Biotic–Abiotic Influences on Modern Ca–Si-Rich Hydrothermal Spring Mounds of the Pastos Grandes Volcanic Caldera (Bolivia)
Abstract
:1. Introduction
2. Settings
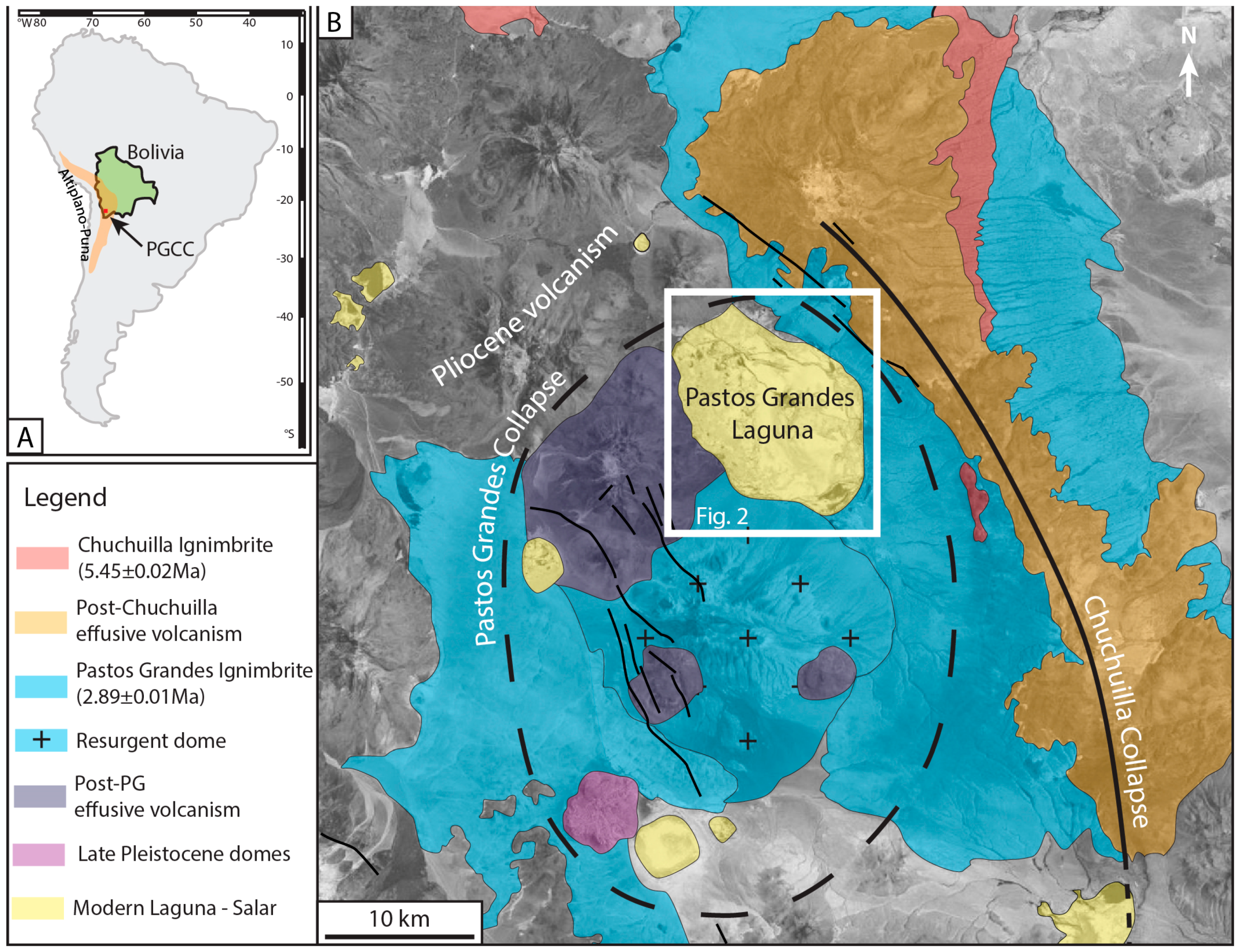
2.1. Geological Setting
2.2. Climate
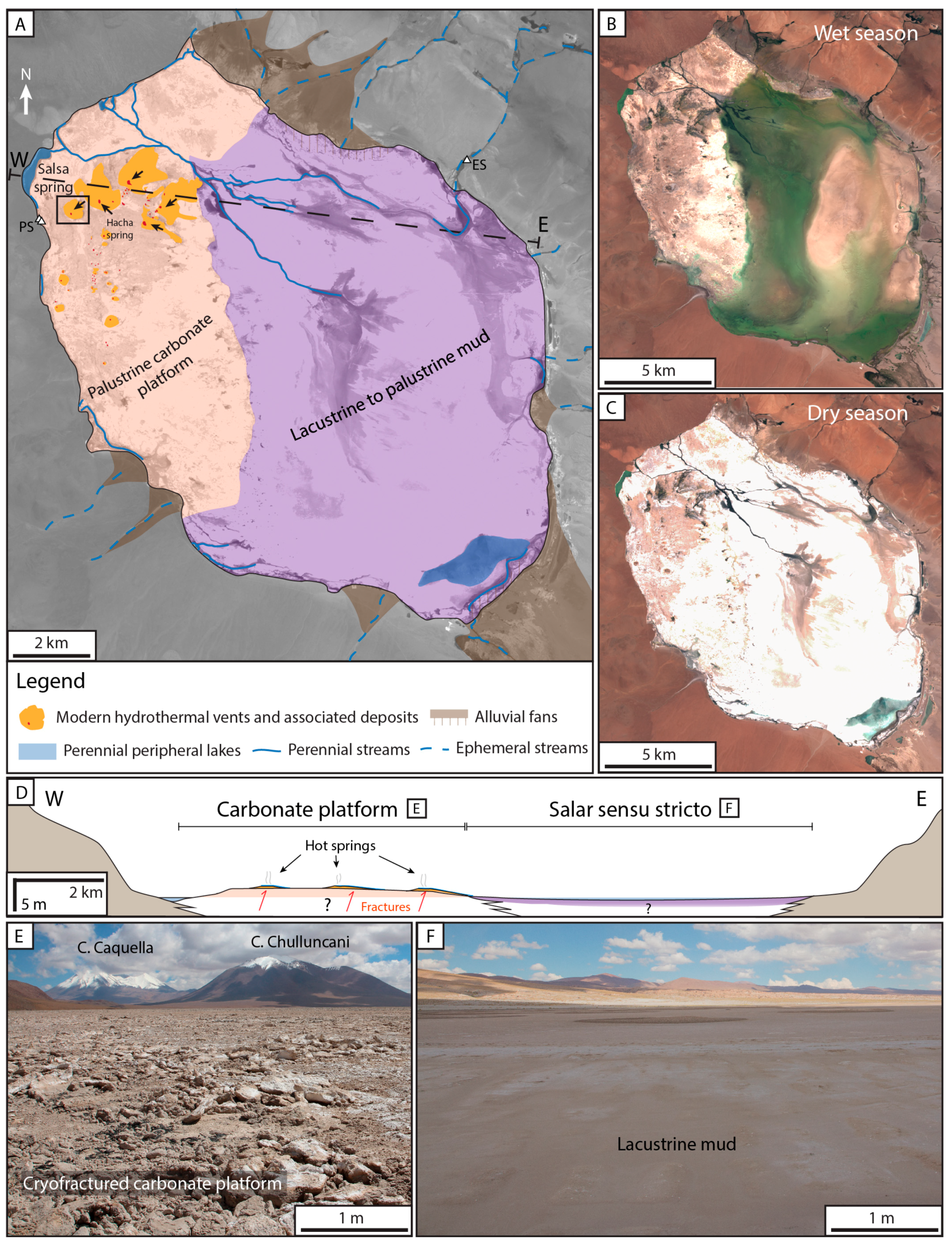
2.3. The Pastos Grandes Laguna
3. Materials and Methods
4. Results
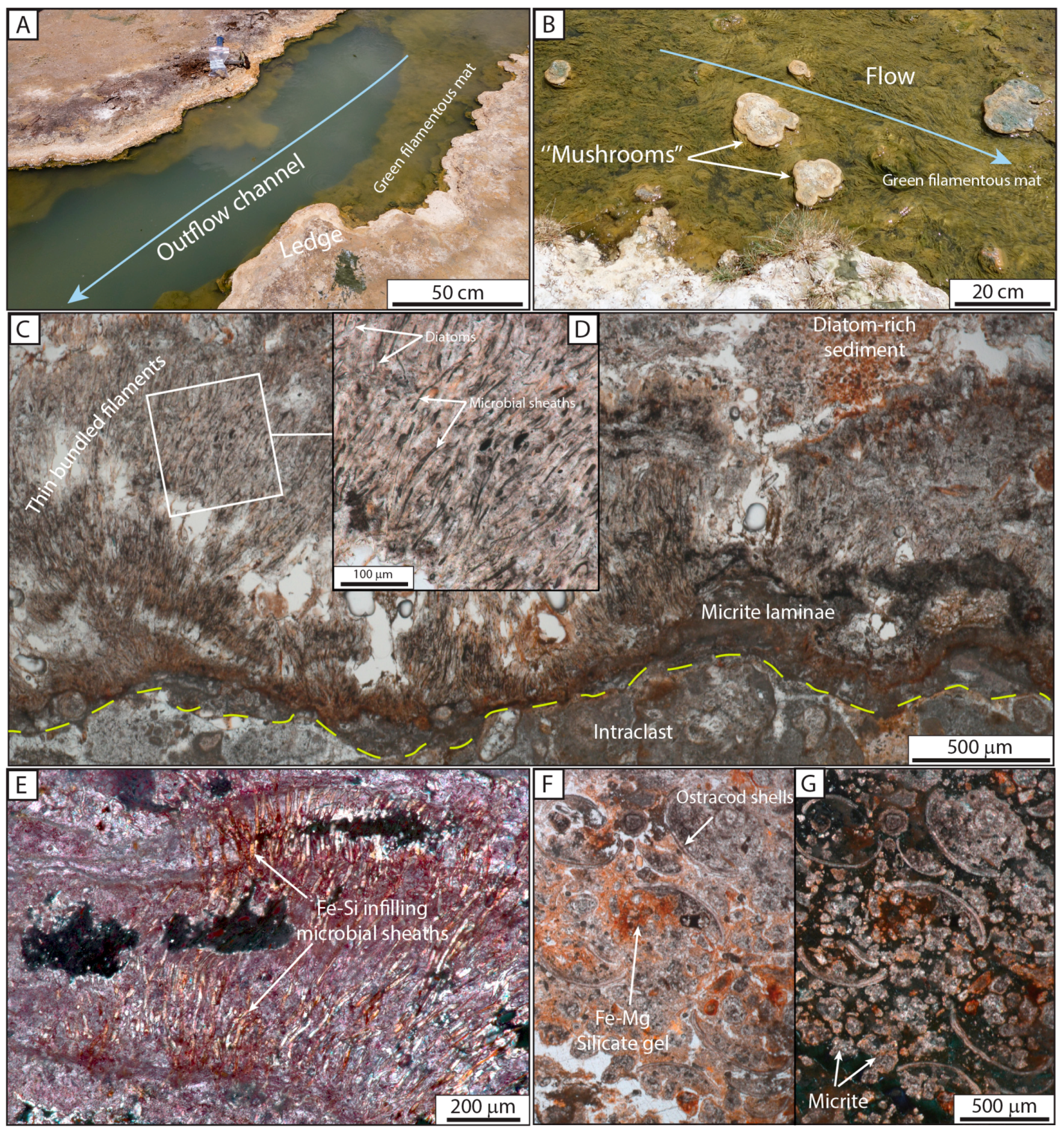
4.1. La Salsa Flat Mound
4.2. La Salsa Main Hydrothermal Discharge
4.2.1. Water Properties
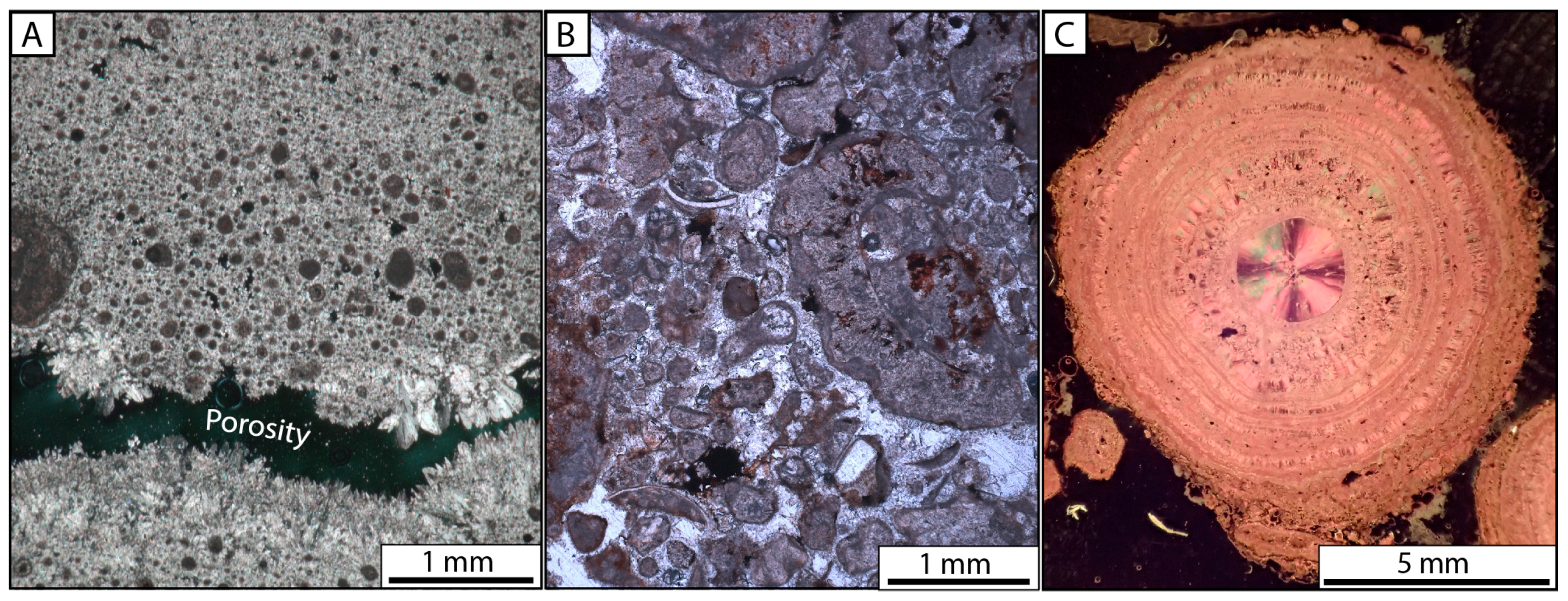
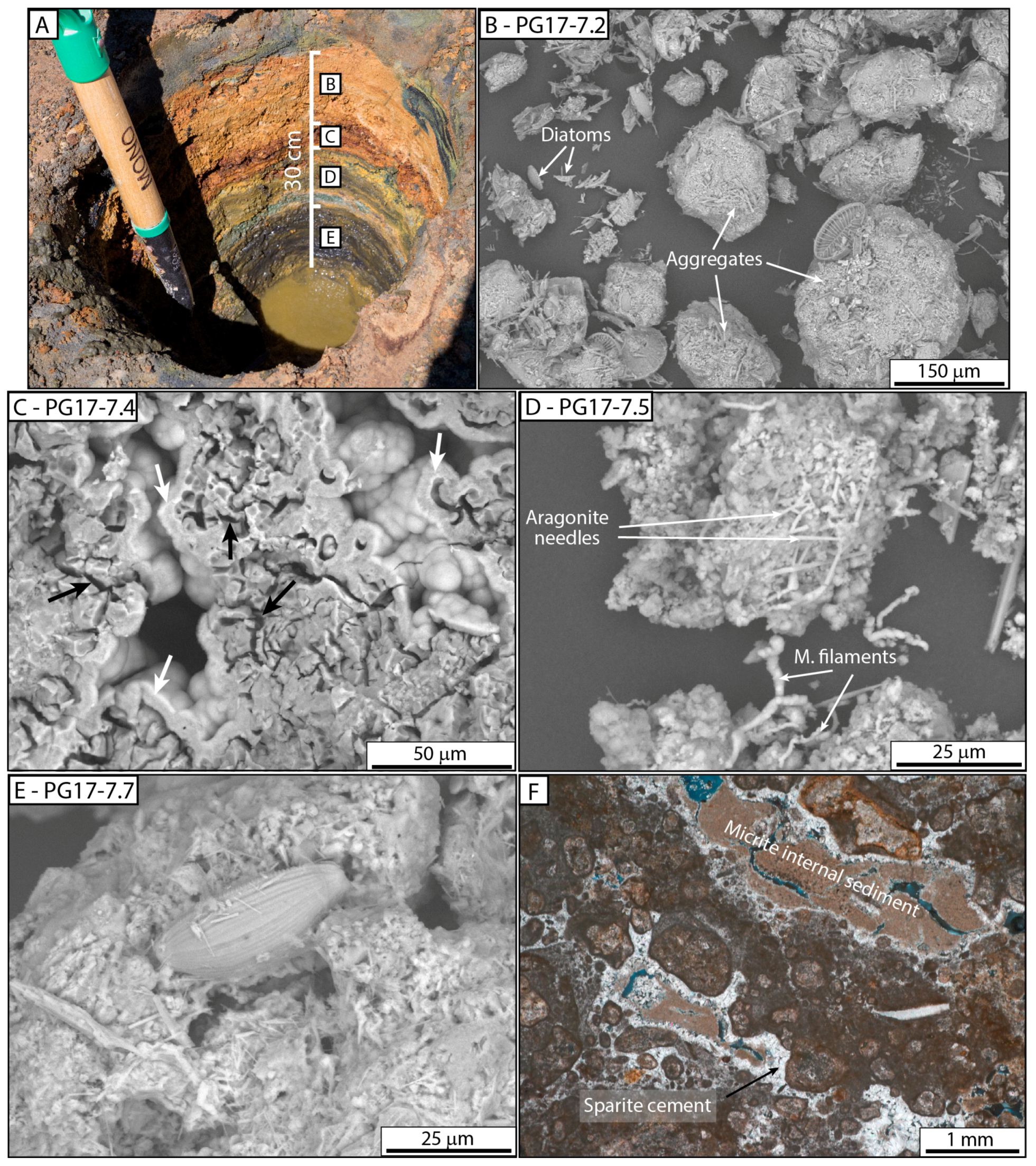
