Pilot-Scale Removal of Arsenic and Heavy Metals from Mining Wastewater Using Adsorption Combined with Constructed Wetland
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbent, Plant, Substrate, and Mining Wastewater
2.2. System Set-Up and Operation
2.3. Sampling
2.4. Analytical Methods
2.5. Calculation of Metal Removal and Accumulation
3. Results
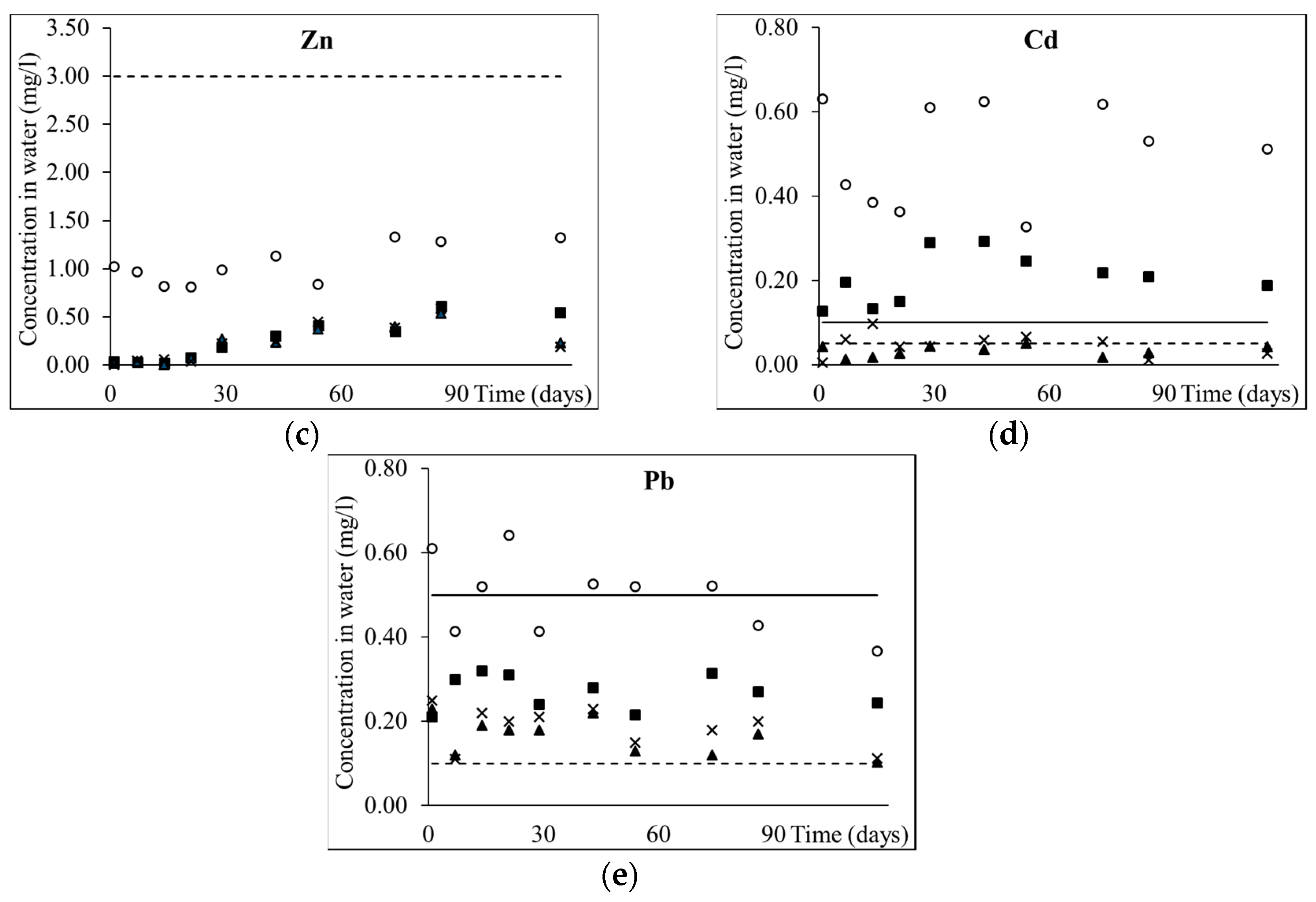
3.1. Removal of As and Heavy Metals from Mining Wastewater
3.2. Accumulation of As and Heavy Metals in the Adsorbent
3.3. Plant Growth and Concentrations of As and Heavy Metals in P. australis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gosar, M. Environmental impacts of metal mining. Mater. Geoenviron. 2004, 51, 2097–2107. [Google Scholar]
- Chockalingam, E.; Subramanian, S. Studies on removal of metal ions and sulphate reduction using rice husk and Desulfotomaculum nigrificans with reference to remediation of acid mine drainage. Chemosphere 2006, 62, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Gunatilake, S.K. Methods of removing heavy metals from industrial wastewater. J. Multidiscip. Eng. Sci. Stud. 2015, 1, 12–18. [Google Scholar]
- Chakravarti, A.K.; Chowdhury, S.B.; Chakrabarty, S.; Chakrabarty, T.; Mukherjee, D.C. Liquid membrane multiple emulsion process of chromium(VI) separation from wastewaters. Colloids Surf. A Physicochem. Eng. Asp. 1995, 103, 59–71. [Google Scholar] [CrossRef]
- Hering, J.G.; Chen, P.J.; Wilkie, J.A.; Elimelech, M. Arsenic removal from drinking water during coagulation. J. Environ. Eng. 1997, 8, 800–807. [Google Scholar] [CrossRef]
- Kim, J.; Benjamin, M.M. Modeling a novel ion exchange process for arsenic and nitrate removal. Water Res. 2004, 38, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.R.; Chaudhari, S.; Khilar, K.C.; Mahajan, S.P. Removal of arsenic from water by electrocoagulation. Chemosphere 2005, 55, 1245–1252. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I. Removal of arsenic from contaminated water sources by sorption onto Iron-coated polymeric materials. Water Res. 2002, 36, 5145–5155. [Google Scholar] [CrossRef]
- Aksu, Z. Determination of equilibirium, kinetic and thermodynamic parameters of the batch biosorption of nickel (II) ions onto Clorella vulgaris. Process Biochem. 2002, 38, 89–99. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Vashi, R.T. COD and BOD removal from Textile wastewater using naturally prepared adsorbents and their activation forms using sulphuric acid. Wastewater Engineering: Advanced Wastewater Treatment Systems. IJSR Publ. 2014, 31–40. [Google Scholar]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Iakovleva, E.; Sillanpaa, M. The use of low-cost adsorbents for wastewater purification in mining industries. Environ. Sci. Pollut. Res. 2013, 20, 7878–7899. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, L.; Guo, B.; He, S. Adsorption of mercury on laterite from Guizhou Province, China. J. Environ. Sci. 2008, 20, 1328–1334. [Google Scholar] [CrossRef]
