; Lee, C.G.; Hong, H.S. Recycling process for recovery of gallium from GaN an e-waste of LED industry through ball milling, annealing and leaching. Environ. Res. 2015, 138, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef]
- Lehner, T. Integrated recycling of non-ferrous metals at Boliden Ltd. Rönnskar smelter. In Proceedings of the 1998 IEEE International Symposium on Electronics and the Environment, Oak Brook, IL, USA, 6 May 1998; pp. 42–47. [Google Scholar]
- Hagelüken, C. Recycling of electronic scrap at Umicore’s integrated metals smelter and refinery. Erzmetall 2006, 59, 152–161. [Google Scholar]
- Nakamura, T. E-scrap recycling system and technologies in Japan. Geosystem Eng. 2014, 17, 104–112. [Google Scholar] [CrossRef]
- Ayres, R.U.; Peiró, L.T. Material efficiency: Rare and critical metals. Phil. Trans. R. Soc. A 2013, 371, 20110563. [Google Scholar] [CrossRef]
- Avarmaa, K.; Yliaho, S.; Taskinen, P. Indium, gallium and tin distributions between copper and slag in WEEE smelting conditions. In Proceedings of the European Metallurgical Conference (EMC) 2017, Leipzig, Germany, 25–28 June 2017; pp. 1485–1500. [Google Scholar]
- Avarmaa, K.; Yliaho, S.; Taskinen, P. Recoveries of rare elements Ga, Ge, In and Sn from waste electric and electronic equipment through secondary copper smelting. Waste Manag. 2017, 71, 400–410. [Google Scholar] [CrossRef]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Paradis, S. Indium, germanium and gallium in volcanic-and sediment-hosted base-metal sulphide deposits. In Proceedings of the Symposium on Strategic and Critical Materials Proceedings, Victoria, BC, Canada, 13–14 November 2015; pp. 23–29. [Google Scholar]
- Yi, W.; Halliday, A.N.; Lee, D.C.; Christensen, J.N. Indium and tin in basalts, sulfides, and the mantle. Geochim. Cosmochim. Acta 1995, 59, 5081–5090. [Google Scholar] [CrossRef]
- Siebert, J.; Corgne, A.; Ryerson, F.J. Systematics of metal–silicate partitioning for many siderophile elements applied to Earth’s core formation. Geochim. Cosmochim. Acta 2011, 75, 1451–1489. [Google Scholar] [CrossRef]
- Holzapfel, C.; Courtial, P.; Dingwell, D.B.; Chakraborty, S.; Palme, H. Experimental determination of partial molar volumes of Ga2O3 and GeO2 in silicate melts: Implications for the pressure dependence of metal–silicate partition coefficients. Chem. Geol. 2001, 174, 33–49. [Google Scholar] [CrossRef]
- Capobianco, C.J.; Drake, M.J.; De’Aro, J. Siderophile geochemistry of Ga, Ge, and Sn: Cationic oxidation states in silicate melts and the effect of composition in iron–nickel alloys. Geochim. Cosmochim. Acta 1999, 63, 2667–2677. [Google Scholar] [CrossRef]
- Schmitt, W.; Palme, H.; Wänke, H. Experimental determination of metal/silicate partition coefficients for P, Co, Ni, Cu, Ga, Ge, Mo, and W and some implications for the early evolution of the Earth. Geochim. Cosmochim. Acta 1989, 53, 173–185. [Google Scholar] [CrossRef]
- Drake, M.J.; Newsom, H.E.; Reed, S.J.; Enright, M.C. Experimental determination of the partitioning of gallium between solid iron metal and synthetic basaltic melt: Electron and ion microprobe study. Geochim. Cosmochim. Acta 1984, 48, 1609–1615. [Google Scholar] [CrossRef]