4.2.2. Microbial and Mud Deposits
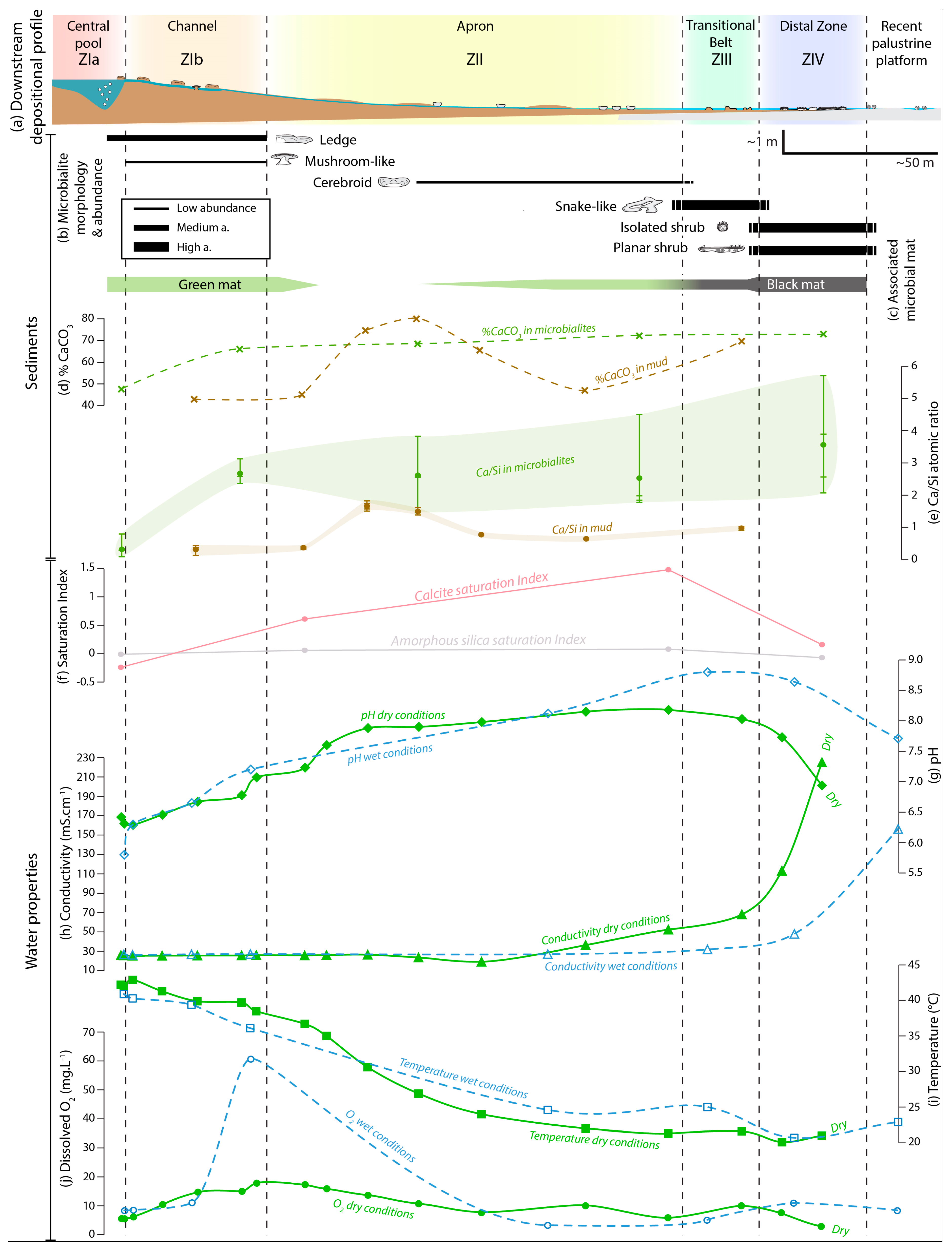
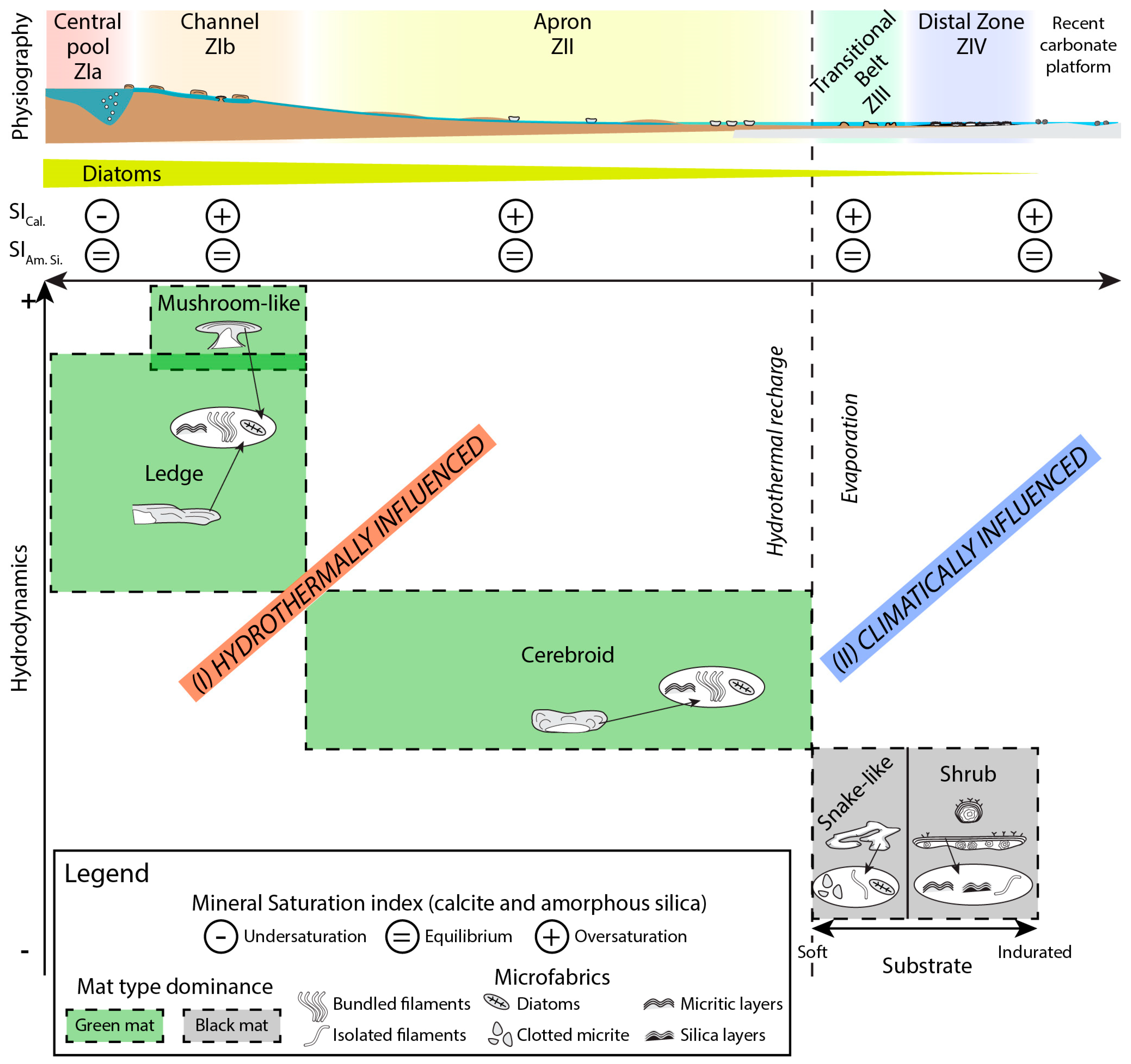
4.2.3. Zonation of the Depositional Environment
Zone I—The Hydrothermal Feeding System
Zone II—The Apron
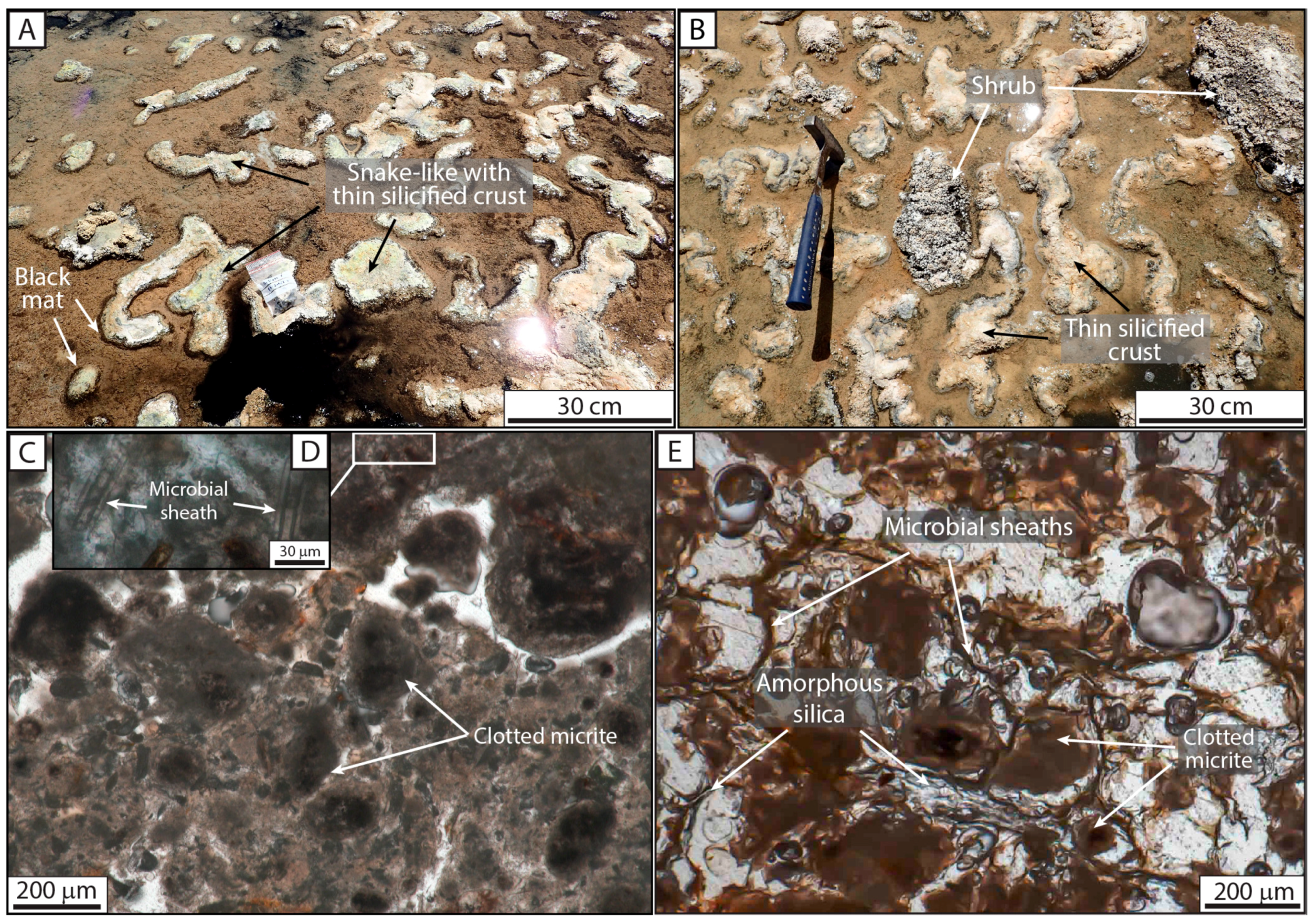
Zone III—The Proximal–Distal Transitional Belt
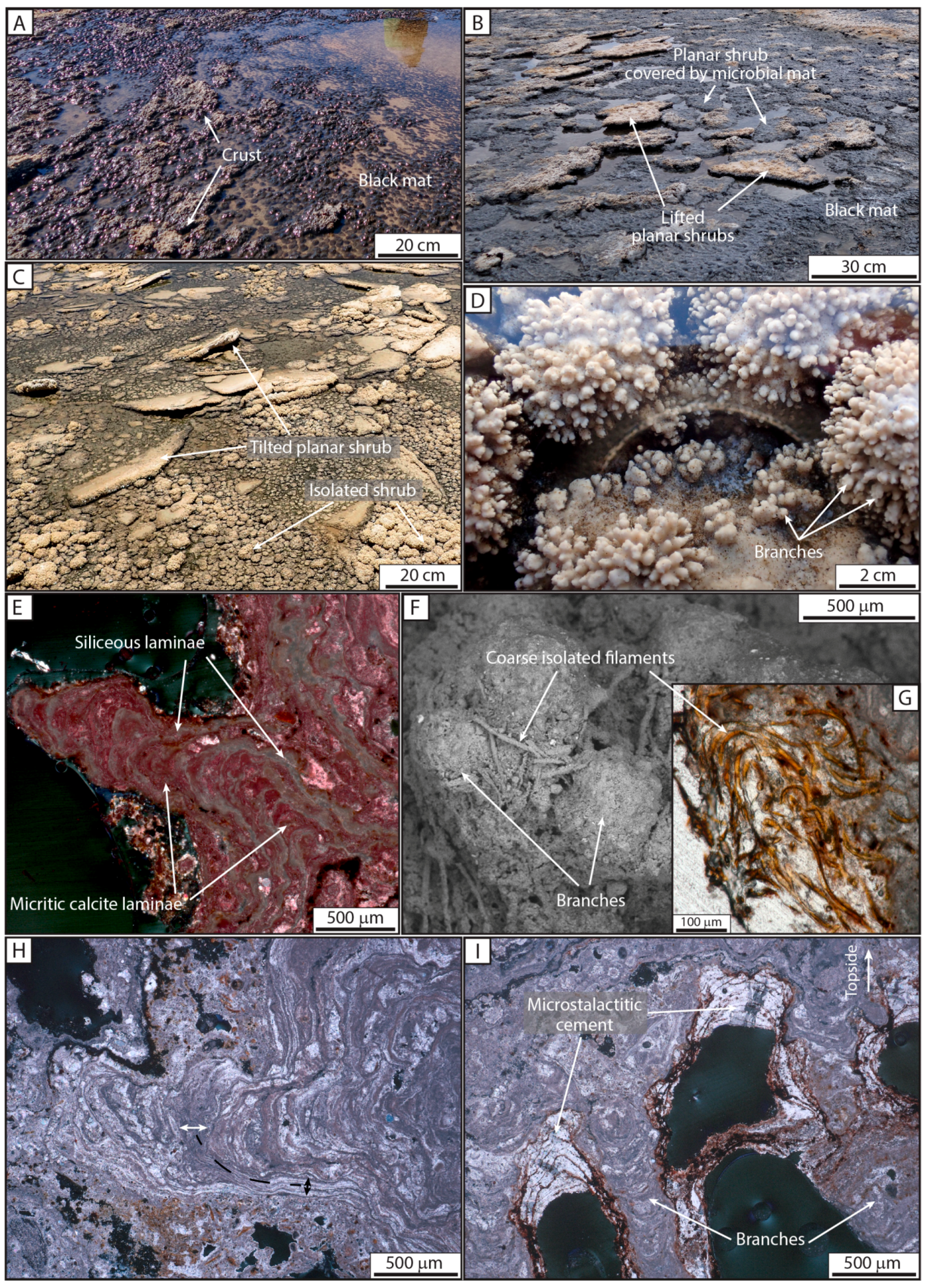
Zone IV—The Distal Zone
The Transient Peripheral Saline Zone
5. Interpretation and Discussion
5.1. Origin of the Flat Mound
5.2. Interaction between Microbialites and their Physical Environment
5.3. Controlling Factors in the Development of Biotic/Abiotic Products
6. Conclusions
- (1)
- The flat mound is dominated by diatoms, calcitic micrite crystals, and diagenetic aragonite needles. Its origin is not yet fully elucidated, but two processes are clearly involved: (i) accumulation of mud during ephemeral flooding around the central pool and (ii) diagenetic precipitation inside the mound, enhancing its elevation by a swelling effect. Despite some morphological signs suggesting mud volcanism, sedimentary observations do not support this hypothesis.