- Chen, T.Y.; Kao, C.M.; Yeh, T.Y.; Chien, H.Y.; Chao, A.C. Application of a constructed wetland for industrial wastewater treatment: A pilot-scale study. Chemesphere 2006, 64, 497–502. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed wetlands for wastewater treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Vymazal, J.; Brezinova, T. Accumulation of heavy metals in aboveground biomass of Phragmites australis in horizontal flow constructed wetlands for wastewater treatment: A review. Chem. Eng. J. 2016, 290, 232–242. [Google Scholar] [CrossRef]
- Masi, F.; Rizzo, A.; Regelsberger, M. The role of constructed wetlands in a new circular economy, resource oriented, and ecosystem services paradigm. J. Environ. Manag. 2017, 216, 275–284. [Google Scholar] [CrossRef]
- Jiang, X.; Wanga, C. Zinc distribution and zinc-binding forms in Phragmites australis under zinc pollution. J. Plant Physiol. 2008, 165, 697–704. [Google Scholar] [CrossRef]
- Chen, M.Z.; Tang, Y.Y.; Li, X.P.; Yu, Z.X. Study on the heavy metals removal efficiencies of constructed wetlands with different substrates. J. Water Resour. Prot. 2009, 1, 22–28. [Google Scholar] [CrossRef]
- Zhu, W.L.; Cui, L.H.; Ouyang, Y.; Long, C.F.; Tang, X.D. Kinetic adsorption of ammonium nitrogen by substrate materials for constructed wetlands. Pedosphere 2011, 21, 454–463. [Google Scholar] [CrossRef]
- Allende, L.K.; McCarthy, D.T.; Fletcher, T.D. The influence of media type on removal of arsenic, iron and boron from acidic wastewater in horizontal flow wetland microcosms planted with Phragmites australis. Chem. Eng. J. 2014, 246, 217–228. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Sakakibara, M.; Sano, S. Accumulation of Indium and other heavy metals by Eleocharis acicularis: An option for phytoremediation and phytomining. Bioresour. Technol. 2011, 102, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.M.; Nguyen, B.Q.; Nguyen, H.T.; Nguyen, H.T.H. Adsorption of arsenic and heavy metals from solutions by unmodified iron-ore sludge. Appl. Sci. 2019, 9, 619. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Anh, B.T.K. The removal of heavy metals by iron mine drainage sludge and Phragmites australis. IOP Conf. Ser. Earth Environ. Sci. 2017, 71, 012–022. [Google Scholar]
- Thao, N.H.P.; Ha, N.T.H.; Thuy, P.T.; Khai, N.M.; Nga, T.T.H. The sorption ability of Tam Duong and Thach That laterite in the treatment of heavy metals and arsenic. Vnu J. Sci. Earth Sci. 2016, 32, 321–326. [Google Scholar]