- Jones, J.H.; Drake, M.J. Geochemical constraints on core formation in the Earth. Nature 1986, 322, 221. [Google Scholar] [CrossRef]
- Mann, U.; Frost, D.J.; Rubie, D.C. Evidence for high-pressure core-mantle differentiation from the metal–silicate partitioning of lithophile and weakly-siderophile elements. Geochim. Cosmochim. Acta 2009, 73, 7360–7386. [Google Scholar] [CrossRef]
- Righter, K.; Nickodem, K.; Pando, K.; Danielson, L.; Boujibar, A.; Righter, M.; Lapen, T.J. Distribution of Sb, As, Ge, and In between metal and silicate during accretion and core formation in the Earth. Geochim. Cosmochim. Acta 2017, 198, 1–16. [Google Scholar] [CrossRef]
- Hidayat, T.; Henao, H.M.; Hayes, P.C.; Jak, E. Phase equilibria studies of Cu-O-Si systems in equilibrium with air and metallic copper and Cu-Me-O-Si systems (Me = Ca, Mg, Al, and Fe) in equilibrium with metallic copper. Metall. Mater. Trans. B 2012, 43, 1290–1299. [Google Scholar] [CrossRef]
- Zhang, R.; Taskinen, P. A phase equilibria study and thermodynamic assessment of the BaO–Al2O3 system. Calphad 2015, 51, 42–50. [Google Scholar] [CrossRef]
- Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Taskinen, P. Urban mining of precious metals via oxidizing copper smelting. Miner. Eng. 2019, 133, 95–102. [Google Scholar] [CrossRef]
- Klemettinen, L.; Avarmaa, K.; Taskinen, P.; Jokilaakso, A. Behavior of nickel as a trace element and time-dependent formation of spinels in WEEE smelting. In Proceedings of the Extraction 2018, Ottawa, ON, Canada, 26–29 August 2018; pp. 1073–1082. [Google Scholar]
- Avarmaa, K.; Taskinen, P. The influence of aluminum on indium and tin behaviour during secondary copper smelting. In Proceedings of the Exctraction 2018, Ottawa, ON, Canada, 26–29 August 2018; pp. 1061–1071. [Google Scholar]
- Gisby, J.; Taskinen, P.; Pihlasalo, J.; Li, Z.; Tyrer, M.; Pearce, J.; Avarmaa, K.; Björklund, P.; Davies, H.; Korpi, M.; et al. MTDATA and the prediction of phase equilibria in oxide systems: 30 years of industrial collaboration. Metall. Mater. Trans. B 2017, 48, 91–98. [Google Scholar] [CrossRef]
- Sylvester, P.J.; Jackson, S.E. A brief history of laser ablation inductively coupled plasma mass spectrometry (LA–ICP–MS). Elements 2016, 12, 307–310. [Google Scholar] [CrossRef]
- Avarmaa, K.; O’Brien, H.; Johto, H.; Taskinen, P. Equilibrium distribution of precious metals between slag and copper matte at 1250–1350 °C. J. Sustain. Metall. 2015, 1, 216–228. [Google Scholar] [CrossRef]
- Sukhomlinov, D.; Klemettinen, L.; Jokilaakso, A.; Taskinen, P. Indium and iridium partitioning between copper matte and iron silicate slag. In Proceedings of the European Metallurgical Conference, Düsseldorf, Germany, 23–26 June 2019. [Google Scholar]
- Jochum, K.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Günther, D. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- GeoREM. Available online: http://georem.mpch-mainz.gwdg.de/sample_query_pref.asp (accessed on 15 April 2019).
