Processes and Conditions of the Origin for Fe3+-Bearing Magnesiowüstite under Lithospheric Mantle Pressures and Temperatures
Abstract
1. Introduction
1.1. Distribution of Mg, Fe-Oxides in the Earth’s Interior
1.2. Crustal Structure and Composition of Mg,Fe-Oxides in the Mantle
1.3. Modern Concepts on the Fe3+-Bearing Magnesiowüstite Formation in the Upper Mantle
2. Materials and Methods
2.1. Methodical Approach for “Sandwich-Type” Experiments
2.2. Methodical Approach for “Mixture-Type” Experiments
2.3. Analytical Procedure
3. Results
3.1. Features of Fe3+-Bearing Magnesiowüstite Formation in the Carbonate-Iron System
3.2. Features of Fe3+-Bearing Magnesiowüstite Formation in the Carbonate-Oxide-Metal System
3.3. Features of Fe3+-Bearing Magnesiowüstite Formation in the Carbonate-Oxide System
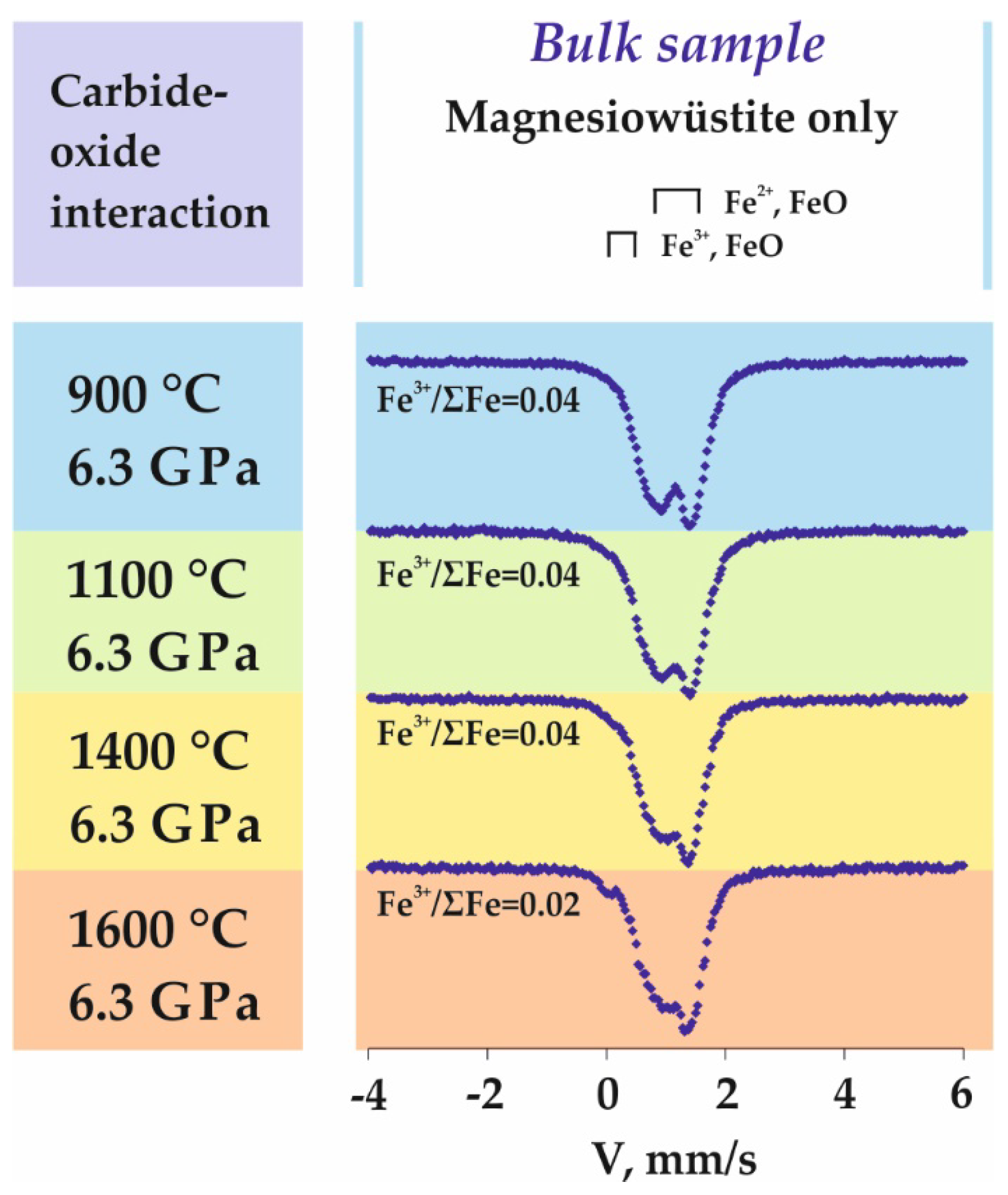
3.4. Features of Fe3+-Bearing Wüstite Formation in the Carbide-Oxide System
3.5. Features of Fe3+-Bearing Magnesiowüstite Formation in the Carbonate-Iron-Sulfur System
4. Discussion
4.1. Processes of Fe3+-Bearing Magnesiowüstite Formation in the Carbonate-Iron System
4.2. Processes of Fe3+-Bearing Magnesiowüstite Formation in the Carbonate-Oxide-Metal System
4.3. Processes of Fe3+-Bearing Wüstite Formation in the Carbonate-Oxide System
4.4. Processes of Fe3+-Bearing Magnesiowüstite Formation in the Carbide-Oxide System
4.5. Processes of Fe3+-Bearing Magnesiowüstite Formation in the Carbonate-Iron-Sulfur System
4.6. Processes of Fe3+-Bearing Magnesiowüstite Formation in the Lithospheric Mantle
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Irifune, T. Absence of an aluminous phase in the upper part of the Earth’s lower mantle. Nature 1994, 370, 131–133. [Google Scholar] [CrossRef]
- Fei, Y.; Bertka, C.M. Phase transitions in the Earth’s mantle and mantle mineralogy. In Mantle Petrology: Field Observations and High Pressure Experimentation: A Tribute to Francis R. (Joe) Boyd; Fei, Y., Bertka, C.M., Mysen, B.O., Eds.; Special Publication No. 6; Geochemical Society: Washington, DC, USA, 1999; pp. 189–207. [Google Scholar]
- Wood, B.J. Phase transformations and partitioning relations in peridotite under lower mantle conditions. Earth Planet. Sci. Lett. 2000, 174, 341–354. [Google Scholar] [CrossRef]
- Irifune, T.; Shinmei, T.; McCammon, C.A.; Miyajima, N.; Rubie, D.C.; Frost, D.J. Iron partitioning and density changes of pyrolite in Earth’s lower mantle. Science 2010, 327, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Ringwood, A.E. Phase transformations and their bearing on the constitution and dynamics of the mantle. Geochim. Cosmochim. Acta 1991, 55, 2083–2110. [Google Scholar] [CrossRef]
- Fei, Y.; Mao, H.K.; Shu, J.; Hu, J. P-V-T equation of state of magnesiowüstite (Mg0.6Fe0.4)O. Phys. Chem. Miner. 1992, 18, 416–422. [Google Scholar] [CrossRef]
- Mao, H.K.; Shu, J.; Fei, Y.; Hu, J.Z.; Hemley, R.J. The wüstite enigma. Phys. Earth Planet. Int. 1996, 96, 135–145. [Google Scholar] [CrossRef]
- Dubrovinsky, L.S.; Dubrovinskaya, N.A.; Annersten, H.; Hålenius, E.; Harryson, H.; Tutti, F.; Rekhi, S.; LeBihan, T. Stability of ferropericlase in the lower mantle. Science 2000, 289, 430–432. [Google Scholar] [CrossRef]
- Lin, J.-F.; Heinz, D.L.; Mao, H.K.; Hemley, R.J.; Devine, J.M.; Li, J.; Shen, G. Stability of magnesiowüstite in Earth’s lower mantle. Proc. Natl. Acad. Sci. USA 2003, 100, 4405–4408. [Google Scholar] [CrossRef]
- Kaminsky, F. Mineralogy of the lower mantle: A review of ‘super-deep’ mineral inclusions in diamond. Earth Sci. Rev. 2012, 110, 127–147. [Google Scholar] [CrossRef]
- Liu, L.-G. Silicate perovskite from phase transformations of pyrope-garnet at high pressure and temperature. Geophys. Res. Lett. 1974, 1, 277–280. [Google Scholar] [CrossRef]
- Liu, L.-G. Post oxide phases of forsterite and enstatite. Geophys. Res. Lett. 1975, 2, 417–419. [Google Scholar] [CrossRef]
