Rare Earth Element Recovery from Acidic Extracts of Florida Phosphate Mining Materials Using Chelating Polymer 1-Octadecene, Polymer with 2,5-Furandione, Sodium Salt
Abstract
1. Introduction
2. Materials and Methods
2.1. Acid Extraction Methods
2.2. REE, Uranium, and Thorium Recovery Methods
2.3. Data Analysis
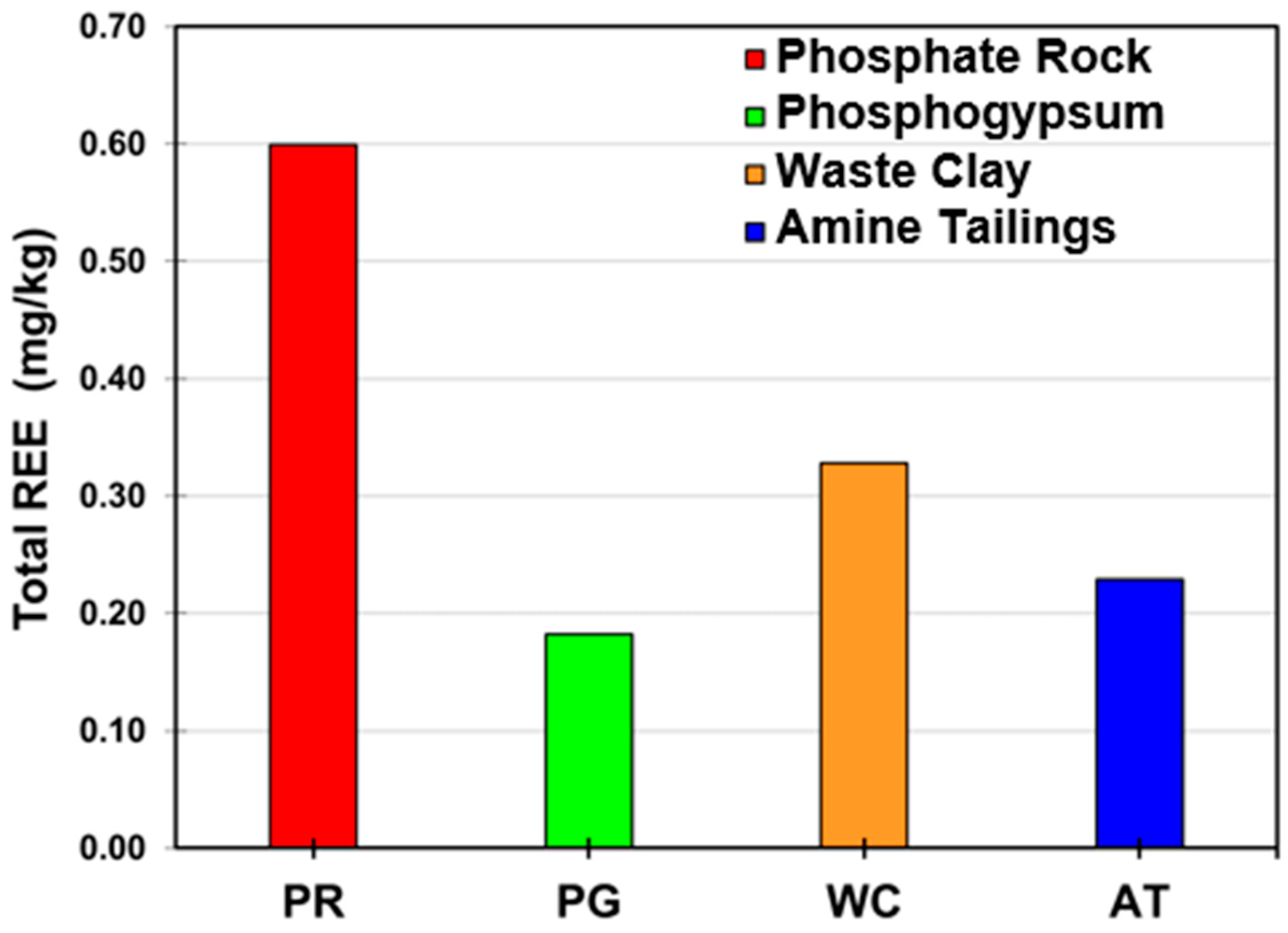
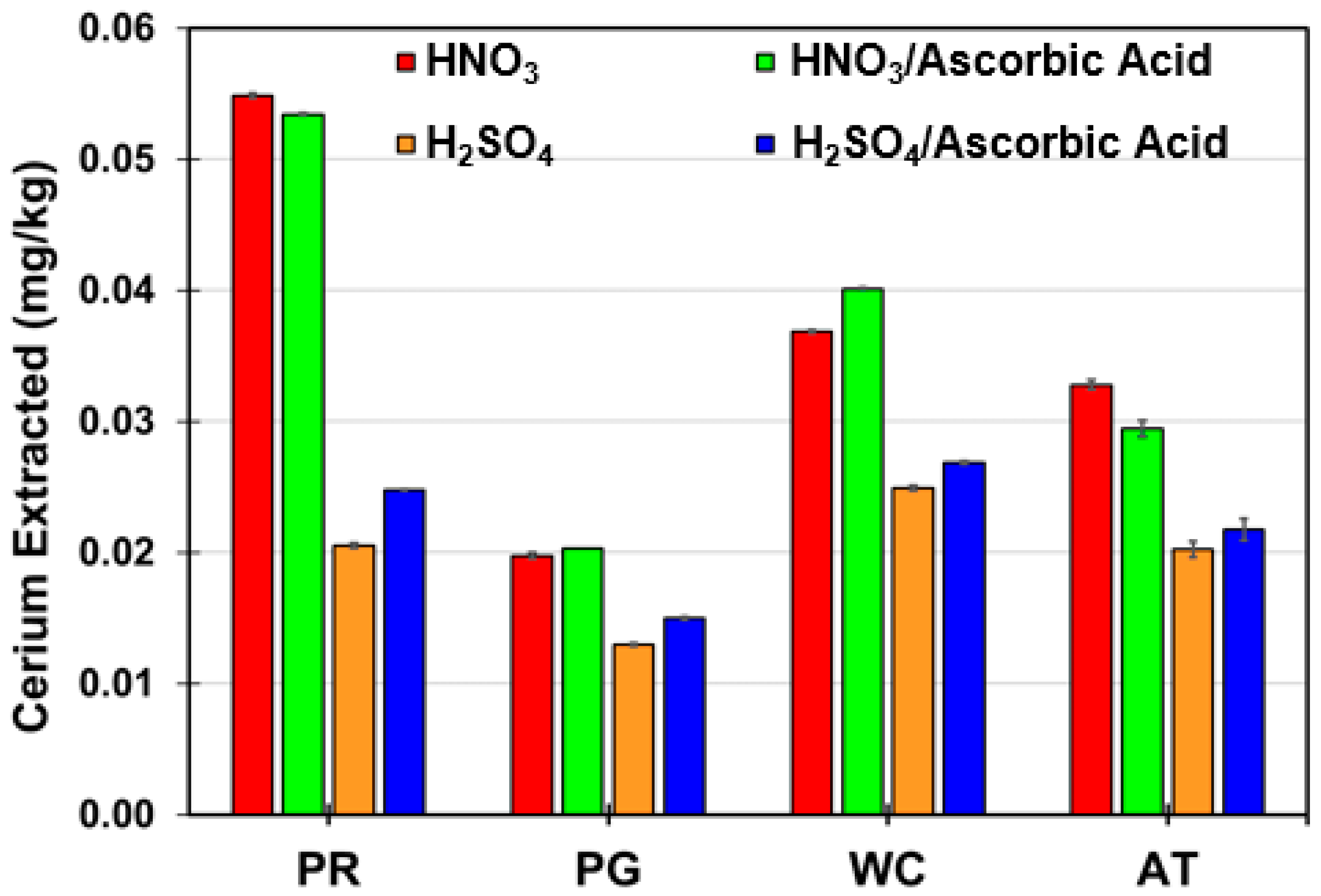
3. Results and Discussion
3.1. Acid Extraction
3.2. REE, Uranium, and Thorium Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, P. Comprehensive recovery and sustainable development of phosphate resources. Procedia Eng. 2014, 83, 37–51. [Google Scholar] [CrossRef]
- Humphries, M. Rare Earth Elements Global Supply Chain; Diane Publishing: Collingdale, PA, USA, 2010. [Google Scholar]