- (2)
- Competition between hydrothermal influence and climatic factors plays a major role in microbialite distribution along the hydrothermal pathway, together with changes in accommodation, hydrodynamics, and substrate. Under proximal hydrothermal influence, in moderate-to-high hydrodynamic conditions, with scarce discontinuous indurated substrates, microbial deposits present ledge and mushroom-like morphologies. In lower hydrodynamic conditions, cerebroid morphologies are observed. Under climatic influence, with low hydrodynamics, snake-like and shrub morphologies dominate. Snake-like microbialites grow on cohesive sediments (with early lithification by drying-up processes) while shrubs grow on inherited carbonate platform slabs and clasts.
- (3)
- The Modern mixed carbonate–silicate deposition observed around the hydrothermal La Salsa spring is a good example of the complex interactions between physical, chemical, and biochemical parameters, controlling mineralogy and microfabrics distribution along a subaerial hydrological pathway. Iron hydroxides, amorphous silica, and calcite precipitate together but result from independent processes: (i) iron hydroxides from oxidation, (ii) amorphous silica during temperature cooling and/or capillary effects, and (iii) calcite when pH increases during CO2 degassing and CO2 assimilation through biological activity.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, B.; Renaut, R.W.; Rosen, M.R. Microbial biofacies in hot-spring sinters: A model based on Ohaaki Pool, North Island, New Zealand. J. Sediment. Res. 1998, 68, 413–434. [Google Scholar] [CrossRef]
- Fernandez-Turiel, J.L.; Garcia-Valles, M.; Gimeno-Torrente, D.; Saavedra-Alonso, J.; Martinez-Manent, S. The hot spring and geyser sinters of El Tatio, Northern Chile. Sediment. Geol. 2005, 180, 125–147. [Google Scholar] [CrossRef]
- Okumura, T.; Takashima, C.; Shiraishi, F.; Nishida, S.; Yukimura, K.; Naganuma, T.; Koike, H.; Arp, G.; Kano, A. Microbial Porcesses Forming Daily Lamination in an Aragonite Travertine, Nagano-yu Hot Spring, Southwest Japan. Geomicrobiol. J. 2011, 28, 135–148. [Google Scholar] [CrossRef]
- Okumura, T.; Takashima, C.; Shiraishi, F.; Kano, A. Textural transition in an aragonite travertine formed under various flow conditions at Pancuran Pitu, Central Java, Indonesia. Sediment. Geol. 2012, 265–266, 195–209. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Phoenix, V.R.; Bottrell, S.H.; Adams, D.G.; Head, I.M. Microbial-silica interactions in Icelandic hot spring sinter: Possible analogues for some Precambrian siliceous stromatolites. Sedimentology 2001, 48, 415–433. [Google Scholar] [CrossRef]
- Renaut, R.W.; Owen, R.B.; Jones, B.; Tiercelin, J.-J.; Tarits, C.; Ego, J.K.; Konhauser, K.O. Impact of lake-level changes on the formation of thermogene travertine in continental rifts: Evidence from Lake Bogoria, Kenya Rift Valley. Sedimentology 2013, 60, 428–468. [Google Scholar] [CrossRef]
- Fouke, B.W.; Farmer, J.D.; Des Marais, D.J.; Pratt, L.; Sturchio, N.C.; Burns, P.C.; Discipulo, M.K. Depositional facies and aquaeous-solid geochemistry of travertine-depositing hot springs (Angel Terrace, Mammoth Hot Springs, Yellowstone National Park, U.S.A.). J. Sediment. Res. 2000, 70, 565–585. [Google Scholar] [CrossRef]
- Teboul, P.-A.; Durlet, C.; Gaucher, E.C.; Virgone, A.; Girard, J.-P.; Curie, J.; Lopez, B.; Camoin, G.F. Origins of elements building travertine and tufa: New perspectives provided by isotopic and geochemical tracers. Sediment. Geol. 2016, 334, 97–114. [Google Scholar] [CrossRef]
- Kele, S.; Demény, A.; Siklósy, Z.; Németh, T.; Tóth, M.; Kovács, M.B. Chemical and stable isotope composition of recent hot-water travertines and associated thermal waters, from Egerszalók, Hungary: Depositional facies and non-equilibrium fractionation. Sediment. Geol. 2008, 211, 53–72. [Google Scholar] [CrossRef]
- Kele, S.; Özkul, M.; Fórizs, I.; Gökgöz, A.; Baykara, M.O.; Alçiçek, M.C.; Németh, T. Stable isotope geochemical study of Pamukkale travertines: New evidences of low-temperature non-equilibrium calcite-water fractionation. Sediment. Geol. 2011, 238, 191–212. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W.; Rosen, M.R. Biogenicity of silica precipitation around geysers and hot-spring vents, North Island, New Zealand. J. Sediment. Res. 1997, 67, 88–104. [Google Scholar] [CrossRef]
- Braunstein, D.; Lowe, D.R. Relationship between spring and geyser activity and the deposition and morphology of high temperature (>73 °C) siliceous sinter, Yellowstone National Park, WY, USA. J. Sediment. Res. 2001, 71, 747–763. [Google Scholar] [CrossRef]
- Renaut, R.W.; Jones, B.; Tiercelin, J.-J. Rapid in situ silicification of microbes at Loburu hot springs, Lake Bogoria, Kenya Rift Valley. Sedimentology 1998, 45, 1083–1103. [Google Scholar] [CrossRef]
- White, D.E.; Brannock, W.W.; Murata, K.J. Silica in hot-spring waters. Geochim. Cosmochim. Acta 1956, 10, 27–59. [Google Scholar] [CrossRef]
- Mountain, B.W.; Benning, L.G.; Boerema, J.A. Experimental studies on New Zealand hot spring sinters: Rates of growth and textural development. Can. J. Earth Sci. 2003, 40, 1643–1667. [Google Scholar] [CrossRef]
- Yee, N.; Phoenix, V.R.; Konhauser, K.O.; Benning, L.G.; Ferris, F.G. The effect of cyanobacteria on silica precipitation at neutral pH: Implications for bacterial silicification in geothermal hot springs. Chem. Geol. 2003, 199, 83–90. [Google Scholar] [CrossRef]
- Benning, L.G.; Phoenix, V.R.; Yee, N.; Konhauser, K.O. The dynamics of cyanobacterial silicification: An infrared micro-spectroscopic investigation. Geochim. Cosmochim. Acta 2004, 68, 743–757. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W.; Rosen, M.R. High-temperature (>90 °C) calcite precipitation at Waikite Hot Springs, North Island, New Zealand. J. Geol. Soc. 1996, 153, 481–496. [Google Scholar] [CrossRef]
- Renaut, R.W.; Jones, B.; Le Turdu, C. Calcite lilypads and ledges at Lorusio Hot Springs, Kenya Rift Valley: Travertine precipitation at the air-water interface. Can. J. Earth Sci. 1999, 36, 649–666. [Google Scholar] [CrossRef]
- Campbell, K.A.; Rodgers, K.A.; Brotheridge, J.M.A.; Browne, P.R.L. An unusual modern silica–carbonate sinter from Pavlova spring, Ngatamariki, New Zealand. Sedimentology 2002, 49, 835–854. [Google Scholar] [CrossRef]
- Smith, D.J.; Jenkin, G.R.T.; Petterson, M.G.; Naden, J.; Fielder, S.; Toba, T.; Chenery, S.R.N. Unusual mixed silica-carbonate deposits from magmatic-hydrothermal hot springs, Savo, Solomon Islands. J. Geol. Soc. 2011, 168, 1297–1310. [Google Scholar] [CrossRef]
- Guido, D.M.; Campbell, K.A. Jurassic hot Spring deposits of the Deseado Massif (Patagonia, Argentina): Characteristics and controls on regional distribution. J. Volcanol. Geotherm. Res. 2011, 203, 35–47. [Google Scholar] [CrossRef]
- Wright, V.P.; Barnett, A.J. An abiotic model for the development of textures in some South Atlantic early Cretaceous lacustrine carbonates. Geol. Soc. Lond. Spec. Publ. 2015, 418, 209–219. [Google Scholar] [CrossRef]
- Teboul, P.-A.; Kluska, J.-M.; Marty, N.C.M.; Debure, M.; Durlet, C.; Virgone, A.; Gaucher, E.C. Volcanic rock alterations of the Kwanza Basin, offshore Angola—Insights from an integrated petrological, geochemical and numerical approach. Mar. Petrol. Geol. 2017, 80, 394–411. [Google Scholar] [CrossRef]
- Risacher, F.; Eugster, H.P. Holocene pisoliths and encrustations associated with spring-fed surface pools, Pastos Grandes, Bolivia. Sedimentology 1979, 26, 253–270. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W. Crystal fabrics and microbiota in large pisoliths from Laguna Pastos Grandes, Bolivia. Sedimentology 1994, 41, 1171–1202. [Google Scholar] [CrossRef]
- Servant-Vildary, S.; Roux, M. Multivariate analysis of diatoms and water chemistry in Bolivian saline lakes. Hydrobiologia 1990, 197, 267–290. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Muller, E.; Ader, M.; Gérard, E.; Virgone, A.; Gaucher, E.; Durlet, C.; Moreira, M.A.; Virgile, R.; Vennin, E.; Agogué, H.; et al. Carbonate Formation and Diagenesis in Pastos Grandes Laguna (Bolivia): Modern Analog for the South Atlantic Cretaceous Presalt Travertinoid Deposits. In Proceedings of the AGU Fall Meeting, New Orleans, LA, USA, 11–15 December 2017. [Google Scholar]