- Gurney, J.J. Diamonds. In Kimberlites and Related Rocks; Ross, J., Jaques, A.L., Fewson, J., Green, D.H., O’Reilly, s.Y., Danchin, R.V., Janse, A.J.A., Eds.; Special Publication 14; Geological Society of Australia: Carlton, Australia, 1989; Volume 2, pp. 935–965. [Google Scholar]
- Bulatov, V.K.; Girnis, A.V.; Brey, G.P.; Woodland, A.B.; Höfer, H.E. Ferropericlase crystallization under upper mantle conditions. Contrib. Mineral. Petrol. 2019, 174, 45. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P.; Joswig, W. Kankan diamonds (Guinea): II. Lower mantle inclusion paragenesis. Contrib. Mineral. Petrol. 2000, 140, 34–47. [Google Scholar] [CrossRef]
- Brey, G.P.; Bulatov, V.; Girnis, A.; Harris, J.W.; Stachel, T. Ferropericlase—A lower mantle phase in the upper mantle. Lithos 2004, 77, 655–663. [Google Scholar] [CrossRef]
- Wicks, J.; Duffy, T.S. Crystal structures of minerals in the lower mantle. In Deep Earth: Physics and Chemistry of the Lower Mantle and Core (Geophysical Monograph Series); John Wiley & Sons, Inc. (American Geophysical Union): New York, NY, USA, 2016; pp. 68–88. [Google Scholar]
- Kaminsky, F.V. The Earth’s Lower Mantle: Composition and Structure; Springer: Geneva, Switzerland, 2017; p. 331. [Google Scholar]
- Dorfman, S.M.; Shieh, S.R.; Meng, Y.; Prakapenka, V.B.; Duffy, T.S. Synthesis and equation of state of perovskites in the (Mg,Fe)3Al2Si3O12 system to 177 GPa. Earth Planet. Sci. Lett. 2012, 357–358, 194–202. [Google Scholar] [CrossRef]
- Duffy, T.S.; Hemley, R.J.; Mao, H.-K. Equation of state and shear strength at multimegabar pressures: Magnesium oxide to 227 GPa. Phys. Rev. Lett. 1995, 74, 1371–1375. [Google Scholar] [CrossRef]
- Fei, Y.; Mao, H.K. In situ determination of the NiAs phase of FeO at high pressure and temperature. Science 1994, 266, 1678–1680. [Google Scholar] [CrossRef]
- Kondo, T.; Ohtani, E.; Hirao, N.; Yagi, T.; Kikegawa, T. Phase transitions of (Mg,Fe)O at megabar pressures. Phys. Earth Planet. Int. 2004, 143–144, 201–213. [Google Scholar] [CrossRef]
- Fischer, R.A.; Campbell, A.J. High pressure melting of wüstite. Am. Miner. 2010, 95, 1473–1477. [Google Scholar] [CrossRef]
- Fischer, R.A.; Campbell, A.J.; Lord, O.T.; Shofner, G.A.; Dera, P.; Prakapenka, V.B. Phase transition and metallization of FeO at high pressures and temperatures. Geophys. Res. Lett. 2011, 38, L24301. [Google Scholar] [CrossRef]
- Fischer, R.A.; Campbell, A.J.; Shofner, G.A.; Lord, O.T.; Dera, P.; Prakapenka, V.P. Equation of state and phase diagram of FeO. Earth Planet. Sci. Lett. 2011, 304, 496–502. [Google Scholar] [CrossRef]
- Ozawa, H.; Hirose, K.; Tateno, S.; Sata, N.; Ohishi, Y. Phase transition boundary between B1 and B8 structures of FeO up to 210 GPa. Phys. Earth Planet. Int. 2010, 179, 157–163. [Google Scholar] [CrossRef]
- Ohta, K.; Fujino, K.; Kuwayama, Y.; Kondo, T.; Shimizu, K.; Ohishi, Y. Highly conductive iron rich (Mg, Fe)O magnesiowüstite and its stability in the Earth’s lower mantle. J. Geophys. Res. Solid Earth 2014, 119, 4656–4665. [Google Scholar] [CrossRef]
- Wicks, J.K.; Jackson, J.M.; Sturhahn, W.; Zhuravlev, K.K.; Tkachev, S.N.; Prakapenka, V.B. Thermal equation of state and stability of (Mg0.06Fe0.94)O. Phys. Earth Planet. Int. 2015, 249, 28–42. [Google Scholar] [CrossRef]
- Katsura, T.; Yoneda, A.; Yamazaki, D.; Yoshino, T.; Ito, E. Adiabatic temperature profile in the mantle. Phys. Earth Planet. Int. 2010, 183, 212–218. [Google Scholar] [CrossRef]
- Harte, B.; Harris, J.W.; Hutchison, M.T.; Watt, G.R.; Wilding, M.C. Lowermantle mineral associations in diamonds from São-Luíz, Brazil. In Mantle Petrology: Field Observations and High Pressure Experimentation: A Tribute to Francis R. (Joe) Boyd; Fei, Y., Bertka, C.M., Mysen, B.O., Eds.; Special Publication No. 6; Geochemical Society: Washington, DC, USA, 1999; pp. 125–153. [Google Scholar]