- QCVN40: 2011-BTNMT Vietnam National Technical Regulation on Industrial Wastewater Quality. Available online: https://moitruong.com.vn/Upload/48/Nam_2017/Thang_3/Ngay_3/QCVN40-2011-BTNMT.pdf (accessed on 24 November 2018).
- Renugadevi, N.; Sreeja, M.; Lalitha, P. Kinetics of chromium(IV) removal from aqueous solution by adsorption technique using activated carbon from pods of wood apple. J. Ultra Chem. 2010, 6, 27–34. [Google Scholar]
- Alessio, G.; Paola, V.; Ezio, R. Removal and accumulation of Cu, Ni, and Zn in horizontal subsurface flow constructed wetlands: Contribution of vegetation and filling medium. Sci. Total Environ. 2010, 408, 5097–5105. [Google Scholar]
- Yeh, T.Y.; Chou, C.C.; Pan, C.T. Heavy metal removal within pilot-scale constructed wetlands receiving river watẻ contaminated by confined swine operations. Desalination 2009, 249, 308–373. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Xu, H.; Wang, D.; Xu, J. Accumulation of Cd, Pb and Zn by 19 wetland plant species in constructed wetland. J. Hazard. Mater. 2007, 147, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Tripathi, B.D. Effect of Phragmites australis and Typha latifolia on biofiltration of heavy metals from secondary treated effluent. Int. J. Environ. Sci. Technol. 2013, 12, 1029–1038. [Google Scholar] [CrossRef]
- Scholes, L.N.L.; Shutes, R.B.E.; Revitt, D.M.; Purchase, D.; Forshaw, M. The removal of urban pollutants by constructed wetlands during wet weather. Water Sci. Technol. 1999, 40, 333–340. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, Q.; Jin, X.; Chen, Z. Removal of Pb (II) from aqueous solution using modified and unmodified kaolinite clay. J. Hazard. Mater. 2009, 170, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W. Arsenate and cadmium co-adsorption and co-precipitation on goethite. J. Hazard. Mater. 2013, 262, 55–63. [Google Scholar] [CrossRef]
- Raul, P.K.; Devi, R.R.; Umlong, I.M.; Thakur, A.J.; Banerjee, S.; Veer, V. Iron oxide hydroxide nanoflower assisted removal of arsenic from water. Mater. Res. Bull. 2014, 49, 360–368. [Google Scholar] [CrossRef]
- Glocheux, Y.; Pasarin, M.M.; Albadarin, A.B.; Allen, S.J.; Walker, G.M. Removal of arsenic from groundwater by adsorption onto an acidified laterite by-product. Chem. Eng. J. 2013, 228, 565–574. [Google Scholar] [CrossRef]
- Pehlivan, E.; Tran, H.T.; Ouédraogo, W.K.I.; Schmidt, C.; Zachmann, D.; Bahadir, M. Sugarcane bagasse treated with hydrous ferric oxide as a potential adsorbent for the removal of As(V) from aqueous solutions. Food Chem. 2013, 138, 133–138. [Google Scholar] [CrossRef]
- Vieira, B.R.C.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S.; Santos, S.C.R. Arsenic removal from water using iron-coated seaweeds. J. Environ. Manag. 2017, 192, 224–233. [Google Scholar] [CrossRef]