- Bougeault, C.; Durlet, C.; Vennin, E.; Muller, E.; Mercuzot, M.; Gérard, E.; Ader, M.; Virgone, A.; Gaucher, E.C. Atypical carbonate-silica mineralisations under biotic and abiotic controls: The Modern hudrothermal example of Pastos Grandes Laguna (Bolivia). In Proceedings of the 20th International Sedimentological Congress, Québec City, QC, Canada, 13–17 August 2018. [Google Scholar]
- Allmendinger, R.W.; Jordan, T.E.; Kay, S.M.; Isacks, B.L. The evolution of the Altiplano-Puna Plateau of the Central Andes. Annu. Rev. Earth Planet. Sci. 1997, 25, 139–174. [Google Scholar] [CrossRef]
- Isacks, B.L. Uplift of the Central Andean Plateau and Bending of the Bolivian Orocline. J. Geophys. Res. 1988, 93, 3211–3231. [Google Scholar] [CrossRef]
- Tibaldi, A.; Corazzato, C.; Rovida, A. Miocene–Quaternary structural evolution of the Uyuni–Atacama region, Andes of Chile and Bolivia. Tectonophysics 2009, 471, 114–135. [Google Scholar] [CrossRef]
- de Silva, S.L. Altiplano–Puna volcanic complex of the central Andes. Geology 1989, 17, 1102–1106. [Google Scholar] [CrossRef]
- de Silva, S.; Zandt, G.; Trumbull, R.; Viramonte, J.G.; Salas, G.; Jiménez, N. Large ignimbrite eruptions and volcano-tectonic depressions in the Central Andes: A thermomechanical perspective. Geol. Soc. Lond. Spec. Publ. 2006, 269, 47–63. [Google Scholar] [CrossRef]
- Salisbury, M.J.; Jicha, B.R.; de Silva, S.L.; Singer, B.S.; Jiménez, N.C.; Ort, M.H. 40Ar/39Ar chronostratigraphy of Altiplano–Puna volcanic complex ignimbrites reveals the development of a major magmatic province. Geol. Soc. Am. Bull. 2011, 123, 821–840. [Google Scholar] [CrossRef]
- Chmielowski, J.; Zandt, G.; Haberland, C. The Central Andean Altiplano-Puna Magmatic Body. Geophys. Res. Lett. 1999, 26, 783–786. [Google Scholar] [CrossRef]
- Ward, K.M.; Zandt, G.; Beck, S.L.; Christensen, D.H.; McFarlin, H. Seismic imaging of the magmatic underpinnings beneath the Altiplano-Puna volcanic complex from the joint inversion of the surface wave dispersion and receiver functions. Earth Planet. Sci. Lett. 2014, 404, 43–53. [Google Scholar] [CrossRef]
- Kaiser, J.F. Understanding Large Resurgent Calderas and Associated Magma Systems: The Pastos Grandes Caldera Complex, Southwest Bolivia. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2014. [Google Scholar]
- Kaiser, J.F.; de Silva, S.; Schmitt, A.K.; Economos, R.; Sunagua, M. Million-year melt-presence in monotonous intermediate magma for a volcanic-plutonic assemblage in the Central Andes: Contrasting histories of crystal-rich and crystal-poor super-sized silicic magmas. Earth Planet. Sci. Lett. 2017, 457, 73–86. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 4, 439–473. [Google Scholar] [CrossRef]
- Risacher, F. Le cadre géochimique des bassins à évaporites des Andes boliviennes. Cah. ORSTOM Sér. Géol. 1978, 10, 37–48. [Google Scholar]
- Iltis, A.; Risacher, F.; Servant–Vildary, S. Contribution à l’étude hydrobiologique des lacs salés du sud de l’Altiplano bolivien. Rev. Hydrobiol. Trop. 1984, 17, 259–273. [Google Scholar]
- Risacher, F.; Fritz, B. Geochemistry of Bolivian salars, Lipez, southern Altiplano: Origin of solutes and brine evolution. Geochim. Cosmochim. Acta 1991, 55, 687–705. [Google Scholar] [CrossRef]
- Ballivian, O.; Risacher, F. Los Salares del Altiplano Boliviano: Métodos de Estudio y Estimación Económica; ORSTOM: Paris, France, 1981; 246p. [Google Scholar]
- Ahlfeld, F. Sodaseen in Lipez (Bolivien). Neues Jb. Miner. Mh. 1956, 6, 128–136. [Google Scholar]
- Badaut, D.; Risacher, F. Authigenic smectite on diatom frustules in Bolivian saline lakes. Geochim. Cosmochim. Acta 1983, 47, 363–375. [Google Scholar] [CrossRef]
- Abdelouas, A. Etude de l’altération de Verres Rhyolitiques au Contact de Saumures Naturelles (Bolivie)—Application à L’étude du Comportement à Long Terme du Verre Nucléaire R7T7. Ph.D. Thesis, Université Louis Pasteur, Strasbourg, France, 1996. [Google Scholar]
- Alonso, R.N.; Viramonte, J.G. Borate deposits in the Andes. In Stratabound Ore Deposits in the Andes; Fontboté, L., Amstutz, G.C., Cardozo, E., Frutos, J., Eds.; Springer: Berlin, Germany, 1990; pp. 721–732. [Google Scholar]
- Kasemann, S.A.; Meixner, A.; Erzinger, J.; Viramonte, J.G.; Alonso, R.N.; Franz, G. Boron isotope composition of geothermal fluids and borate minerals from salar deposits (central Andes/NW Argentina). J. S. Am. Earth Sci. 2004, 16, 685–697. [Google Scholar] [CrossRef]
- Rouchy, J.M.; Servant, M.; Fournier, M.; Causse, C. Extensive carbonate algal bioherms in upper Pleistocene saline lakes of the central Altiplano of Bolivia. Sedimentology 1996, 43, 973–993. [Google Scholar] [CrossRef]
- Sylvestre, F.; Servant, M.; Servant-Vildary, S.; Causse, C.; Fournier, M.; Ybert, J.-P. Lake–Level Chronology on the Southern Bolivian Altiplano (18–23°) during Late-Glacial Time and the Early Holocene. Quat. Res. 1999, 51, 54–66. [Google Scholar] [CrossRef]
- Blard, P.-H.; Sylvestre, F.; Tripati, A.K.; Claude, C.; Causse, C.; Coudrain, A.; Condom, T.; Seidel, J.-L.; Vimeux, F.; Moreau, C.; et al. Lake highstands on the Altiplano (Tropical Andes) contemporaneous with Heinrich 1 and the Younger Dryas: New insights from 14C, U–Th dating and δ18O of carbonates. Quat. Sci. Rev. 2011, 30, 3973–3989. [Google Scholar] [CrossRef]
- Placzek, C.J.; Quade, J.; Patchett, P.J. A 130 ka reconstruction of rainfall on the Bolivian Altiplano. Earth Planet. Sci. Lett. 2013, 363, 97–108. [Google Scholar] [CrossRef]
- Fontes, J.C.; Servant, M. Dataciones Radiometricas Sobre el Cuaternario Reciente del Altiplano de Bolivia; Primer Congreso Geologico de Boliva-Potosi: Potosi, Bolivia, 1976. [Google Scholar]
- Shapiro, R.S. A Comment on the Systematic Confusion of Thrombolites. Palaios 2000, 15, 166–169. [Google Scholar] [CrossRef]
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentary deposits of benthic microbial communities. Palaios 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation, Batch Reaction, one Dimensional Transport, and Inverse Geochemical Calculations; 6-A43; U.S. Geological Survey Techniques and Methods: Reston, VA, USA, 2013; 497p. [Google Scholar]
- Blanc, P.; Lassin, A.; Piantone, P.; Azaroual, M.; Jacquemet, N.; Fabbri, A.; Gaucher, E.C. Thermoddem: A geochemical database focused on low temperature water/rock interactions and waste materials. Appl. Geochem. 2012, 27, 2107–2116. [Google Scholar] [CrossRef]
- Mazzullo, S.J.; Birdwell, B.A. Syngenetic formation of grainstones and pisolites from fenestral carbonates in peritidal settings. J. Sediment. Res. 1989, 59, 605–611. [Google Scholar] [CrossRef]
- Wright, V.P. Paleosols in shallow marine carbonate sequences. Earth Sci. Rev. 1994, 35, 367–395. [Google Scholar] [CrossRef]
- Flügel, E. Microfacies of Carbonate Rocks: Analysis, Interpretation and Application; Springer: Berlin, Germany, 2004; 976p. [Google Scholar]
- Eugster, H.P.; Jones, B.F. Behavior of major solutes during closed-basin brine evolution. Am. J. Sci. 1979, 279, 609–631. [Google Scholar] [CrossRef]
- Pace, A.; Bourillot, R.; Bouton, A.; Vennin, E.; Galaup, S.; Bundeleva, I.; Patrier, P.; Dupraz, C.; Thomazo, C.; Sansjofre, P.; et al. Microbial and diagenetic steps leading to the mineralisation of Great Salt Lake microbialites. Sci. Rep. 2016, 6, 31495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, A.; Bourillot, R.; Bouton, A.; Vennin, E.; Braissant, O.; Dupraz, C.; Duteil, T.; Bundeleva, I.; Patrier, P.; Galaup, S.; et al. Formation of stromatolite lamina at the interface of oxygenic-anoxygenic photosynthesis. Geobiology 2018, 16, 378–398. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.J.; Mlewski, C.; Boidi, F.J.; Farías, M.E.; Gérard, E. Calcium Carbonate Precipitation in Diatom-rich Microbial Mats: The Laguna Negra Hypersaline Lake, Catamarca, Argentina. J. Sediment. Res. 2018, 88, 727–742. [Google Scholar] [CrossRef]
- Mlewski, E.C.; Pisapia, C.; Gomez, F.; Lecourt, L.; Rueda, E.S.; Benzerara, K.; Ménez, B.; Borensztajn, S.; Jamme, F.; Réfrégiers, M.; et al. Characterization of Pustular Mats and Related Rivularia-Rich Laminations in Oncoids From the Laguna Negra Lake (Argentina). Front. Microbiol. 2018, 9, 996. [Google Scholar] [CrossRef]
- Servant–Vildary, S. Les diatomées des sédiments superficiels de quelques lacs salés de Bolivie. Bull. Sci. Géol. 1983, 36, 249–253. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Folk, R.L. Travertines: Depositional morphology and the bacterially constructed constituents. J. Sediment. Res. 1984, 54, 289–316. [Google Scholar] [CrossRef]