- McCammon, C. Deep diamond mysteries. Science 2001, 293, 813–814. [Google Scholar] [CrossRef]
- McCammon, C.A.; Stachel, T.; Harris, J.W. Iron oxidation state in lower mantle mineral assemblages: II. Inclusions in diamonds from Kankan, Guinea. Earth Planet. Sci. Lett. 2004, 222, 423–434. [Google Scholar] [CrossRef]
- Harte, B. Diamond formation in the deep mantle: The record of mineral inclusions and their distribution in relation to mantle dehydration zones. Mineral. Mag. 2010, 74, 189–215. [Google Scholar] [CrossRef]
- Wirth, R.; Dobrzhinetskaya, L.; Harte, B.; Schreiber, A.; Green, H.W. High-Fe (Mg,Fe)O inclusion in diamond apparently from the lowermost mantle. Earth Planet. Sci. Lett. 2014, 404, 365–375. [Google Scholar] [CrossRef]
- Svicero, D.P. Distribution and origin of diamonds in Brazil: An overview. J. Geodyn. 1995, 20, 493–514. [Google Scholar] [CrossRef]
- McCammon, C.A.; Hutchison, M.; Harris, J. Ferric iron content of mineral inclusions in diamonds from São-Luíz: A view into the lower mantle. Science 1997, 278, 434–436. [Google Scholar] [CrossRef]
- Harte, B.; Hudson, N.F.C. Mineral associations in diamonds from the lowermost upper mantle and uppermost lower mantle. In Proceedings of the 10th International Kimberlite Conference, Bangalore, India, 6–11 February 2012; Geological Society of India: Bangalore, India, 2013; Volume 1, pp. 235–253. [Google Scholar]
- Hayman, P.C.; Kopylova, M.G.; Kaminsky, F.Y. Lower mantle diamonds from Rio Soriso (Juina area, Mato Grosso, Brazil). Contrib. Mineral. Petrol. 2005, 149, 430–445. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Kagi, H.; Shatsky, V.S.; Ragozin, A.L. Local variations of carbon isotope composition in diamonds from Sao-Luis (Brazil): Evidence for heterogenous carbon reservoir in sublithospheric mantle. Chem. Geol. 2014, 240, 114–124. [Google Scholar] [CrossRef]
- Bulanova, G.P. The formation of diamond. J. Geochem. Explor. 1995, 53, 2–23. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib. Mineral. Petrol. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Wirth, R.; Schreiber, A. A microinclusion of lower-mantle rock and some other lower-mantle inclusions in diamond. Can. Mineral. 2015, 53, 83–104. [Google Scholar] [CrossRef]
- Fei, Y. Crystal Chemistry of FeO at High Pressure and Temperature; Special Publication 5; Geochemical Society: Washington, DC, USA, 1996; pp. 243–254. [Google Scholar]
- Jacobsen, S.D.; Reichmann, H.J.; Spetzler, H.; Mackwell, S.J.; Smyth, J.R.; Angel, R.J.; McCammon, C.A. Structure and elasticity of single-crystal (Mg,Fe)O and a new method of generating shear waves for gigahertz ultrasonic interferometry. J. Geophys. Res. 2002, 107, 5867–5871. [Google Scholar] [CrossRef]
- Nestola, F.; Burnham, A.D.; Peruzza, L.; Tauro, L.; Alvaro, M.; Walter, M.J.; Gunter, M.; Anzolini, C.; Kohn, S.C. Tetragonal almandine-pyrope phase, TAPP: Finally a name for it, the new mineral jeffbenite. Mineral. Mag. 2016, 80, 1219–1232. [Google Scholar] [CrossRef]
- Longo, M.; McCammon, C.A.; Jacobsen, S.D. Microanalysis of the iron oxidation state in (Mg, Fe)O and application to the study of microscale processes. Contrib. Mineral. Petrol. 2011, 162, 1249–1257. [Google Scholar] [CrossRef]
- Ringwood, A.E. Composition of core and implications for origin of Earth. Geochem. J. 1977, 11, 111–135. [Google Scholar] [CrossRef]
- Sherman, D.M. The nature of pressure-induced metallization of FeO and its implications to the core-mantle boundary. Geophys. Res. Lett. 1989, 16, 515–518. [Google Scholar] [CrossRef]
- McWilliams, R.S.; Spaulding, D.K.; Eggert, J.H.; Celliers, P.M.; Hicks, D.G.; Smith, R.F.; Collins, G.W.; Jeanloz, R. Phase transformations and metallization of magnesium oxide at high pressure and temperature. Science 2012, 338, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Cohen, R.E.; Hirose, K.; Haule, K.; Shimizu, K.; Ohishi, Y. Experimental and theoretical evidence for pressure-induced metallization in FeO with rocksalt-type structure. Phys. Rev. Lett. 2012, 108, 026403. [Google Scholar] [CrossRef] [PubMed]