- Ramana, D.K.V.; Jamuna, K.; Satyanarayana, B.; Venkateswarlu, B.; Rao, M.M.; Seshaiah, K. Removal of heavy metals from aqueous solutions using activated carbon prepared from Cicer arietinum. Toxicol. Environ. Chem. 2010, 92, 1447–1460. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Immobilization of Pb (II), Cd (II) and Ni (II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J. Environ. Manag. 2008, 87, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Giménez, J.; De Pablo, J.; Martínez, M.; Rovira, M.; Valderrama, C. Reactive transport of arsenic (III) and arsenic (V) on natural hematite: Experimental and modeling. J. Colloid Interface Sci. 2010, 348, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Donald, L.S. Environmental Soil Chemistry, 2nd ed.; Academic Press: London, UK, 2003; ISBN 0126564469. [Google Scholar]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of adsorbents and recovery of heavy metals: A review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef]
- Wambu, E.W.; Muthakia, G.K.; Shiundu, P.M.; Thiongo, K.J. Kinetics of copper desorption from regenerated spent bleaching earth. Am. Eurasian J. Sci. Res. 2009, 4, 317–323. [Google Scholar]
- Jalali, R.; Ghafourian, H.; Asef, Y.; Davarpanah, S.J.; Sepeh, S. Removal and recovery of lead using nonliving biomass of marine algae. J. Hazard. Mater. 2002, 92, 253–262. [Google Scholar] [CrossRef]
- Alishir, A.; Mohammad, M.; Abdolmajid, L.; Ebrahim, P.; Hossein, S. Mercury and arsenic accumulation by three species of aquatic plants in Dezful, Iran. Afr. J. Agric. Res. 2011, 6, 5391–5397. [Google Scholar] [CrossRef]
- Ye, Z.H.; Baker, A.J.M.; Wong, M.H.; Willis, A.J. Zinc, lead and Cadmium tolerance, Uptake and Accumulation by the Common reed, Phragmites australis (Cav.) Trin. Ex Steudel. Ann. Bot. 1997, 80, 363–370. [Google Scholar] [CrossRef]
- Vymazal, J.; Lenka, K.; Jaroslav, S.; Vladislav, C.; Jana, S. Trace elements in Phragmites australis growing in constructed wetlands for treatment of municipal wastewater. Ecol. Eng. 2009, 35, 303–309. [Google Scholar] [CrossRef]
- Diego, C.F.; Manuel, P.F.; Jose, A.E.; Blanca, A.L. Long-term (two annual cycles) phytoremediation of heavy metal- contaminated estuarine sediments by Phragmites australis. New Biotechnol. 2016, 38, 56–64. [Google Scholar]
- Lesage, E.; Rousseau, D.P.L.; Meers, E.; Tack, F.M.G.; Pauw, N.D. Accumulation of metals in a horizontal subsurface flow constructed wetland treating domestic wastewater in Flanders, Belgium. Sci. Total Environ. 2007, 380, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Schierup, H.H.; Larsen, V.J. Macrophyte cycling of zinc, copper, lead and cadmium in the littoral zone of a polluted and non-polluted lake. I. Availability, uptake, and translocation of heavy metals in Phragmites australis (Cav.) Trin. Aquat. Bot 1981, 11, 197–210. [Google Scholar] [CrossRef]