- Guidry, S.A.; Chafetz, H.S. Anatomy of siliceous hot springs: Examples from Yellowstone National Park, Wyoming, USA. Sediment. Geol. 2003, 157, 71–106. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W. Impact of seasonal changes on the formation and accumulation of soft siliceous sediments on the discharge apron of Geysir, Iceland. J. Sediment. Res. 2010, 80, 17–35. [Google Scholar] [CrossRef]
- Pentecost, A. Travertine; Springer: Berlin, Germany, 2005; 445p. [Google Scholar]
- French, H.M. The Periglacial Environment, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2007; 458p. [Google Scholar]
- Trombotto, D.T. Survey of cryogenic processes, periglacial forms and permafrost conditions in South America. Rev. Inst. Geol. 2000, 21, 33–55. [Google Scholar] [CrossRef] [Green Version]
- Francou, B.; Le Méhauté, N.; Jomelli, V. Factors Controlling Spacing Distances of Sorted Stripes in a Low-Latitude, Alpine Environment (Cordillera Real, 16 °S, Bolivia). Permafr. Periglac. 2001, 12, 367–377. [Google Scholar] [CrossRef]
- Kessler, M.A.; Werner, B.T. Self-Organization of Sorted Patterned Ground. Science 2003, 299, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Husen, S.; Taylor, R.; Smith, R.B.; Healser, H. Changes in geyser eruption behavior and remotely triggered seismicity in Yellowstone National Park produced by the 2002 M7.9 Denali fault earthquake, Alaska. Geology 2004, 32, 537–540. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Manga, M. Earthquakes and Water; Springer: Berlin, Germany, 2009; 249p. [Google Scholar]
- Wotton, R.S. The ubiquity and many roles of exopolymers (EPS) in aquatic systems. Sci. Mar. 2004, 68, 13–21. [Google Scholar] [CrossRef]
- Gratier, J.P.; Frery, E.; Deschamps, P.; Røyne, A.; Renard, F.; Dysthe, D.; Ellouz-Zimmerman, N.; Hamelin, B. How travertine veins grow from top to bottom and lift the rocks aboves them: The effect of crystallization force. Geology 2012, 40, 1015–1018. [Google Scholar] [CrossRef]
- Frery, E.; Gratier, J.P.; Ellouz-Zimmerman, N.; Deschamps, P.; Blamart, D.; Hamelin, B.; Swennen, R. Geochemical transect through a travertine mount: A detailed record of CO2-enriched fluid leakage from Late Pleistocene to present-day—Little Grand Wash fault (Utah, USA). Quat. Int. 2017, 437, 98–106. [Google Scholar] [CrossRef]
- Mazzini, A.; Svensen, H.; Hovland, M.; Plancke, S. Comparison and implications from strikingly different authigenic carbonates in a Nyegga complex pockmark, G11, Norwegian Sea. Mar. Geol. 2006, 231, 89–102. [Google Scholar] [CrossRef]
- Mazzini, A.; Ivanov, M.K.; Nermoen, A.; Bahr, A.; Bohrmann, G.; Svensen, H.; Plancke, S. Complex plumbing systems in the near subsurface: Geometries of authigenic carbonates from Dolgovskoy Mound (Black Sea) constrained by analogue experiments. Mar. Petrol. Geol. 2008, 25, 457–472. [Google Scholar] [CrossRef]
- Vanneste, H.; Kastner, M.; James, R.H.; Connelly, D.P.; Fisher, R.E.; Kelly-Gerreyn, B.A.; Heeschen, K.; Haeckel, M.; Mills, R.A. Authigenic carbonates from the Darwin Mud Volcano, Gulf of Cadiz: A record of palaeo-seepage of hydrocarbon bearing fluids. Chem. Geol. 2012, 300–301, 24–39. [Google Scholar] [CrossRef]
- Hedberg, H. Relation of Methane Generation to Undercompacted Shales, Shale Diapirs, and Mud Volcanoes. AAPG Bull. 1974, 58, 661–673. [Google Scholar]
- Mazzini, A.; Etiope, G. Mud volcanism: An updated review. Earth-Sci. Rev. 2017, 168, 81–112. [Google Scholar] [CrossRef] [Green Version]
- Svensen, H.; Karlsen, D.A.; Sturz, A.; Backer-Owe, K.; Banks, D.A.; Plancke, S. Processes controlling water and hydrocarbon composition in seeps from the Salton Sea geothermal system, California, USA. Geology 2007, 35, 85–88. [Google Scholar] [CrossRef]
- Onderdonk, N.; Mazzini, A.; Shafer, L.; Svensen, H. Controls on the geomorphic expression and evolution of gryphons, pools, and caldera features at hydrothermal seeps in the Salton Sea Geothermal Field, southern California. Geomorphology 2011, 130, 327–342. [Google Scholar] [CrossRef]
- Galli, P. New empirical relationships between magnitude and distance for liquefaction. Tectonophysics 2000, 324, 169–187. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Manga, M.; Wong, A. Floods on Mars released from groundwater by impact. Icarus 2005, 175, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-Y.; Wong, A.; Dreger, D.S.; Manga, M. Liquefaction Limit during Earthquakes and Underground Explosions: Implications on Ground-Motion Attenuation. Bull. Seismol. Soc. Am. 2006, 96, 355–363. [Google Scholar] [CrossRef]
- Bonini, M. Mud volcano eruptions and earthquakes in the Northern Apennines and Sicily, Italy. Tectonophysics 2009, 474, 723–735. [Google Scholar] [CrossRef]
- Rudolph, M.L.; Manga, M. Mud volcano response to the 4 April 2010 El Mayor-Cucapah earthquake. J. Geophys. Res. 2010, 115, B12211. [Google Scholar] [CrossRef]
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, K.A. Mineralogical and textural changes accompanying of silica sinter. Miner. Deposita 2000, 35, 48–62. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W. Microstructural changes accompanying the opal-A to opal-CT transition: New evidence from the siliceous sinters of Geysir, Haukadalur, Iceland. Sedimentology 2007, 54, 921–948. [Google Scholar] [CrossRef]
- Williams, L.A.; Parks, G.A.; Crerar, D.A. Silica Diagenesis, I. Solubility controls. J. Sediment. Res. 1985, 55, 301–311. [Google Scholar] [CrossRef]
- Lewin, J.C. The dissolution of silica from diatom walls. Geochim. Cosmochim. Acta 1961, 21, 182–198. [Google Scholar] [CrossRef]
- Ryves, D.B.; Battarbee, R.W.; Juggins, S.; Fritz, S.C.; Anderson, N.J. Physical and chemical predictors of diatom dissolution in freshwater and saline lake sediments in North America and West Greenland. Limnol. Oceanogr. 2006, 51, 1355–1368. [Google Scholar] [CrossRef] [Green Version]
- Dupraz, C.; Fowler, A.; Tobias, C.; Visscher, P.T. Stromatolitic knobs in Storr’s Lake (San Salvador, Bahamas): A model system for formation and alteration of laminae. Geobiology 2013, 11, 527–548. [Google Scholar] [CrossRef]
- Jahnert, R.J.; Collins, L.B. Controls on microbial activity and tidal flat evolution in Shark Bay, Western Australia. Sedimentology 2013, 60, 1071–1099. [Google Scholar] [CrossRef]
- Bouton, A.; Vennin, E.; Pace, A.; Bourillot, R.; Dupraz, C.; Thomazo, C.; Brayard, A.; Désaubliaux, G.; Visscher, P.T. External controls on the distribution, fabrics and mineralization of modern microbial mats in a coastal hypersaline lagoon, Cayo Coco (Cuba). Sedimentology 2016, 63, 972–1016. [Google Scholar] [CrossRef]
- Roche, A.; Vennin, E.; Bouton, A.; Olivier, N.; Wattinne, A.; Bundeleva, I.; Deconinck, J.-F.; Virgone, A.; Gaucher, E.C.; Visscher, P.T. Oligo-Miocene lacustrine microbial and metazoan buildups from the Limagne Basin (French Massif Central). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 504, 34–59. [Google Scholar] [CrossRef]
- Warden, J.G.; Coshell, L.; Rosen, M.R.; Breecker, D.O.; Ruthrof, K.X.; Omelon, C.R. The importance of groundwater flow to the formation of modern thrombolytic microbialites. Geobiology 2019. [Google Scholar] [CrossRef] [PubMed]
- Andres, M.S.; Reid, R.P. Growth morphologies of modern marine stromatolites: A case study from Highborne Cay, Bahamas. Sediment. Geol. 2006, 185, 319–328. [Google Scholar] [CrossRef]
- Casanova, J. Les Stromatolites Continentaux: Paléoécologie, Paléohydrologie, Paléoclimatologie. Application au Rift Gregory. Ph.D. Thesis, University Aix-Marseille II, Marseille, France, 1986. [Google Scholar]
- Casanova, J.; Hillaire-Marcel, C. Late Holocene hydrological history of Lake Tanganyika, East Africa, from isotopic data on fossil stromatolites. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1992, 91, 35–48. [Google Scholar] [CrossRef]
- Ginsburg, R.N.; Planavsky, N.J. Diversity of Bahamian Microbialite Substrates. In Links Between Geological Processes, Microbial Activites & Evolution of Life; Dylek, Y., Furnes, H., Muehlenbachs, K., Eds.; Springer: Berlin, Germany, 2008; pp. 177–195. [Google Scholar]
- Bouton, A.; Vennin, E.; Boulle, J.; Pace, A.; Bourillot, R.; Thomazo, C.; Brayard, A.; Désaubliaux, G.; Goslar, T.; Yokoyama, Y.; et al. Linking the distribution of microbial deposits from the Great Salt Lake (Utah, USA) to tectonic and climatic processes. Biogeosciences 2016, 13, 5511–5526. [Google Scholar] [CrossRef] [Green Version]