- Tremper, R.T.; Giddings, R.A.; Hodge, J.D.; Gordon, R.S. Creep of polycrystalline MgO–FeO–Fe2O3 solid–solutions. J. Am. Ceram. Soc. 1974, 57, 421–428. [Google Scholar] [CrossRef]
- Wood, B.J.; Nell, J. High-temperature electrical-conductivity of the lower-mantle phase (Mg,Fe)O. Nature 1991, 351, 309–311. [Google Scholar] [CrossRef]
- Mackwell, S.; Bystricky, M.; Sproni, C. Fe–Mg interdiffusion in (Mg,Fe)O. Phys. Chem. Mineral. 2005, 32, 418–425. [Google Scholar] [CrossRef]
- Otsuka, K.; Longo, M.; McCammon, C.A.; Karato, S.-I. Ferric iron content of ferropericlase as a function of composition, oxygen fugacity, temperature and pressure: Implications for redox conditions during diamond formation in the lower mantle. Earth Planet. Sci. Lett. 2013, 365, 7–16. [Google Scholar] [CrossRef]
- Otsuka, K.; McCammon, C.A.; Karato, S. Tetrahedral occupancy of ferric iron in (Mg,Fe)O: Implications for point defects in the Earth’s lower mantle. Phys. Earth Planet. Int. 2010, 180, 179–188. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Ryabchikov, I.D.; McCammon, C.A.; Longo, M.; Abakumov, A.M.; Turner, S.; Heidari, H. Oxidation potential in the Earth’s lower mantle as recorded by ferropericlase inclusions in diamond. Earth Planet. Sci. Lett. 2015, 417, 49–56. [Google Scholar] [CrossRef]
- Biellmann, C.; Gillet, P.; Guyot, F.; Peyronneau, J.; Reynard, B. Experimental evidence for carbonate stability in the Earth’s lower mantle. Earth Planet. Sci. Lett. 1993, 118, 31–41. [Google Scholar] [CrossRef]
- Brenker, F.E.; Vollmer, C.; Vincze, L.; Vekemans, B.; Szymanski, A.; Janssens, K.; Szaloki, I.; Nasdala, L.; Joswig, W.; Kaminsky, F. Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet. Sci. Lett. 2007, 260, 1–9. [Google Scholar] [CrossRef]
- Stagno, V.; Tange, Y.; Miyajima, N.; McCammon, C.A.; Irifune, T.; Frost, D.G. The stability of magnesite in the transition zone and the lower mantle as function of oxygen fugacity. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Boulard, E.; Gloter, A.; Corgne, A.; Antonangeli, D.; Auzende, A.-L.; Perrillat, J.-P.; Guyot, F.; Fiquet, G. New host for carbon in the deep Earth. Proc. Natl. Acad. Sci USA 2011, 10, 5184–5187. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, F.V.; Wirth, R.; Schreiber, A. Carbonatitic inclusions in deep mantle diamond from Juina, Brazil: New minerals in the carbonate–halide association. Can. Mineral. 2013, 51, 447–466. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Li, S.; Wu, T.-H.; Peng, S.-Y. Reduction of carbon dioxide into carbon by the active wüstite and the mechanism of the reaction. Mater. Chem. Phys. 1999, 58, 139–145. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle–slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef] [PubMed]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Bayukov, O.A. The role of rocks saturated with metallic iron in the formation of ferric carbonate–silicate melts: Experimental modeling under PT-conditions of lithospheric mantle. Russ. Geol. Geophys. 2015, 56, 143–154. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Bayukov, O.A. Wüstite stability in the presence of a CO2-fluid and a carbonate-silicate melt: Implications for the graphite/diamond formation and generation of Fe-rich mantle metasomatic agents. Lithos 2016, 244, 20–29. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Bayukov, O.A.; Sobolev, N.V. Conditions for diamond and graphite formation from iron carbide at the P-T parameters of lithospheric mantle. Russ. Geol. Geophys. 2016, 57, 176–189. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Novoselov, I.D.; Bayukov, O.A. An effect of reduced S-rich fluids on diamond formation under mantle-slab interaction. Lithos 2019, 336–337, 27–39. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Bayukov, O.A.; Sobolev, N.V. The formation of graphite upon the interaction of subducted carbonates and sulfur with metal-bearing rocks of the lithospheric mantle. Dokl. Earth Sci. 2016, 466, 88–91. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Sokol, A.G. Effect of nitrogen impurity on diamond crystal growth processes. Cryst. Growth Des. 2010, 10, 3169–3175. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F. Fluid-bearing alkaline carbonate melts as the medium for the formation of diamonds in the Earth’s mantle: An experimental study. Lithos 2002, 60, 145–159. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Aldon, L.; Jumas, J.-C. Lithium-induced conversion reaction in wüstite Fe1-xO studied by 57Fe Mössbauer spectroscopy. Solid State Sci. 2012, 14, 354–361. [Google Scholar] [CrossRef][Green Version]
- Greenwood, N.N.; Howe, A.T. Mössbauer studies of Fe1-xO. Part I. The defect structure of quenched samples. J. Chem. Soc. 1972, 1, 110–116. [Google Scholar] [CrossRef]
- Manning, P.G.; Jones, W.; Birchall, T. Mössbauer spectral studies of iron-enriched sediments from Homilton Harbor, Ontario. Can. Miner. 1980, 18, 291–299. [Google Scholar]
- Dasgupta, R.; Buono, A.; Whelan, G.; Walker, D. High-pressure melting relations in Fe–C–S systems: Implications for formation, evolution and structure of metallic cores in planetary bodies. Geochim. Cosmochim. Acta 2009, 73, 6678–6691. [Google Scholar] [CrossRef]
- Pal’yanov, Y.; Borzdov, Y.; Kupriyanov, I.; Gusev, V.; Khokhryakov, A.; Sokol, A. High-pressure synthesis and characterization of diamond from a sulfur-carbon system. Diam. Relat. Mater. 2001, 10, 2145–2152. [Google Scholar]
- Palyanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Pal’yanova, G.A.; Borzdov, Y.M.; Sobolev, N.V. Diamond and graphite crystallization in COH fluid at P-T parameters of the natural diamond formation. Dokl. Earth Sci. 2000, 375, 1395–1398. [Google Scholar]
- Brey, G.P.; Ryabchikov, I.D. Carbon dioxide in strongly undersaturated melts and origin of kimberlitic magmas. Neues Jahrb. Mineral. Monatsh.. 1994, 10, 449–463. [Google Scholar]
- Rohrbach, A.; Ballhaus, C.; Golla-Schindler, U.; Ulmer, P.; Kamenetsky, V.S.; Kuzmin, D.V. Metal saturation in the upper mantle. Nature 2007, 449, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A.; Schmidt, M.W. Redox freezing and melting in the Earth’s deep mantle resulting from carbon—Iron redox coupling. Nature 2011, 472, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Oganov, A.R.; Hemley, R.J.; Hazen, R.M.; Jones, A.P. Structure, bonding and mineralogy of carbon at extreme conditions. Rev. Mineral. Geochem. 2013, 75, 47–77. [Google Scholar] [CrossRef]
- Martirosyan, N.S.; Shatskiy, A.; Chanyshev, A.D.; Litasov, K.D.; Podborodnikov, I.V.; Yoshino, T. Effect of water on the magnesite–iron interaction, with implications for the fate of carbonates in the deep mantle. Lithos 2019, 326–327, 435–445. [Google Scholar] [CrossRef]














| Run N | P, GPa | T, °C | t, h | Sample Zone | Phase Assemblage | Magnesiowüstite and Wüstite Composition |
|---|---|---|---|---|---|---|
| Carbonate-metal system | ||||||
| 1541 | 7.5 | 1000 | 60 | Reduced | Mws (17) + Coh (81) + Gr (2) | Mg0.12–0.35Fe2+0.65–0.88O |
| Oxidized | Mws (62) + Marg (36) + Gr (2) | Mg0.38Fe2+0.42–0.57Fe3+0.02–0.15O | ||||
| 1525 | 7.5 | 1400 | 60 | Reduced | Mws (57) + Lcarb (37) + Gr (3) + Dm (3) | Mg0.37Fe0.60Fe3+0.03O |
| Oxidized | Mws (34) + Lcarb (25) + Fms (25) + Gr (3) + Dm (3) | Mg0.38Fe2+0.55Fe3+0.06O | ||||
| 1250 | 6.5 | 1450 | 20 | Reduced | Mws (61) + Coh (37) + Dm (2) | Mg0.27-0.30Fe2+0.62-0.68Fe3+0.05O |
| Oxidized | Mws (15) + Lcarb (82) + Gr (2) + Dm (1) | Mg0.33-0.39Fe2+0.42-0.44Fe3+0.19O | ||||
| 1515 | 7.5 | 1550 | 20 | Reduced | Mws (80) + Lcarb (12) + Gr (6) + Dm (2) | Mg0.42Fe2+0.51Fe3+0.05O |