- Phillips, D.P.; Human, L.R.D.; Adams, J.B. Wetland plants as indicators of heavy metal contamination. Mar. Pollut. Bull. 2015, 92, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G.; Giudice, R.L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. J. 2009, 10, 639–645. [Google Scholar] [CrossRef]
- Nga, T.T.H.; Ha, N.T.H. Simultaneous removal of some heavy metals and arsenic from aqueous solutions by Phragmites australis. Vietnam J. Sci. Technol. 2016, 54, 259–264. [Google Scholar]
- Selinus, O.; Alloway, B.J.; Centeno, J.A.; Finkelman, R.B.; Fuge, R.; Lindh, U.; Smedley, P. Essentials of Medical Geology: Impacts of the Natural Environment on Public Health; Elsevier Academic Press: London, UK, 2005. [Google Scholar]
- Marchand, L.; Mench, M.; Jacob, D.L.; Otte, M.L. Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: A review. Environ. Pollut. 2010, 158, 3447–3461. [Google Scholar] [CrossRef]
- Anh, T.T.; Gaskov, I.V.; Hoa, T.T.; Nevolko, P.A.; Dung, P.T.; Nien, B.A.; Can, P.N. Mineralogical and geochemical characteristics and forming conditions of lead—Zinc deposits in Lo Gam structure, northern Vietnam. Vietnam J. Earth Sci. 2011, 33, 393–408. (In Vietnamese) [Google Scholar]





| Material Characteristics | Adsorbent (SBC2-400-10S) | Substrates | ||
|---|---|---|---|---|
| Limestone | Laterite | |||
| Mineral composition (%) | Quartz | 43 * | - | 39 ** |
| Muscovite | 13 * | - | - | |
| Illite | 13 * | - | 2 ** | |
| Kaolinite | 12 * | - | 36 ** | |
| Hematite | 7 * | - | 7 ** | |
| Goethite | 4 * | - | 15 ** | |
| Calcite | - | 14 | - | |
| Ankerite | - | 86 | - | |
| BET (m2/g) | 39.5 | - | - | |
| PCD (mmolc (-)∙kg−1) | 88.7 | - | 52 ** | |
| Module | Filtration Materials (Substrates) | Dimension (Height (mm) × Width (mm) × Length) (mm)) | Working Volume (m3) |
|---|---|---|---|
| MD1 | 2000 × 1500 × 1500 | 4.5 | |
| MD2 | SBC2-400-10S | 2000 × 420 × 840 | 0.71 |
| MD3–MD6 | Limestone | 1000 × 1000 × 4100 | 4.1 |
| MD7 | Limestone | 1000 × 500 × 4100 | 2.05 |
| MD8 | Laterite | 1000 × 500 × 4100 | 2.05 |
| Moduls | As | Mn | Zn | Cd | Pb |
|---|---|---|---|---|---|
| MD1 | 9.56 | 2.64 | 27.2 | 13.7 | 28.1 |
| MD2 | 49.6 | 54.3 | 50.0 | 43.9 | 16.0 |
| MD3-7 | 30.7 | 42.6 | 2.87 | 35.7 | 22.7 |
| MD3-7 * | 75.1 | 98.8 | 30.2 | 83.7 | 42.8 |
| MD3-6 + MD8 | 34.7 | 32.1 | 2.39 | 31.9 | 18.3 |
| MD2 + MD3-7 | 80.3 | 96.9 | 52.9 | 79.6 | 38.7 |
| MD1 + MD2 + MD3-7 | 89.9 | 99.5 | 80.1 | 93.3 | 66.8 |
| Metals | Authors | Type of Wetland | Substrates | Length × width × depth (m) | Retention Time | Hydraulic Loading Rate | Operation Duration (Months) | Influent Concentration (mg/L) | Removal Efficiency (%) | Metal Content in P. australis (mg/kg-DW) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Belowground Biomass | Aboveground Biomass | ||||||||||