- Manzo, E.; Perri, E.; Tucker, M.E. Carbonate deposition in a fluvial tufa system: Processes and products (Corvino Valley–Southern Italy). Sedimentology 2012, 59, 553–577. [Google Scholar] [CrossRef]
- Pedley, M.; Rogerson, M.; Middleton, R. Freshwater calcite precipitates from in vitro mesocosm flume experiments: A case for biomediation of tufas. Sedimentology 2009, 56, 511–527. [Google Scholar] [CrossRef]
- Roche, A.; Vennin, E.; Bundeleva, I.; Payandi-Rolland, D.; Bouton, A.; Gaucher, E.C.; Amiotte-Suchet, P.; Courvoisier, H.; Visscher, P.T. The role of substrate on the mineralization potential of microbial mats in a modern freshwater river (Villiers-le-Bâcle, France). Minerals 2019, 9, 359. [Google Scholar] [CrossRef]
- Dill, R.F. Subtidal stromatolites, ooids and crusted-lime muds at the Great Bahama Bank Margin. In From Shoreline to Abyss; Osborn, R.F., Ed.; SEPM: Tulsa, OK, USA, 1991; Volume 46, pp. 147–171. [Google Scholar]
- Basso, D.; Bracchi, V.A.; Favalli, A.N. Microbialite formation in southern Sinai (Egypt). Facies 2013, 59, 7–18. [Google Scholar] [CrossRef]
- Leggitt, V.L.; Cushman, R.A., Jr. Complex caddisfly-dominated bioherms from the Eocene Green River Formation. Sediment. Geol. 2001, 145, 377–396. [Google Scholar] [CrossRef]
- Della Porta, G. Carbonate build-ups in lacustrine, hydrothermal and fluvial settings: Comparing depositional geometry, fabric types and geochemical signature. Geol. Soc. Lond. Spec. Publ. 2015, 418, 17–68. [Google Scholar] [CrossRef]
- Stockner, J.G. Observations of Thermophilic Algal Communities in Mount Rainier and Yellowstone National Parks. Limnol. Oceanogr. 1967, 12, 13–17. [Google Scholar] [CrossRef]
- Owen, R.B.; Renaut, R.W.; Hover, V.C.; Ashley, G.M.; Muasya, A.M. Swamps, springs and diatoms: Wetlands of the semi-arid Bogoria-Baringo Rift, Kenya. Hydrobiologia 2004, 518, 59–78. [Google Scholar] [CrossRef]
- Owen, R.B.; Renaut, R.W.; Jones, B. Geothermal diatoms: A comparative study of floras in hot spring systems of Iceland, New Zealand, and Kenya. Hydrobiologia 2008, 610, 175–192. [Google Scholar] [CrossRef]
- Winsborough, B.M.; Golubić, S. The role of diatoms in stromatolite growth: Two examples from modern freshwater settings. J. Phycol. 1987, 23, 195–201. [Google Scholar] [CrossRef]
- Gomez, F.J.; Kah, L.C.; Bartley, J.K.; Astini, R.A. Microbialites in a high-altitude Andean lake: Multiple controls on carbonate precipitation and lamina accretion. Palaios 2014, 29, 233–249. [Google Scholar] [CrossRef]
- Arp, G.; Wedemeyer, N.; Reitner, J. Fluvial Tufa Formation in a Hard-Water Creek (Deinschwanger Bach, Franconian Alb, Germany). Facies 2001, 44, 1–22. [Google Scholar] [CrossRef]
- Phoenix, V.R.; Konhauser, K.O.; Ferris, F.G. Experimental study of iron and silica immobilization by bacteria in mixed Fe-Si systems: Implications for microbial silicification in hot springs. Can. J. Earth. Sci. 2003, 40, 1669–1678. [Google Scholar] [CrossRef]
- Walther, J.V.; Helgeson, H.C. Calculation of the thermodynamic properties of aqueous silica and the solubility of quartz and its polymorphs at high pressures and temperatures. Am. J. Sci. 1977, 277, 1315–1351. [Google Scholar] [CrossRef]
- Williams, L.A.; Crerar, D.A. Silica diagenesis, II. General mechanisms. J. Sediment. Res. 1985, 55, 312–321. [Google Scholar] [CrossRef]
- Visscher, P.T.; Reid, R.P.; Bebout, B.M.; Hoeft, S.E.; Macintyre, I.G.; Thompson, J.A., Jr. Formation of lithified micritic laminae in modern marine stromatolites (Bahamas): The role of sulfur cycling. Am. Mineral. 1998, 83, 1482–1493. [Google Scholar] [CrossRef]
- Visscher, P.T.; Reid, R.P.; Bebout, B.M. Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 2000, 28, 919–922. [Google Scholar] [CrossRef]
- Dupraz, C.; Visscher, P.T.; Baumgartner, L.K.; Reid, R.P. Microbe-mineral interactions: Early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas). Sedimentology 2004, 51, 745–765. [Google Scholar] [CrossRef]
- Freytet, P.; Verrecchia, E. Les carbonates continentaux du pourtour méditerranéen: Microfaciès et milieux de formation. Méditerranée 1989, 68, 5–28. [Google Scholar] [CrossRef]
- Riding, R. Abiogenic, microbial and hybrid authigenic carbonate crusts: Components of Precambrian stromatolites. Geol. Croat. 2008, 61, 73–103. [Google Scholar]






| Facies | Composition | Structure | Extension | Depositional Environment | Zone | |
|---|---|---|---|---|---|---|
| F1: Facies of the carbonate platform | F1a: Laminated grainstone | Recent carbonate crust composed of detrital grains, intraclasts (micrite–sparite), ostracod valves, microbial filaments, and pisolites; Dominant components depend on the position on the carbonate platform, affected by cemention (sparite or micrite, locally iron hydroxide) and dissolutions | Porosity organized in laminae (birdeyes?) in F1a and scarce porosity in F1b; micritization of grains; circumgranular fractures; calcitic spar-cement infilling, local iron hydroxide cements | Up to 30 cm thick | Pedogenic (diagenetic grain and caliche formations) of lacustrine facies | Recent carbonate platform and locally observed in Zone IV |
| F1b: Massive grainstone | ||||||
| F1c:Pisolites | Successive micritic and sparitic laminae developing around a nucleus (intraclast, spherulite, detrital grains) | Discoid to spherical pisolites | From few mm to 20 cm in diameter | Developed on carbonate platform | ||
| F2: Mud to wacke sediments | F2a: Unconsolidated micritic mud | Orange to green layered mud, mainly aggregates composed of micrite, diatoms, cyanobacterial filaments, ostracod valves, and aragonite needles | Crudely layered | cm to dm thick | Domal mound facies | I |
| F2b: Diatom mud | Reddish to black muds mainly composed of diatoms, micrite (and rare filaments) | Accumulation | mm to cm thick | Central pool | I | |
| F2c: Ostracod-rich mud | Brown to black mud composed of disarticulated ostracods, micrite, diatoms organized in aggregates, and rare filaments | Accumulation | Several hundred m2 | Along the slope from the channel to the proximal–distal transitional belt | II and III | |
| F3: Amorphous deposits | Reddish crust composed of mineralized EPS in Si–Fe–Mg–As, affected by shrinkage cracks, with micritic intraclasts, coated by isopachous to pustular amorphous silica cements | Diagenetic structures | 2–3 cm thick | Domal mound facies | Flat mound | |
| F4: Pedogenic packstones-floatstones | Broken and corroded micritic intraclasts mixed with F2a, affected by circumgranular cracking, stained by Fe, porosity infilled by sparite cements and micrite | Clotted | cm to dm thick with intraclasts of 50 μm to 1 mm) | Reworked facies in pedogenized domal mound | I and II | |
| F5: Microbial and diatomaceous bindstone | Ledge | Microbial laminae | Bordering edges | Central pool and channel | I | |
| Mushroom-like | Scarce | Channel | I | |||
| Cerebroid | Scarce | Apron | II | |||
| F6: Diatomaceous and peloidal micropackstone | Snake-like | Microbial laminae | Covering more than 8000 m2 | Transitional proximal–distal belt | III | |
| F7: Shrub | Isolated shrub, Planar shrub | Microbial laminae | Covering more than 46,000 m2 | Distal zone | IV | |
| IV | ||||||
| Points | Zone | Latitude | Longitude | pH | Conductivity (mS∙cm−1) | Temperature (°C) | Oxygen (mg∙L−1) |
|---|---|---|---|---|---|---|---|
| Dry conditions (January 2016) | |||||||
| PG1-1 | Z-Ia | 21.61934°S | 67.84842°W | 6.42 | 25.90 | 42.20 | 5.51 |
| PG1-2 | Z-Ia | 21.61934°S | 67.84836°W | 6.31 | 25.80 | 42.00 | 5.50 |
| PG1-3 | Z-Ib | 21.61934°S | 67.84832°W | 6.29 | 25.40 | 42.90 | 6.15 |
| PG1-4 | Z-Ib | 21.61941°S | 67.84825°W | 6.46 | 25.50 | 41.30 | 10.42 |
| PG1-5 | Z-Ib | 21.61950°S | 67.84817°W | 6.67 | 25.60 | 39.90 | 14.70 |
| PG1-6 | Z-Ib | 21.61951°S | 67.84805°W | 6.78 | 25.70 | 39.70 | 15.00 |
| PG1-7 | Z-II | 21.61955°S | 67.84801°W | 7.07 | 26.00 | 38.50 | 17.80 |
| PG1-8 | Z-II | 21.61962°S | 67.84782°W | 7.23 | 25.80 | 36.70 | 17.30 |
| PG1-9 | Z-II | 21.61960°S | 67.84771°W | 7.60 | 26.10 | 35.00 | 15.90 |
| PG1-10 | Z-II | 21.61974°S | 67.84759°W | 7.88 | 26.50 | 30.60 | 13.60 |
| PG1-11 | Z-II | 21.61987°S | 67.84746°W | 7.90 | 23.60 | 26.90 | 10.70 |
| PG1-12 | Z-II | 21.61978°S | 67.84722°W | 7.98 | 19.28 | 24.00 | 7.60 |
| PG1-13 | Z-II | 21.61955°S | 67.84689°W | 8.15 | 36.40 | 22.00 | 10.00 |