| Oxidized | Mws (45) + Lcarb (15) + Fms (36) + Gr (2) + Dm (2) | Mg0.44Fe2+0.51Fe3+0.05O | ||||
| Carbonate-oxide-metal system | ||||||
| 1287 | 6.3 | 1250 | 20 | Reduced | Mws (60) + Coh (8) + Gr (2) + Grt (30) | Mg0.17Fe2+0.76Fe3+0.07O |
| Oxidized | Mws (56) + Grt (28) + Ol (8) + Gr (2) + Carb (6) | Mg0.19Fe2+0.74Fe3+0.07O | ||||
| 1250-2 | 6.3 | 1450 | 20 | Reduced | Mws (60) + Coh (2) + Gr (4) + Grt (34) | Mg0.24Fe2+0.69Fe3+0.07O |
| Carbonate-oxide system | ||||||
| 1289 | 6.3 | 1150 | 20 | Reduced | Mws (100) | Mg0.01Fe2+0.95Fe3+0.04O |
| 1287-2 | 6.3 | 1250 | 20 | Reduced | Mws (100) | Mg0.12Fe2+0.80Fe3+0.08O |
| 1283 | 6.3 | 1350 | 20 | Reduced | Mws (100) | Mg0.13Fe2+0.73Fe3+0.14O |
| Carbide-oxide system | ||||||
| 1623-A4 | 6.3 | 900 | 18 | n/a | Ws (93) + Gr (1) + Coh (5) | Fe2+0.96Fe3+0.04O |
| 1620-A4 | 6.3 | 1100 | 18 | n/a | Ws (93) + Gr (1) + Coh (5) | Fe2+0.96Fe3+0.04O |
| 1618-A4 | 6.3 | 1400 | 18 | n/a | Ws (93) + Gr (1) +LFe–C (5) + DG | Fe2+0.96Fe3+0.04O |
| 1602-A4 | 6.3 | 1600 | 18 | n/a | Ws (93) + Gr (1) + LFe–C (5) + DG | Fe2+0.96Fe3+0.04O |
| Carbonate-metal-sulphur system | ||||||
| MSC-23 | 6.3 | 900 | 18 | n/a | Po (16) + Mws (22) + Coh (6) + Carb (55) +Gr (1) | Mg0.32–0.37Fe2+0.58–0.63Fe3+0.04Ca0.02O |
| MSC-20 | 6.3 | 1200 | 18 | n/a | Mws (36) + Po (63) + Gr (1) | Mg0.29–0.30Fe2+0.58Fe3+0.05Ca0.08O |
| MSC-18 | 6.3 | 1400 | 18 | n/a | Mws (58) + Gr (2) + LFe–S–C (16) + LFe–S–O (24) | Mg0.34Fe2+0.63Fe3+0.03O |
| MSC-02 | 6.3 | 1600 | 18 | n/a | Mws (58) + Gr (2) + LFe–S–C (16) + LFe–S–O (24) | Mg0.44Fe0.51Ca0.06O Mg0.17Fe2+0.76Fe3+0.07O |
| Run N | P, GPa | T, °C | t, h | Sample Zone | Phase | Mass Concentrations, wt.% | |||
|---|---|---|---|---|---|---|---|---|---|
| FeO + Fe2O3 * | MgO | CaO | Total | ||||||
| Carbonate-metal system | |||||||||
| 1541 | 7.5 | 1000 | 60 | Reduced | Mws | 77.1 | 22.6 | 0.1 | 99.8 |
| 88.0 | 13.0 | - | 101.0 | ||||||
| 93.5 | 7.0 | - | 100.5 | ||||||
| Oxidized | Mws | 74.9 | 25.1 | 0.2 | 100.1 | ||||
| 1525 | 7.5 | 1400 | 60 | Reduced | Mws | 74.2 | 24.5 | 0.1 | 99.0 |
| Oxidized | Mws | 74.3 | 25.5 | 0.2 | 100.0 | ||||
| 1250 | 6.5 | 1450 | 20 | Reduced | Mws | 79.9 | 19.5 | 0.5 | 99.9 |
| 82.4 | 16.7 | 0.4 | 99.6 | ||||||
| Oxidized | Mws | 72.3 | 25.3 | - | 99.9 | ||||
| 73.6 | 21.3 | 4.4 | 99.4 | ||||||
| 1515 | 7.5 | 1550 | 20 | Reduced | Mws | 71.2 | 28.5 | - | 99.7 |
| Oxidized | Mws | 69.7 | 30.1 | 0.1 | 99.8 | ||||
| Carbonate-oxide-metal system | |||||||||
| 1287 | 6.3 | 1250 | 20 | Reduced | Mws | 88.9 | 10.3 | - | 99.2 |
| Oxidized | Mws | 87.7 | 11.8 | - | 99.5 | ||||
| 1250-2 | 6.3 | 1450 | 20 | Reduced | Mws | 85.0 | 14.6 | - | 99.6 |
| Carbonate-oxide-metal system | |||||||||
| 1289 | 6.3 | 1150 | 20 | Reduced | Mws | 99.3 | 0.6 | - | 99.9 |
| 1287-2 | 6.3 | 1250 | 20 | Reduced | Mws | 93.9 | 6.9 | - | 100.9 |
| 1283 | 6.3 | 1350 | 20 | Reduced | Mws | 91.7 | 7.5 | - | 99.2 |
| Carbonate-metal-sulphur system | |||||||||
| MSC-23 | 6.3 | 900 | 18 | n/a | Mws | 77.2 | 20.7 | 1.8 | 99.8 |
| MSC-20 | 6.3 | 1200 | 18 | n/a | Mws | 76.6 | 20.2 | 2.6 | 99.4 |
| MSC-18 | 6.3 | 1400 | 18 | n/a | Mws | 76.8 | 22.7 | 0.1 | 99.6 |
| MSC-02 | 6.3 | 1600 | 18 | n/a | Mws center | 63.3 | 30.8 | 5.6 | 99.0 |
| Mws rim | 89.6 | 10.1 | - | 99.7 | |||||
| Run N | Sample Zone | Phase | Fe Positions | A, % (± 2) | IS, mm/s ±0.02 | QS, mm/s ±0.04 | W, mm/s ±0.04 |
|---|---|---|---|---|---|---|---|
| 1541 | Reduced | Ws | Fe2+ in FeO | 26 | 1.02 | 0.35 | 0.37 |
| Mws | Fe2+ in Fe1 − yMgyO | 40 | 1.00 | 0.68 | 0.29 | ||
| Fe2+ in (Fe1 − yMgy)xO | 14 | 0.95 | 1.02 | 0.22 | |||
| Fe2+ in (Fe1 − yMgy)x1O, high y | 20 | 0.88 | 1.19 | 0.42 | |||
| Oxidized | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 37 | 1.02 | 0.49 | 0.32 | |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 44 | 1.02 | 0.79 | 0.32 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 9 | 1.00 | 1.10 | 0.22 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 3) | 5 | 1.02 | 1.37 | 0.27 | |||