- Laurino, J.; Huba, Z.; Mustacato, J.; Zhang, P. The Recovery of Rare Earth Elements from Phosphate Rock and Phosphate Mining Waste Products Using a Novel Water-Insoluble Adsorption Polymer. In Proceedings of the Beneficiation of Phosphates VIII, Cape Town, South Africa, 29 April–4 May 2018. [Google Scholar]
- Rychkov, V.N.; Kirillov, E.V.; Kirillov, S.V.; Semenishchev, V.S.; Bunkov, G.M.; Botalov, M.S.; Smyshlyaev, D.V.; Malyshev, A.S. Recovery of rare earth elements from phosphogypsum. J. Clean. Prod. 2018, 196, 674–681. [Google Scholar] [CrossRef]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; du Bray, E.A.; Koenig, A.E. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Edahbi, M.; Benzaazoua, M.; Plante, B.; Doire, S.; Kormos, L. Mineralogical characterization using QEMSCAN® and leaching potential study of REE within silicate ores: A case study of the Matamec project, Quebec, Canada. J. Geochem. Explor. 2018, 185, 64–73. [Google Scholar] [CrossRef]
- Soltani, F.; Abdollahy, M.; Petersen, J.; Ram, R.; Javad Koleini, S.M.; Moradkhani, D. Leaching and recovery of phosphate and rare earth elements from an iron-rich fluorapatite concentrate: Part II: Selective leaching of calcium and phosphate and acid baking of the residue. Hydrometallurgy 2019, 184, 29–38. [Google Scholar] [CrossRef]
- May, A.; Sweeney, J.W. Assessment of environmental impacts associated with phosphogypsum in Florida. In The Chemistry and Technology of Gypsum; Kuntze, R.A., Ed.; ASTM International: West Conshohocken, PA, USA, 1984; pp. 116–139. ISBN 0-8031-0219-4. [Google Scholar]
- Van Kauwenberg, S.J.; Cathcart, J.B.; McClellan, G.H. Mineralogy and Alteration of the Phosphate Deposits of Florida; U.S. Government Printing Office: Washington, DC, USA, 1914.
- Weber, R.J.; Reisman, D.J. Rare Earth Elements: A Review of Production, Processing, Recycling, and Associated Environmental Issues; US EPA Region: Washington, DC, USA, 2012.