- Jochum, K.; Willbold, M.; Raczek, I.; Stoll, B.; Herwig, K. Chemical characterization of the USGS reference glasses GSA-1G, GSC-1G, GSD-1G, GSE-1G, BCR-2G, BHVO-2G and BIR-1G using EPMA, ID-TIMS ID-ICP-MS and LA-ICP-MS. Geostand. Geoanal. Res. 2005, 29, 285–302. [Google Scholar] [CrossRef]
- Van Achterbergh, E.; Ryan, C.G.; Jackson, S.E.; Griffin, W.L. Data reduction software for LA-ICP-MS. In Laser Ablation-ICP-Mass Spectrometry in the Earth Sciences: Principles and Applications; Short Course Series; Mineralogical Association of Canada: Ottawa, ON, Canada, 2001; Volume 29, pp. 239–243. [Google Scholar]
- Belissont, R.; Boiron, M.C.; Luais, B.; Cathelineau, M. LA-ICP-MS analyses of minor and trace elements and bulk Ge isotopes in zoned Ge-rich sphalerites from the Noailhac–Saint-Salvy deposit (France): Insights into incorporation mechanisms and ore deposition processes. Geochim. Cosmochim. Acta 2014, 126, 518–540. [Google Scholar] [CrossRef]
- Yazawa, A.; Nakazawa, S.; Takeda, Y. Distribution behavior of various elements in copper smelting systems. Adv. Sulfide Smelt. 1983, 1, 99–117. [Google Scholar] [CrossRef]
- Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Taskinen, P. The behavior of tin in black copper smelting conditions with different iron-silicate based slags. In Proceedings of the European Metallurgical Conference, Düsseldorf, Germany, 23–26 June 2019. [Google Scholar]
- Germani, M.S.; Small, M.; Zoller, W.H.; Moyers, J.L. Fractionation of elements during copper smelting. Environ. Sci. Technol. 1981, 15, 299–305. [Google Scholar] [CrossRef]
- Ke, J.J.; Qiu, R.; Chen, C.Y. Recovery of metal values from copper smelter flue dust. Hydrometallurgy 1984, 12, 217–224. [Google Scholar] [CrossRef]
- Makipirtti, S.A. Process for the Refining of Sulfidic Complex and Mixed Ores or Concentrates. U.S. Patent No 4,169,725, 2 October 1979. [Google Scholar]