| Fe3+ (IV) in (Fe1 − yMgy)xO | 5 | 0.24 | 0.23 | 0.49 | |||
| Oxidized | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 42 | 1.02 | 0.61 | 0.33 | |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 15 | 0.96 | 1.09 | 0.21 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 5 | 0.81 | 1.81 | 0.20 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1 + Fe3+) | 8 | 0.97 | 2.26 | 0.21 | |||
| Fe3+ (VI) in (Fe1 − yMgy)xO | 30 | 0.43 | 0 | 0.74 | |||
| 1250 | Reduced | Mws | Fe2+ in (Fe1 − yMgy)O (NCV=0) | 28 | 1.00 | 0.45 | 0.24 |
| Fe2+ in (Fe1 − yMgy)xO (NCV=1) | 31 | 1.01 | 0.69 | 0.23 | |||
| Fe2+ in (Fe1-yMgy)xO (NCV=2) | 21 | 1.00 | 0.93 | 0.22 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV=3) | 17 | 0.99 | 1.21 | 0.28 | |||
| Fe3+ (VI) in (Fe1 − yMgy)xO | 3 | 0.52 | 0.68 | 0.22 | |||
| Oxidized | Mws | Fe2+ in (Fe1 − yMgy)xO | 66 | 1.09 | 1.22 | 0.56 | |
| Fe3+ (IV) in (Fe1 − yMgy)xO | 34 | 0.13 | - | 0.39 | |||
| 1525 | Reduced | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 34 | 0.98 | 0.51 | 0.32 |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 26 | 0.98 | 0.77 | 0.24 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 22 | 0.97 | 1.05 | 0.23 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 3) | 9 | 0.96 | 1.40 | 0.22 | |||
| Fe3+ (IV) in (Fe1-yMgy)xO | 9 | 0.23 | 0.23 | 0.24 | |||
| Oxidized | Mws | Fe2+ in (Fe1-yMgy)O (NCV = 0) | 56 | 1.19 | 1.79 | 0.22 | |
| Fe2+ in (Fe1-yMgy)xO (NCV = 1) | 19 | 0.99 | 0.54 | 0.27 | |||
| Fe2+ in (Fe1-yMgy)xO (NCV = 2) | 10 | 0.98 | 0.82 | 0.20 | |||
| Fe2+ in (Fe1-yMgy)xO (NCV = 3) | 12 | 0.97 | 1.11 | 0.23 | |||
| Fe3+ (IV) in (Fe1 − yMgy)xO | 3 | 0.15 | 0.11 | 0.08 | |||
| 1515 | Reduced | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 38 | 1.01 | 0.52 | 0.39 |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 20 | 1.00 | 0.78 | 0.27 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 19 | 0.99 | 1.04 | 0.28 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 3) | 8 | 1.00 | 1.38 | 0.27 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1 + Fe3+) | 4 | 1.01 | 2.10 | 0.27 | |||
| Fe3+ (IV) in (Fe1-yMgy)xO | 11 | 0.27 | 0.17 | 0.27 | |||
| Oxidized | Mws | Fe2+ in (Fe1-yMgy)O (NCV=0) | 47 | 1.00 | 0.52 | 0.28 | |
| Fe2+ in (Fe1-yMgy)xO (NCV=1) | 22 | 1.00 | 0.78 | 0.21 | |||
| Fe2+ in (Fe1-yMgy)xO (NCV=2) | 18 | 1.00 | 1.04 | 0.23 | |||
| Fe3+ (IV-VI) in (Fe1-yMgy)xO | 13 | 0.27 | 0.29 | 0.23 |
| Run N | Sample Zone | Phase | Fe Positions | A, % (± 2) | IS, mm/s ±0.02 | QS, mm/s ±0.04 | W, mm/s ±0.04 |
|---|---|---|---|---|---|---|---|
| Carbonate-oxide-metal system | |||||||
| 1287 | Reduced | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 21 | 0.99 | 0.44 | 0.28 |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 16 | 0.97 | 0.70 | 0.27 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 55 | 0.96 | 0.96 | 0.46 | |||
| Fe3+ (IV) in (Fe1 − yMgy)xO | 7 | 0.25 | 0.18 | 0.25 | |||
| Oxidized | Mws | Fe2+ in (Fe1 − yMgy)xO | 93 | 0.97 | 0.75 | 0.49 | |
| Fe3+ (IV) in (Fe1 − yMgy)xO | 7 | 0.26 | 0.17 | 0.19 | |||
| 1250-2 | Reduced | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 20 | 1.02 | 0.49 | 0.32 |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 39 | 1.02 | 0.79 | 0.32 | |||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 36 | 1.00 | 1.10 | 0.22 | |||
| Fe3+ (IV) in (Fe1 − yMgy)xO | 5 | 1.02 | 1.37 | 0.27 | |||
| Carbonate-oxide system | |||||||
| 1289 | Reduced | Mws | Fe2+ in (Fe1 − yMgy)xO | 95 | 1.06 | 0.73 | 0.46 |
| Fe3+ (IV) in (Fe1 − yMgy)xO | 5 | 0.16 | 0.53 | 0.21 | |||
| 1287-2 | Reduced | Mws | Fe2+ in (Fe1 − yMgy)xO | 93 | 1.03 | 0.94 | 0.50 |
| Fe3+ (IV) in (Fe1 − yMgy)xO | 7 | 0.16 | 0.19 | 0.23 | |||
| 1283 | Reduced | Mws | Fe2+ in (Fe1 − yMgy)xO | 88 | 1.06 | 0.71 | 0.46 |