- Zhang, P.; Liang, H.; Jin, Z.; DePaoli, D. The ultimate mineral processing challenge: Recovery of rare earths, phosphorus and uranium from Florida phosphatic clay. Miner. Metall. Process. 2017, 34, 183–188. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare-earth leaching from Florida phosphate rock in wet-process phosphoric acid production. Miner. Metall. Process. 2017, 34, 146–153. [Google Scholar] [CrossRef]
- Canovas, C.R.; Chapron, S.; Arrachart, G.; Pellet-Rostaing, S. Leaching of rare earth elements (REEs) and impurities from phosphogypsum: A preliminary insight for further recovery of critical raw materials. J. Clean. Prod. 2019, 219, 225–235. [Google Scholar] [CrossRef]
- Goyne, K.W.; Brantley, S.L.; Chorover, J. Rare earth element release from phosphate minerals in the presence of organic acids. Chem. Geol. 2010, 278, 1–14. [Google Scholar] [CrossRef]
- Kolokolnikov, V.A.; Kovalev, M.I. Processing Rare-Earth Element Concentrate Obtained from Phosphogypsum. Chem. Sustain. Dev. 2009, 17, 261–266. [Google Scholar]
- Wang, L.; Long, Z.; Huang, X.; Yu, Y.; Cui, D.; Zhang, G. Recovery of rare earths from wet-process phosphoric acid. Hydrometallurgy 2010, 101, 41–47. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Zhang, P. REE extraction from phosphoric acid, phosphoric acid sludge, and phosphogypsum. Miner. Process. Extr. Metall. 2015, 124, 143–150. [Google Scholar] [CrossRef]
- Battsengel, A.; Batnasan, A.; Haga, K.; Watanabe, Y.; Shibayama, A. Magnetic separation and leaching study of rare earth elements from apatite-iron ore. Int. J. Soc. Mater. Eng. Resour. 2018, 23, 88–92. [Google Scholar] [CrossRef]
- Silva, R.G.; Morais, C.A.; Oliveira, E.D. Selective precipitation of rare earths from non-purified and purified sulfate liquors using sodium sulfate and disodium hydrogen phosphate. Miner. Eng. 2019, 134, 402–416. [Google Scholar] [CrossRef]
- Rychkov, V.N.; Kirillov, E.V.; Kirillov, S.V.; Bunkov, G.M.; Mashkovtsev, M.A.; Botalov, M.S.; Volkovich, V.A.; Semenishchev, V.S. Selective ion exchange recovery of rare earth elements from uranium mining solutions. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2016. [Google Scholar]
- Edebali, S.; Pehlivan, E. Evaluation of Cr(III) by ion-exchange resins from aqueous solution: Equilibrium, thermodymanics and kinetics. Desalin. Water Treat. 2014, 52, 7143–7153. [Google Scholar] [CrossRef]
- Felipe, E.; Silva, G.; Vidigal, B.; Ladeira, A.C. Recovery of rare earth elements from acid mine drainage. In Sustainable Industrial Processing Summit 2017 Volume 1: Barrios Intl. Symp/Non-ferrous Smelting & Hydro/Electrochemical Processing; Flogen Star Outreach: Mont-Royal, QC, Canada, 2017; ISBN 978-1-987820-61-4. [Google Scholar]
- Laurino, J.P. Removal of Lead (II) Ions by Poly (2-octadecyl butanedioic acid): Isothermal and Kinetic Studies. J. Macromol. Sci. Part A Pure Appl. Chem. 2008, 45, 612–619. [Google Scholar] [CrossRef]
- Zhang, P.; Miller, J.; DePaoli, D.; Yang, M. Rare Earths Occurrence in Florida Phosphate Ore and Their Fate during Mining and Chemical Processing. In Beneficiation of Phosphates VIII; Zhang, P., Miller, J., Filho, L., Porteus, M., Snyders, N., Wingate, E., Akdogan, G., Eds.; ECI Symposium Series; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 2018. [Google Scholar]