- Rick, C.E. Production of Group IV–A Metals. U.S. Patent No. 2,773,787, 11 December 1956. [Google Scholar]
- Lisowyj, B.; Hitchcock, D.C.; Epstein, H. Process for the Recovery of Gallium and Germanium from Coal Fly Ash. U.S. Patent No. 4,678,647, 7 July 1987. [Google Scholar]
- Klemettinen, L.; Avarmaa, K.; Taskinen, P. Trace element distributions in black copper smelting. Erzmetall 2017, 70, 257–264. [Google Scholar]
- Sukhomlinov, D.; Avarmaa, K.; Virtanen, O.; Taskinen, P.; Jokilaakso, A. Slag-copper equilibria of selected trace elements in black-copper smelting. Part II. Trace element distributions. Miner. Process. Extr. Metall. Rev. 2019. Accepted for publication (10 April 2019). [Google Scholar]
- Anindya, A.; Swinbourne, D.R.; Reuter, M.A.; Matusewicz, R.W. Distribution of elements between copper and FeOx–CaO–SiO2 slags during pyroprocessing of WEEE: Part 2–indium. Miner. Process. Extr. Metall. 2014, 123, 43–52. [Google Scholar] [CrossRef]
- Kegler, P.; Holzheid, A. Determination of the formal Ge-oxide species in silicate melts at oxygen fugacities applicable to terrestrial core formation scenarios. Eur. J. Mineral. 2011, 23, 369–378. [Google Scholar] [CrossRef]
- Morales, P.; Aravena, J.; Salas, J.; Sanchez, G.; Roman, J. Germanium, rhenium and selenium: New valuable by-products to be recovered at Chuquicamata. In Proceedings of the Copper 1991, Hydrometallurgy and Electrometallurgy of Copper, Ottawa, ON, Canada, 18–21 August 1991; pp. 269–280. [Google Scholar]
- Shuva, M.A.H.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.; Reuter, M.A. Thermodynamics behavior of germanium during equilibrium reactions between FeOx-CaO-SiO2-MgO slag and molten copper. Metall. Mater. Trans. B 2016, 47, 2889–2903. [Google Scholar] [CrossRef]
- Yan, S.; Swinbourne, D.R. Distribution of germanium under lead smelting conditions. Miner. Process. Extr. Metall. 2003, 112, 75–80. [Google Scholar] [CrossRef]