| As | This study | HSSF | Limestone | 1 × 1 × 4.1 + 1 × 0.5x4.1 | 2 d | 5 m3/d | 4 | 0.1 | 75.1 | 21.9 | 17.8 |
| Laterite | 86.0 | ||||||||||
| [24] | HSSF | Zeolite | 0.6 × 0.2 × 0.65 | 11 d | 30 mm/d | 5.5 | 2.6 | 99.9 | 0.00286 | 0.00234 | |
| [27] * | SF | Soil | 1.2 × 0.23 | 2 d | 0.05 m3/day | 1 | 0.4 | 80.5 | - | - | |
| HSSF | Limestone | 1.2 × 0.28 | 83.1 | ||||||||
| [49] | - | Sand | 1 × 0.6 | 10 d | - | - | 0.2 | - | 119.55 | 6.36 | |
| [50] | VSSF | Sand | 1 × 1 × 1.5 | 2–3w | 28 mm/d | 24 | - | 1.65 | 0.58 | ||
| [51] | HSSF | - | - | - | - | - | - | - | 4.95 | 0.18 | |
| [52] | - | Sediment | - | - | - | - | 37.6 | - | 1.2–2.4 | 0.1–0.6 | |
| Mn | This study | HSSF | Limestone | 1 × 1 × 4.1 + 1 × 0.5 × 4.1 | 2 d | 5 m3/day | 4 | 1.34 | 98.8 | 2200 | 506 |
| [27] * | SF | Soil | 1.2 × 0.23 | 2 d | 0.05m3/day | 1 | 4 | 91.2 | - | - | |
| HSSF | Limestone | 1.2 × 0.28 | 94.1 | ||||||||
| [51] | HSSF | - | - | - | - | - | - | - | 266 | 175 | |
| [52] | - | Sediment | - | - | - | - | 2606 | - | 264.7–1178 | 193.3–361.9 | |
| [53] | VSSF | Goetextile | 12.5 × 30 × 0.6 | 2 d | 184 m3/d | 10 | - | 52 | 146 | ||
| HSSF | Gravel | 50 × 13 × 0.5 | 6.7 d | 22.5 m3/d | 58 | 60 | |||||
| [54] | - | Gravel | 30 × 3 × 0.6 | 15 d | 2000 l/d | 5 | 3.47 | - | 266 | 87.5 | |
| Zn | This study | HSSF | Limestone | 1 × 1 × 4.1 + 1 × 0.5 × 4.1 | 2 d | 5 m3/day | 4 | 0.26 | 30.2 | 188 | 144 |
| [22] | - | Coke | 1.93 × 0.4 × 0.6 | 12.68 d | 300mL/min | 2 | 48.5 | 91.18 | - | 0.1016 | |
| Gravel | 9.6 d | 5.268 | 61.57 | 0.009 | |||||||
| [27] | SF | Soil | 1.2 × 0.23 | 2 d | 0.05 m3/day | 1 | 1.5 | 78.9 | - | - | |
| HSSF | Limestone | 1.2 × 0.28 | 81.7 | ||||||||
| [31] | HSSF | Gravel | 12 × 2.5 × (1.5–2) | - | 8 m3/d | 8 | 0.5 | 27 | 66.2 | 59.1 | |
| [32] | SF | Gravel and sand | 1.8 × 0.5 × 0.5 | 1.25 d | 16 cm/d | 4 | 0.366 | 92 | 160 | 60 | |
| [35] | SSP | Soil | 1 × 2 × 0.25 | 2 | 5 | 94.2 | 302.53 | 149.56 | |||
| [55] | HSSF | - | - | - | - | - | - | - | 86 | 20.5 | |
| [52] | - | Sediment | - | - | - | - | 516 | - | 69.6–198 | 45.7–154.6 | |
| [53] | VSSF | Goetextile | 12.5 × 30 × 0.6 | 2 d | 184 m3/d | - | - | - | 132 | 70 | |
| HSSF | Gravel | 50 × 13 × 0.5 | 6.7 d | 22.5 m3/d | 10 | - | - | 129 | 23 | ||
| [54] | - | Gravel | 30 × 3 × 0.6 | 15 d | 2000 L/d | 5 | 0.12 | - | 46.3 | 16.6 | |
| [55] | - | - | - | - | - | - | - | - | 54.99–176.7 | 20.65–32.4 | |
| Cd | This study | HSSF | Limestone | 1 × 1 × 4.1 + 1 × 0.5 × 4.1 | 2d | 5 m3/day | 4 | 0.21 | 83.7 | 0.784 | 1.1 |
| [33] | - | PVC | 0.5 × 0.5 × 0.3 | 16 d | - | 2 | 4.55 | 44.2 | - | 2.47 | |
| [34] | - | - | 360 m2 | - | - | 4 | 1.73 | 72 | - | 0.007–0.04 | |
| [35] | SSP | Soil | 1 × 2 × 0.25 | 2 | 0.5 | 92.2 | 33.26 | 11.42 | |||
| [50] | VSSF | Sand | 1 × 1 × 1.5 | 2–3 w | 28 mm/d | 24 | - | 0.739 | <0.03 | ||