| Fe3+ (IV) in (Fe1 − yMgy)xO | 12 | 0.18 | 0.52 | 0.33 | |||
| Run N | Phase | Fe Positions | A, % ±2 | IS, mm/s ±0.02 | QS, mm/s ±0.04 | W, mm/s ±0.04 |
|---|---|---|---|---|---|---|
| Carbide-oxide system | ||||||
| 1623-A4 | Ws | Fe2+ in FeO (NCV = 0) | 27 | 0.96 | 0.38 | 0.32 |
| Fe2+ in FexO (NCV = 1) | 11 | 1.14 | 0.38 | 0.22 | ||
| Fe2+ in FexO (NCV = 2) | 32 | 0.89 | 0.61 | 0.41 | ||
| Fe2+ in FexO (NCV = 3) | 26 | 0.92 | 1.04 | 0.41 | ||
| Fe3+ (VI) in FexO | 4 | 0.36 | 1.02 | 0.21 | ||
| 1620-A4 | Ws | Fe2+ in FeO (NCV = 0) | 30 | 0.95 | 0.35 | 0.32 |
| Fe2+ in FexO (NCV = 1) | 15 | 1.12 | 0.38 | 0.24 | ||
| Fe2+ in FexO (NCV = 2) | 24 | 0.91 | 0.64 | 0.33 | ||
| Fe2+ in FexO (NCV = 3) | 27 | 0.93 | 1.06 | 0.37 | ||
| Fe3+ (VI) in FexO | 4 | 0.32 | 0.75 | 0.31 | ||
| 1618-A4 | Ws | Fe2+ in FeO (NCV = 0) | 26 | 0.96 | 0.31 | 0.36 |
| Fe2+ in FexO (NCV = 1) | 7 | 1.09 | 0.31 | 0.22 | ||
| Fe2+ in FexO (NCV = 2) | 23 | 0.98 | 0.62 | 0.38 | ||
| Fe2+ in FexO (NCV = 3) | 40 | 0.93 | 0.96 | 0.48 | ||
| Fe3+ (VI) in FexO | 4 | 0.17 | 0.58 | 0.53 | ||
| 1602-A4 | Ws | Fe2+ in FeO (NCV = 0) | 20 | 0.97 | 0.34 | 0.36 |
| Fe2+ in FexO (NCV = 1) | 8 | 1.09 | 0.40 | 0.24 | ||
| Fe2+ in FexO (NCV = 2) | 38 | 0.94 | 0.60 | 0.43 | ||
| Fe2+ in FexO (NCV = 3) | 32 | 0.94 | 1.02 | 0.43 | ||
| Fe3+ (VI) in FexO | 2 | 0.40 | 0.80 | 0.33 | ||
| Carbonate-metal-sulphur system | ||||||
| MCS-23 | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 27 | 1.07 | 0.52 | 0.31 |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 49 | 1.06 | 0.82 | 0.35 | ||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 16 | 1.08 | 1.22 | 0.30 | ||
| Fe3+ (IV) in (Fe1 − yMgy)xO | 8 | 0.11 | 0.38 | 0.34 | ||
| MCS-20 | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 19 | 1.07 | 0.61 | 0.29 |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 47 | 1.07 | 0.99 | 0.41 | ||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 20 | 1.06 | 1.48 | 0.39 | ||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 3) | 8 | 1.02 | 2.12 | 0.27 | ||
| Fe3+ (IV and VI) in (Fe1 − yMgy)xO | 5 | 0.27 | 0.39 | 0.30 | ||
| 0.25 | 0.78 | 0.20 | ||||
| MCS-18 | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 35 | 1.04 | 0.55 | 0.33 |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 45 | 1.04 | 0.83 | 0.33 | ||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 5 | 1.04 | 1.27 | 0.23 | ||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 3) | 8 | 1.00 | 1.37 | 0.40 | ||
| Fe3+ (IV) in (Fe1 − yMgy)xO | 7 | 0.19 | 0.60 | 0.23 | ||
| MCS-02 | Mws | Fe2+ in (Fe1 − yMgy)O (NCV = 0) | 52 | 1.01 | 0.59 | 0.34 |
| Fe2+ in (Fe1 − yMgy)xO (NCV = 1) | 18 | 1.01 | 0.90 | 0.24 | ||
| Fe2+ in (Fe1 − yMgy)xO (NCV = 2) | 16 | 1.00 | 1.27 | 0.30 | ||
| Fe3+ (VI) in (Fe1 − yMgy)xO | 13 | 0.38 | 0.91 | 0.35 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataleva, Y.; Palyanov, Y.; Borzdov, Y.; Bayukov, O. Processes and Conditions of the Origin for Fe3+-Bearing Magnesiowüstite under Lithospheric Mantle Pressures and Temperatures. Minerals 2019, 9, 474. https://doi.org/10.3390/min9080474
Bataleva Y, Palyanov Y, Borzdov Y, Bayukov O. Processes and Conditions of the Origin for Fe3+-Bearing Magnesiowüstite under Lithospheric Mantle Pressures and Temperatures. Minerals. 2019; 9(8):474. https://doi.org/10.3390/min9080474
Chicago/Turabian StyleBataleva, Yuliya, Yuri Palyanov, Yuri Borzdov, and Oleg Bayukov. 2019. "Processes and Conditions of the Origin for Fe3+-Bearing Magnesiowüstite under Lithospheric Mantle Pressures and Temperatures" Minerals 9, no. 8: 474. https://doi.org/10.3390/min9080474
APA StyleBataleva, Y., Palyanov, Y., Borzdov, Y., & Bayukov, O. (2019). Processes and Conditions of the Origin for Fe3+-Bearing Magnesiowüstite under Lithospheric Mantle Pressures and Temperatures. Minerals, 9(8), 474. https://doi.org/10.3390/min9080474