- Zhang, P.; Bogan, M. Recovery of phosphate from Florida beneficiation slimes. Miner. Eng. 1995, 8, 523–534. [Google Scholar] [CrossRef]
- Miller, J.; Lin, C.; Crossman, R. Isolation and Characterization of Rare Earth Mineral Particles in Florida Phosphate Rock by de Rapid Scan Radiography and HRXMT; Florida Industrial and Phosphate Research Institute: Bartow, FL, USA, 2015. [Google Scholar]
- Preston, J.S.; Cole, P.M.; Craig, W.M.; Feather, A.M. The recovery of rare earth oxides from a phosphoric acid by-product. Part 1: Leaching of rare earth values and recovery of a mixed rare earth oxide by solvent extraction. Hydrometallurgy 1996, 41, 1–20. [Google Scholar] [CrossRef]
- Walawalkar, M.; Nichol, C.K.; Azimi, G. Process investigation of the acid leaching of rare earth elements from phosphogypsum using HCl, HNO3, and H2SO4. Hydrometallurgy 2016, 166, 195–204. [Google Scholar] [CrossRef]
- Mishelevich, A.; Apelblat, A. Solubilities of magnesium-L-ascorbate, calcium-L-ascorbate, magnesium-L-glutamate, magnesium-D-gluconate, calcium-D-gluconate, calcium-D-heptagluconate, L-aspartic acid, and 3-nitrobenzoic acid in water. J. Chem. Thermodyn. 2008, 40, 897–900. [Google Scholar] [CrossRef]
- Ellis, R.J.; Brigham, D.M.; Delmau, L.; Ivanov, A.S.; Williams, N.J.; Vo, M.N.; Reinhart, B.; Moyer, B.A.; Bryantsev, V.S. “Straining” to separate the rare earths: How the lanthanide contraction impacts chelation by diglycolamide ligands. Inorg. Chem. 2017, 56, 1152–1160. [Google Scholar] [CrossRef] [PubMed]



| Acid Extraction Solution | Acid Composition |
|---|---|
| 1 | 2.5% HNO3 |
| 2 | 2.5% H2SO4 |
| 3 | 1.25% HNO3 + 1.25% H2SO4 (2.5% total) |
| 4 | 2.5% HNO3 + 5% Citric Acid |
| 5 | 2.5% HNO3 + 5% Ascorbic Acid |
| 6 | 2.5% H2SO4 + 5% Citric Acid |
| 7 | 2.5% H2SO4 + 5% Citric Acid |
| Sample Description | Sample Appearance | Average Moisture Content (before Drying) | Average Moisture Content (after Drying) | Average Particle Size (d50) |
|---|---|---|---|---|
| Phosphate Rock | Fine gray sand | 1.14% | 1.14% | 257.3 µm |
| Phosphogypsum | Gray to beige powder | 19.36% | 0.89% | 72.45 µm |
| Amine Tailings | Gray to brown wet powder | 20.77% | 0.23% | 168.8 µm |
| Waste Clay | Gray | 60.64% | 1.70% | Not Determined |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurino, J.P.; Mustacato, J.; Huba, Z.J. Rare Earth Element Recovery from Acidic Extracts of Florida Phosphate Mining Materials Using Chelating Polymer 1-Octadecene, Polymer with 2,5-Furandione, Sodium Salt. Minerals 2019, 9, 477. https://doi.org/10.3390/min9080477
Laurino JP, Mustacato J, Huba ZJ. Rare Earth Element Recovery from Acidic Extracts of Florida Phosphate Mining Materials Using Chelating Polymer 1-Octadecene, Polymer with 2,5-Furandione, Sodium Salt. Minerals. 2019; 9(8):477. https://doi.org/10.3390/min9080477
Chicago/Turabian StyleLaurino, Joseph P., Jack Mustacato, and Zachary J. Huba. 2019. "Rare Earth Element Recovery from Acidic Extracts of Florida Phosphate Mining Materials Using Chelating Polymer 1-Octadecene, Polymer with 2,5-Furandione, Sodium Salt" Minerals 9, no. 8: 477. https://doi.org/10.3390/min9080477
APA StyleLaurino, J. P., Mustacato, J., & Huba, Z. J. (2019). Rare Earth Element Recovery from Acidic Extracts of Florida Phosphate Mining Materials Using Chelating Polymer 1-Octadecene, Polymer with 2,5-Furandione, Sodium Salt. Minerals, 9(8), 477. https://doi.org/10.3390/min9080477