| [51] | HSSF | - | - | - | - | - | - | - | 0.21 | 0.57–0.9 | |
| [52] | - | Sediment | - | - | - | - | 0.1 | - | 0.2–1.3 | <0.1 | |
| [53] | VSSF | Goetextile | 12.5 × 30 × 0.6 | 2 d | 184 m3/d | - | - | - | 0.21 | 0.014 | |
| HSSF | Gravel | 50 × 13 × 0.5 | 6.7 d | 22.5 m3/d | 10 | 0.47 | 0.083 | ||||
| [54] | - | Gravel | 30 × 3 × 0.6 | 15 d | 2000 L/d | 5 | <0.01 | - | 0.25 | 0.05 | |
| Pb | This study | HSSF | Limestone | 1 × 1 × 4.1 + 1 × 0.5x4.1 | 2 d | 5 m3/day | 4 | 0.27 | 42.8 | 115 | 80.9 |
| [22] | - | Coke | 1.93 × 0.4 × 0.6 | 12.68 d | 300 mL/min | - | 27.44 | 99.66 | - | 0.0763 | |
| Gravel | 9.6 d | 49.89 | 99.53 | 0.0736 | |||||||
| [27] | SF | Soil | 1.2 × 0.23 | 2 d | 0.05 m3/day | 1 | 0.6 | 73.5 | - | - | |
| HSSF | Limestone | 1.2 × 0.28 | 81.1 | ||||||||
| [34] | - | - | 360 m2 | - | - | 4 | 7.58 | 69 | - | 36-108 | |
| [35] | SSP | Soil | 1 × 2 × 0.25 | 2 | 2 | 96.4 | 152.88 | 54.3 | |||
| [50] | VSSF | Sand | 1 × 1 × 1.5 | 2–3 w | 28 mm/d | 24 | - | - | 17.63 | 0.98 | |
| [51] | HSSF | - | - | - | - | - | - | - | 7.1 | 0.11 | |
| [52] | - | Sediment | - | - | - | - | 186 | - | 9.1–79.1 | 0.7–1.1 | |
| [53] | VSSF | Goetextile | 12.5 × 30 × 0.6 | 2 d | 184 m3/d | - | - | - | 10 | 0.39 | |
| HSSF | Gravel | 50 × 13 × 0.5 | 6.7 d | 22.5 m3/d | 10 | 20 | 0.53 | ||||
| [54] | - | Gravel | 30 × 3 × 0.6 | 15 d | 2000 L/d | 5 | 0.06 | - | 12.2 | 0.1 | |
| [55] | - | - | - | - | - | - | - | - | 21.49–90.94 | 1.14–2.18 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.H.; Nguyen, B.Q.; Duong, T.T.; Bui, A.T.K.; Nguyen, H.T.A.; Cao, H.T.; Mai, N.T.; Nguyen, K.M.; Pham, T.T.; Kim, K.-W. Pilot-Scale Removal of Arsenic and Heavy Metals from Mining Wastewater Using Adsorption Combined with Constructed Wetland. Minerals 2019, 9, 379. https://doi.org/10.3390/min9060379
Nguyen HTH, Nguyen BQ, Duong TT, Bui ATK, Nguyen HTA, Cao HT, Mai NT, Nguyen KM, Pham TT, Kim K-W. Pilot-Scale Removal of Arsenic and Heavy Metals from Mining Wastewater Using Adsorption Combined with Constructed Wetland. Minerals. 2019; 9(6):379. https://doi.org/10.3390/min9060379
Chicago/Turabian StyleNguyen, Ha T. H., Bien Q. Nguyen, Thuy T. Duong, Anh T. K. Bui, Hang T. A. Nguyen, Ha T. Cao, Nhuan T. Mai, Khai M. Nguyen, Thuy T. Pham, and Kyoung-Woong Kim. 2019. "Pilot-Scale Removal of Arsenic and Heavy Metals from Mining Wastewater Using Adsorption Combined with Constructed Wetland" Minerals 9, no. 6: 379. https://doi.org/10.3390/min9060379
APA StyleNguyen, H. T. H., Nguyen, B. Q., Duong, T. T., Bui, A. T. K., Nguyen, H. T. A., Cao, H. T., Mai, N. T., Nguyen, K. M., Pham, T. T., & Kim, K.-W. (2019). Pilot-Scale Removal of Arsenic and Heavy Metals from Mining Wastewater Using Adsorption Combined with Constructed Wetland. Minerals, 9(6), 379. https://doi.org/10.3390/min9060379