| PG1-14 | Z-II | 21.61929°S | 67.84652°W | 7.96 | 55.00 | 21.70 | n.d. |
| PG1-16 | Z-II | 21.61939°S | 67.84614°W | 8.18 | 52.40 | 21.30 | 5.85 |
| PG1-15 | Z-III | 21.61928°S | 67.84630°W | 8.03 | 68.00 | 21.60 | 10.00 |
| PG1-17 | Z-IV | 21.61947°S | 67.84598°W | 7.73 | 113.00 | 20.10 | 7.62 |
| PG1-18 | Z-IV | 21.61949°S | 67.84583°W | 6.94 | 225.00 | 21.00 | 2.83 |
| Wet conditions (March 2017) | |||||||
| PG17-101 | Z-Ia | 21.61934°S | 67.84835°W | 5.8 | 26.7 | 40.9 | 8.29 |
| PG17-102 | Z-Ib | 21.61936°S | 67.84832°W | 6.3 | 26.3 | 40.3 | 8.45 |
| PG17-103 | Z-Ib | 21.61948°S | 67.84824°W | 6.65 | 26.9 | 39.4 | 11.02 |
| PG17-105 | Z-Ib | 21.61954°S | 67.84804°W | 7.2 | 27.2 | 36.1 | 60.6 |
| PG17-106 | Z-II | 21.61965°S | 67.84732°W | 8.12 | 27 | 24.6 | 3.21 |
| PG17-107 | Z-III | 21.62001°S | 67.84693°W | 8.8 | 31.9 | 25 | 5.02 |
| PG17-108 | Z-IV | 21.62012°S | 67.84660°W | 8.64 | 48 | 20.7 | 10.87 |
| PG17-110 | 1CP | 21.62035°S | 67.84591°W | 7.71 | 156.3 | 22.9 | 8.31 |
| Points | PG1-1 | PG1-8 | PG1-16 | PG1-18 | ||||
|---|---|---|---|---|---|---|---|---|
| Zone | Z-Ia | Z-II | Z-II | Z-IV | ||||
| pH | 6.42 | 7.23 | 8.18 | 6.94 | ||||
| Alkalinity (meq∙L−1) | 10.7 | 9.84 | 1.08 | 5.21 | ||||
| Na+ | 5063 | 5.53 × 10−1 | 5073 | 5.59 × 10−1 | 8816 | 5.50 × 10−1 | 71,673 | 5.39 × 10−1 |
| K+ | 523 | 5.71 × 10−2 | 522 | 5.75 × 10−2 | 936 | 5.84 × 10−2 | 6809 | 5.11 × 10−2 |
| Ca2+ | 457 | 4.99 × 10−2 | 456 | 5.03 × 10−2 | 589 | 3.67 × 10−2 | 3093 | 2.32 × 10−2 |
| Mg2+ | 132 | 1.44 × 10−2 | 137 | 1.51 × 10−2 | 224 | 1.39 × 10−2 | 1571 | 1.18 × 10−2 |
| SO42− | 283 | 3.09 × 10−2 | 270 | 2.97 × 10−2 | 498 | 3.11 × 10−2 | 2941 | 2.21 × 10−2 |
| Cl− | 9147 | - | 9068 | - | 16,028 | - | 133,044 | - |
| HCO3− | 381 | 4.16 × 10−2 | 591.4 | 6.52 × 10−2 | 173.4 | 1.08 × 10−2 | 237.8 | 1.78 × 10−3 |
| Li+ | 69 | 7.54 × 10−3 | 71 | 7.83 × 10−3 | 113 | 7.05 × 10−3 | 789 | 5.9 × 10−3 |
| Sr2+ | 9.7 | 1.06 × 10−3 | 9.8 | 1.00 × 10−3 | 15 | 9.3 × 10−4 | 111 | 8.34× 10−4 |
| Ba2+ | 0.19 | 2.07× 10−5 | 0.23 | 2.53 × 10−5 | 0.38 | 2.37 × 10−5 | 1.7 | 1.27 × 10−5 |
| Fe | 0.17 | 1.86 × 10−5 | 0.08 | 8.82 × 10−6 | <0.04 | - | <0.04 | - |
| B | 35 | 3.82 × 10−3 | 35 | 3.86 × 10−3 | 64 | 3.99 × 10−3 | 396 | 2.97 × 10−3 |
| Si | 72 | 7.87 × 10−3 | 67 | 7.38 × 10−3 | 62 | 3.86 × 10−3 | 30 | 2.25 × 10−4 |
| Br− | 4.8 | 5.24 × 10−4 | 4.7 | 5.18 × 10−4 | 8 | 4.99 × 10−4 | 61 | 4.58 × 10−4 |
| SIAmorphous. silica | −0.01 | 0.06 | 0.08 | −0.07 | ||||
| SICalcite | −0.24 | 0.61 | 1.48 | 0.16 | ||||
| SIFe(OH)3 (2L) | 1.83 | 2.57 | 3.09 | 0.98 | ||||
| SIFeOOH | 4.70 | 5.51 | 6.18 | 4.12 | ||||
| SIGypsum | −1.27 | −1.68 | −1.59 | −0.59 |
| Macrofabric | Composition | Structure | Dimension | Biological Community | Substrate | Depositional Environment | Zone and Facies |
|---|---|---|---|---|---|---|---|
| Ledge | Alternating laminae composed of micrite, bundles of microbial sheaths and diatoms, coating F4. The upper and lateral parts of the structure show a transition from bundles of microbial sheaths to micritic laminae, sheltered parts infilled by diatoms and ostracods | Planar laminated structure; growth in stairs | 2 to 5 cm thick; 5–20 cm long | Green mat; diatoms | F4 | Central pool and channel | I, F5 |
| Mushroom-like | Planar to curved laminae | 5–10 cm in diameter | Green mat; diatoms | F4 | Channel | I, F5 | |
| Cerebroid | Alternating laminae composed of micrite, bundles of microbial sheaths and diatoms, coating F4. The upper and lateral parts of cerebroids show a transition from bundles of bacterial sheaths to micritic laminae, bundles composed of cast and mold of filaments | Irregular ovoid structure, composed of planar and columnar laminae | 2 to 20 cm in diameter | Green mat; diatoms | F4 | Apron | II, F5 |
| Snake-like | Alternation of densely packed and loosely packed micritic peloid laminae with diatoms, top structure showing siliceous cements and gypsum | Laminated and clotted hemispherical and tortuous structures showing a white crust on their upper part; white crusts locally covered by mm micrite and silica-rich branches | 10 cm to 1 m long | Black mat; diatoms | Associated with F2b, resting on F1 | Transitional proximal–distal belt | III, F6 |
| Isolated shrub | Alternation of micritic to sparitic laminae, and siliceous laminae, coating F1. Transition from F1 to shrub laminae is marked by a corroded surface coated with iron oxides. Microstalactitic cements precipitating between downward-facing microstromatolites. | Planar to columnar (finger-like) structures | 2 to 5 cm in diameter | Black mat; diatoms | F1 | Distal zone | IV, F7 |
| Planar shrub | Planar, with columnar structure at the edges, and with a smoothed central part | Ranging from 5 cm to 50 cm | Black mat; diatoms | F1 | IV, F7 |
| Microfabric Components | Main Characteristics | Description | Size | Macro | Zone |
|---|---|---|---|---|---|
| Diatoms | - | Accumulation of well-preserved or fragmented diatoms organized in clusters or layers, or observed as mats (slime) | 10 to 150 μm | Accumulation as layers or trapped in ledge, mushroom-like, cerebroid, snake-like morphologies | I, II and III |
| Ostracods | - | Clusters composed of ostracods associated with diatoms, micrite, and iron hydroxide, infilling dissolution cavities or trapped within microbial or diatomaceous layers, complete and disarticulated ostracods | 200 to 600 μm | In association with mushroom-like and cerebroid morphologies | I, II and III |
| Micritic aggregates | - | Aggregates of micritic crystals incorporating diatoms | 20 to 500 μm | Diatomaceous mud | I, II and III |
| Microbial sheaths | Bundles | Microbial sheaths organized in bundles and associated with diatoms, preserved as internal fossil molds embedded with micrite or as filament casts filled by Fe–(Mg–As) silicate gel | Bundles up to 2 mm; more than 2 mm width molds 7–10 μm in diameter and over 200 μm long; Casts 13–20 μm in diameter and up to 300 μm long | Bundles developed on hard substrate (previous growth phases) organized in ledge, mushroom-like and cerebroid morphologies | I and II |
| Isolated | Single microbial sheaths embedded in micritic peloids Well-preserved casts of filaments filled by Fe silicate gel | 13–17 μm in diameter and up to 300 μm long; rare broken fragments | Isolated microbial sheathss incorporated in snake-like and shrub morphologies | III and IV | |
| Peloids | -- | Irregular micritic peloids organized in clots; their density can vary, forming darker or lighter layers with associated diatoms or calcite/silica cement | 100 to 200 μm; can exceed 1 mm | Accumulation of peloids in snake-like morphologies | III |
| Laminae | Micritic and micritic to sparitic | Irregular or continuous wavy to planar micritic laminae (microbial origin?)/transitional micritic to sparitic laminae | 30 to 500 μm | In ledge, mushroom-like, cerebroid, and shrub morphologies | I, II and IV |
| Siliceous | Thin discontinuous laminae of amorphous silica | 20 to 200 μm | In shrubs | IV |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougeault, C.; Vennin, E.; Durlet, C.; Muller, E.; Mercuzot, M.; Chavez, M.; Gérard, E.; Ader, M.; Virgone, A.; Gaucher, E.C. Biotic–Abiotic Influences on Modern Ca–Si-Rich Hydrothermal Spring Mounds of the Pastos Grandes Volcanic Caldera (Bolivia). Minerals 2019, 9, 380. https://doi.org/10.3390/min9060380
Bougeault C, Vennin E, Durlet C, Muller E, Mercuzot M, Chavez M, Gérard E, Ader M, Virgone A, Gaucher EC. Biotic–Abiotic Influences on Modern Ca–Si-Rich Hydrothermal Spring Mounds of the Pastos Grandes Volcanic Caldera (Bolivia). Minerals. 2019; 9(6):380. https://doi.org/10.3390/min9060380
Chicago/Turabian StyleBougeault, Cédric, Emmanuelle Vennin, Christophe Durlet, Elodie Muller, Mathilde Mercuzot, Marco Chavez, Emmanuelle Gérard, Magali Ader, Aurélien Virgone, and Eric C. Gaucher. 2019. "Biotic–Abiotic Influences on Modern Ca–Si-Rich Hydrothermal Spring Mounds of the Pastos Grandes Volcanic Caldera (Bolivia)" Minerals 9, no. 6: 380. https://doi.org/10.3390/min9060380
APA StyleBougeault, C., Vennin, E., Durlet, C., Muller, E., Mercuzot, M., Chavez, M., Gérard, E., Ader, M., Virgone, A., & Gaucher, E. C. (2019). Biotic–Abiotic Influences on Modern Ca–Si-Rich Hydrothermal Spring Mounds of the Pastos Grandes Volcanic Caldera (Bolivia). Minerals, 9(6), 380. https://doi.org/10.3390/min9060380