- Henao, H.M.; Hayes, P.C.; Jak, E.; Richards, G.G. Research on indium and germanium distributions between lead bullion and slag at selected process conditions. In Proceedings of the Lead Zinc 2010, Vancouver, BC, Canada, 3–6 October 2010; pp. 1145–1160. [Google Scholar]
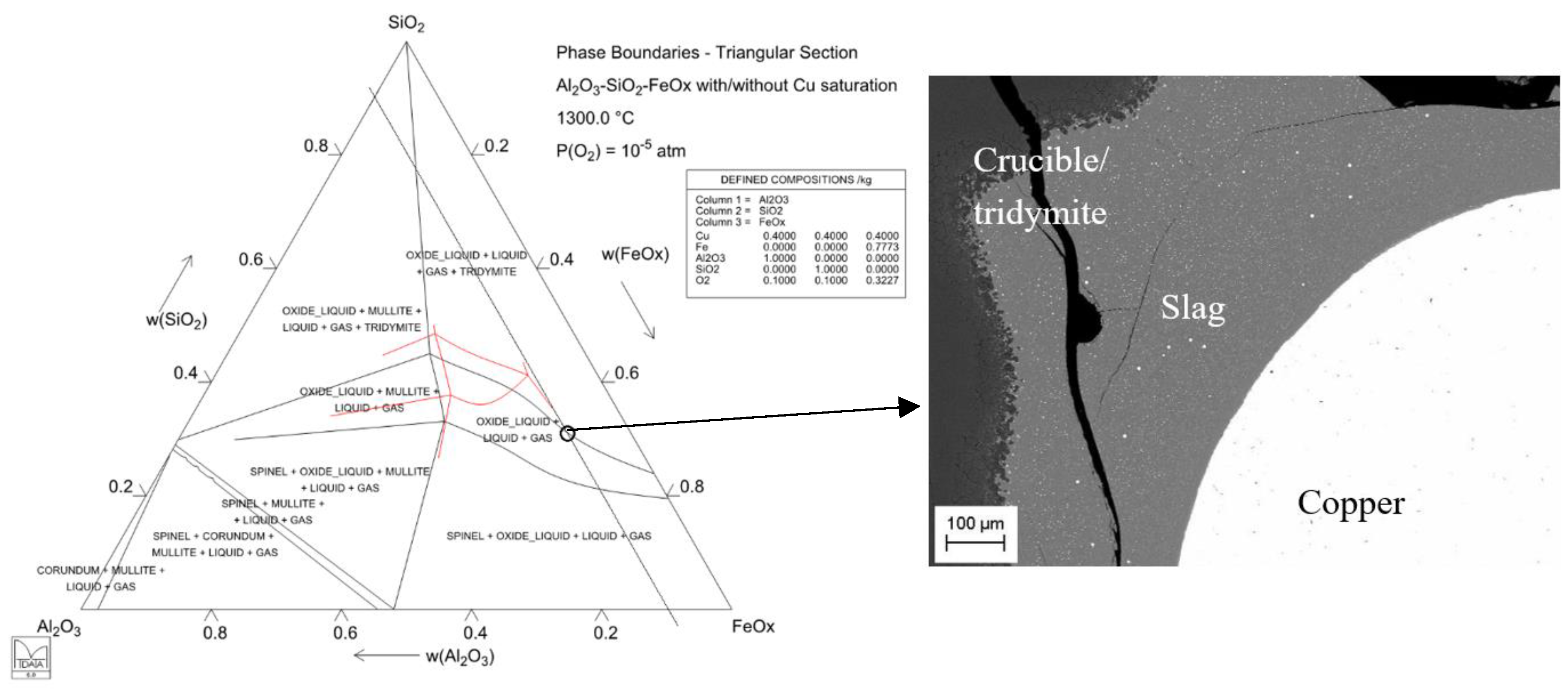



| Ga | Ge | In | |
|---|---|---|---|
| Cu | 211 ppm | 274 ppm | 153 ppm |
| Slag EPMA | 168 ppm | 212 ppm | 124 ppm |
| Slag LA-ICP-MS | 69Ga, 71Ga 8.6, 9.8 ppb | 74Ge 34.7 ppb | 115In 2.2 ppb |
| Standard | Ga | Ge | In |
|---|---|---|---|
| NIST 612 SRM | 36.9 ± 1.5 | 36.1 ± 3.8 | 38.9 ± 2.1 |
| NIST 610 SRM | 433 ± 13 | 447 ± 78 | 434 ± 19 |
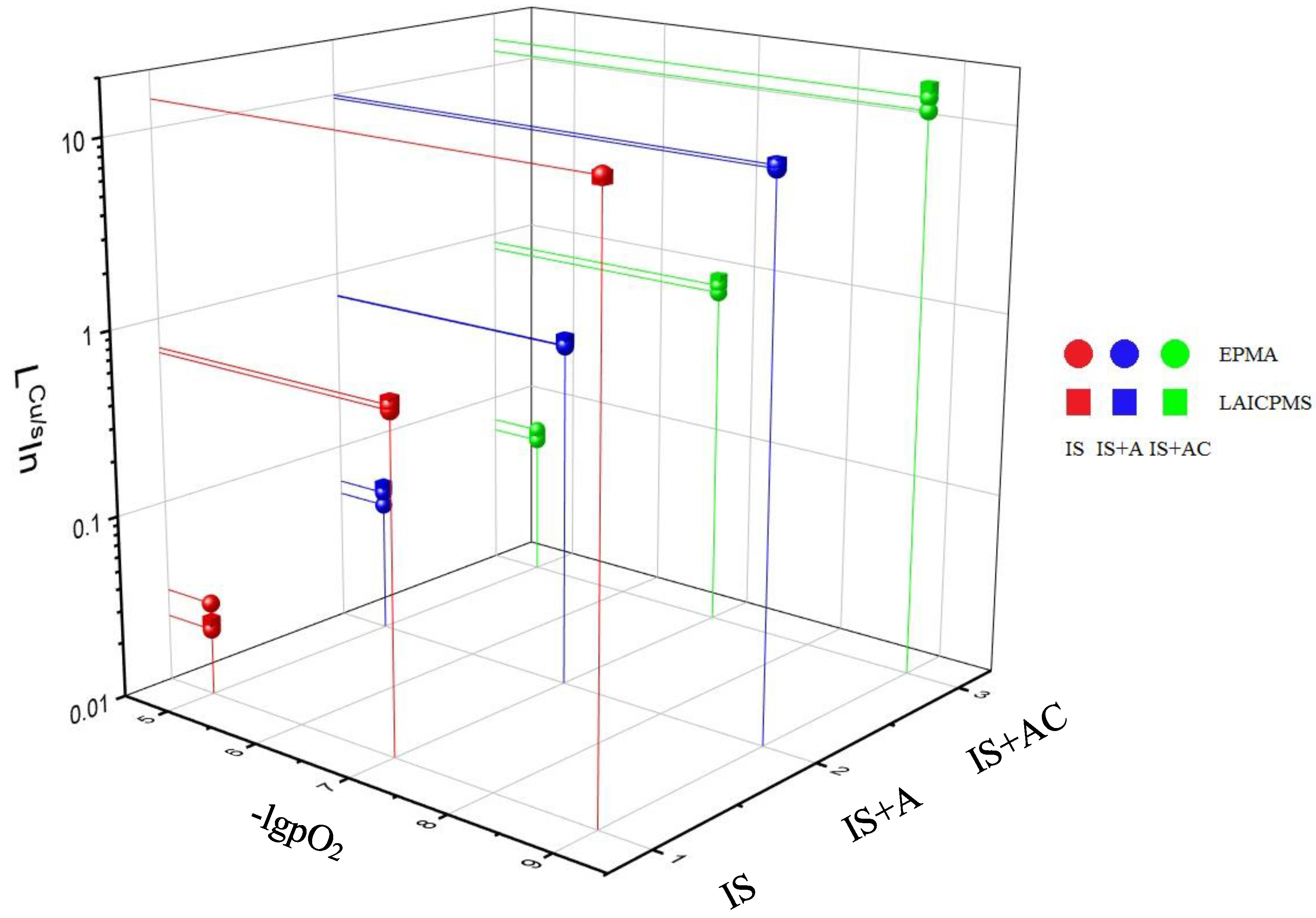
| System1 | lgpO2 | Copper Phase | Slag Phase, EPMA | Slag Phase, LAICPMS 612 | Slag Phase, LAICPMS 610 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In | Ga2 | Ge | In | Ga | Ge | In | Ga | Ge | In | Ga | Ge | ||
| IS | −5 | 110±30 | 60 | n.d. | 4620 ± 50 | 4180 ± 110 | n.d. | 3970 ± 100 | 3310 ± 80 | 4.2 ± 0.5 | 4170 ± 110 | 3630 ± 90 | 4.4 ± 0.5 |
| 160 ± 40 | 40 | n.d. | 4950 ± 60 | 4670 ± 120 | n.d. | ||||||||
| −7 | 840 ± 50 | 70 | n.d. | 1290 ± 100 | 5920 ± 150 | n.d. | 1130 ± 30 | 4690 ± 70 | 0.8 ± 0.2 | 1160 ± 30 | 5000 ± 100 | 0.8 ± 0.2 | |
| 690 ± 80 | 30 | n.d. | 990 ± 50 | 6110 ± 60 | n.d. | ||||||||
| −9 | 1790 ± 130 | 460 ± 60 | n.d. | 130 ± 20 | 4420 ± 70 | n.d. | 120 ± 10 | 4330 ± 50 | 0.5 ± 0.3 | 130 ± 10 | 3760 ± 60 | 0.5 ± 0.3 | |
| IS+A | −5 | 270 ± 70 | 40 | n.d. | 4310 ± 90 | 4390 ± 80 | n.d. | 3850 ± 90 | 3600 ± 80 | 0.5 ± 0.2 | 3970 ± 100 | 3840 ± 90 | 0.5 ± 0.2 |
| 220 ± 80 | 40 | n.d. | 4190 ± 40 | 4430 ± 50 | n.d. | ||||||||
| −7 | 2000 ± 150 | 90 | n.d. | 2700 ± 70 | 6070 ± 50 | n.d. | 2400 ± 60 | 4910 ± 130 | 1.3 ± 0.8 | 2490 ± 70 | 5280 ± 130 | 1.4 ± 0.8 | |
| 1590 ± 160 | 50 | n.d. | 2120 ± 120 | 6310 ± 70 | n.d. | ||||||||
| −9 | 3430 ± 450 | 660 ± 40 | n.d. | 340 ± 70 | 5390 ± 80 | n.d. | 330 ± 40 | 4190 ± 80 | 1.9 ± 1.5 | 340 ± 40 | 4590 ± 90 | 2.0 ± 1.6 | |
| 3410 ± 150 | 670 ± 50 | n.d. | 360 ± 70 | 5530 ± 90 | n.d. | ||||||||
| IS+AC | −5 | 240 ± 70 | 80 | n.d. | 3820 ± 120 | 3850 ± 110 | n.d. | 3500 ± 150 | 3520 ± 110 | 0.5 ± 0.2 | 3660 ± 150 | 3860 ± 120 | 0.5 ± 0.2 |
| 300 ± 120 | 50 | n.d. | 4150 ± 90 | 4480 ± 60 | n.d. | ||||||||
| −7 | 1500 ± 150 | 80 | n.d. | 1680 ± 70 | 4940 ± 160 | n.d. | 1490 ± 90 | 4600 ± 110 | 1.4 ± 1.1 | 1560 ± 90 | 4960 ± 100 | 1.5 ± 1.2 | |
| 1660 ± 90 | 60 | n.d. | 2030 ± 60 | 5800 ± 110 | n.d. | ||||||||
| −9 | 4090 ± 380 | 370 ± 70 | n.d. | 290 ± 20 | 5630 ± 90 | n.d. | 250 ± 10 | 4510 ± 80 | 0.9 ± 0.4 | 260 ± 10 | 4930 ± 90 | 0.9 ± 0.5 | |
| 3240 ± 330 | 380 ± 70 | n.d. | 270 ± 50 | 5630 ± 80 | n.d. | 4.4 ± 0.5 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Taskinen, P.; Jokilaakso, A. Critical Metals Ga, Ge and In: Experimental Evidence for Smelter Recovery Improvements. Minerals 2019, 9, 367. https://doi.org/10.3390/min9060367
Avarmaa K, Klemettinen L, O’Brien H, Taskinen P, Jokilaakso A. Critical Metals Ga, Ge and In: Experimental Evidence for Smelter Recovery Improvements. Minerals. 2019; 9(6):367. https://doi.org/10.3390/min9060367
Chicago/Turabian StyleAvarmaa, Katri, Lassi Klemettinen, Hugh O’Brien, Pekka Taskinen, and Ari Jokilaakso. 2019. "Critical Metals Ga, Ge and In: Experimental Evidence for Smelter Recovery Improvements" Minerals 9, no. 6: 367. https://doi.org/10.3390/min9060367
APA StyleAvarmaa, K., Klemettinen, L., O’Brien, H., Taskinen, P., & Jokilaakso, A. (2019). Critical Metals Ga, Ge and In: Experimental Evidence for Smelter Recovery Improvements. Minerals, 9(6), 367. https://doi.org/10.3390/min9060367






