Simultaneous Leaching of Seafloor Massive Sulfides and Polymetallic Nodules
Abstract
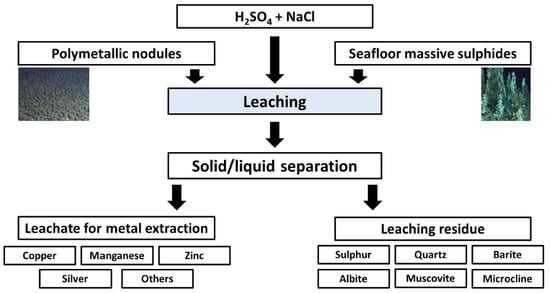
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Material Characterization
3.2. Leaching
3.3. Mineralogical Analyses of Feed and Residues
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lusty, P.A.J.; Murton, B.J. Deep-ocean mineral deposits: metal resources and windows into Earth processes. Elements 2018, 14, 301–306. [Google Scholar] [CrossRef]
- Sharma, R. Deep-Sea Mining: Current Status and Future Considerations. In Deep-Sea Mining Resource Potential, Technical and Environmental Considerations; Sharma, R., Ed.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Lodge, M.W.; Verlaan, P.A. Deep-sea mining: International regulatory challenges and responses. Elements 2018, 14, 331–336. [Google Scholar] [CrossRef]
- Drzymala, J. Mineral Processing. Foundations of Theory and Practice of Metallurgy; Oficyna Wydawnicza Politechniki Wroclawskiej: Wroclaw, Poland, 2007. [Google Scholar]
- Gupta, C.K. Chemical Metallurgy: Principles and Practice; John Wiley & Sons: New Jersey, NJ, USA, 2006. [Google Scholar]
- Nakajima, Y.; Uto, S.; Kanada, S.; Yamamoto, J.; Takahashi, J.; Otabe, S.; Sadaki, J.; Okaya, K.; Matsuo, S.; Fujita, T. Concept of seafloor mineral processing for development of seafloor massive sulfides. In Proceedings of the ASME 30th International Conference on Ocean, offshore and Arctic Engineering, Rotterdam, The Netherlands, 19–24 June 2011. [Google Scholar]
- Kowalczuk, P.B.; Snook, B.; Kleiv, R.A.; Aasly, K. Efficient extraction of copper and zinc from seafloor massive sulphide rock samples from the Loki’s Castle area at the Arctic Mid-Ocean Ridge. Miner. Eng. 2018, 115, 106–116. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Manaig, D.O.; Drivenes, K.; Snook, B.; Aasly, K.; Kleiv, R.A. Galvanic leaching of seafloor massive sulphides using MnO2 in H2SO4-NaCl media. Minerals 2018, 8, 235. [Google Scholar] [CrossRef]
- Havlik, T.; Laubertova, M.; Miskufova, A.; Kondas, J.; Vranka, F. Extraction of copper, zinc, nickel and cobalt in acid oxidative leaching of chalcopyrite at the presence of deep-sea manganese nodules as oxidant. Hydrometallurgy 2005, 77, 51–59. [Google Scholar] [CrossRef]
- Nakazawa, H.; Hareyama, W. Galvanic leaching of chalcopyrite using manganese oxides in spent zinc-carbon batteries. Resour. Process. 2016, 63, 3–11. [Google Scholar] [CrossRef]
- Wang, S.F.; Xiao, L.; Li, Y.Q.; Fang, Z.; Qiu, G.Z.; Li, J. Electronegative leaching for sphalerite-MnO2 in the presence of acidithiobacillus ferrooxidants. J. Therm. Anal. Calorim. 2009, 95, 601–604. [Google Scholar] [CrossRef]
- Nayak, B.B.; Mishra, K.G.; Paramguru, R.K. Kinetics and mechanism of MnO2 dissolution in H2SO4 in the presence of pyrite. J. Appl. Electrochem. 1999, 29, 191–200. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Jeffrey, M.I.; Lawson, F. The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometall. 2000, 56, 189–202. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; MacDonald, R.J.C.; Ingraham, T.R. The kinetics of dissolution of cubanite in aqueous acidic ferric sulfate solutions. Metall. Trans. 1970, 1, 3083–3088. [Google Scholar]
- Veloso, T.C.; Peixoto, J.J.M.; Pereira, M.S.; Leao, V.A. Kinetics of chalcopyrite leaching in either ferric sulphate or cupric sulphate media in the presence of NaCl. Int. J. Miner. Process. 2016, 148, 147–154. [Google Scholar] [CrossRef]
- Pedersen, R.B.; Rapp, H.T.; Thorseth, I.H.; Lilley, M.D.; Barriga, F.J.A.S.; Baumberger, T.; Flesland, K.; Fonseca, R.; Fruh-Green, G.L.; Jorgensen, S.L. Discovery of a black smoker vent field and vent fauna at the Arctic Mid-Ocean Ridge. Nat. Commun. 2010, 1, 126. [Google Scholar] [CrossRef]
- Ludvigsen, M.; Aasly, K.; Ellefemo, S.; Hilario, A.; Ramirez-Llodra, E.; Søreide, F.; Falcon-Suarez, I.; Juliani, C.; Kieswetter, A.; Lim, A.; et al. NTNU Cruise Reports 2016 No 1 MarMine Arctic Mid Ocean Ridge 15.08.2016–05.09.2016; NTNU: Trondheim, Norway, 2016; ISSN 2535-2520. [Google Scholar]
- Snook, B.; Drivenes, K.; Rollinson, G.; Aasly, K. Characterisation of Mineralised Material from the Loki’s Castle Hydrothermal Vent on the Mohn’s Ridge. Minerals 2018, 8, 576. [Google Scholar] [CrossRef]
- Bouzahzah, H.; Benzaazoua, M.; Mermillod-Blondin, R.; Pirard, E. A novel procedure for polished section preparation for automated mineralogy avoiding internal particle settlement. In Proceedings of the 12th International Congress for Applied Mineralogy (ICAM), Istanbul, Turkey, 10–12 August 2015. [Google Scholar]
- Santoro, L.; Tshipeng, S.; Pirard, E.; Bouzahzah, H.; Kaniki, A.; Herrington, R. Mineralogical reconciliation of cobalt recovery from the acid leaching of oxide ores from five deposits in Katanga (DRC). Miner. Eng. 2019, 137, 277–289. [Google Scholar] [CrossRef]
- Abramovski, T.; Stefanova, V.P.; Causse, R.; Romanchuk, A. Technologies for the processing of polymetallic nodules from Clarion Clipperton Zone in the Pacific Ocean. J. Chem. Technol. Metall. 2017, 52, 258–269. [Google Scholar]
- Mineralogy Database. Available online: http://webmineral.com/ (accessed on 5 July 2019).
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Miner. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Senanayake, G. Acid leaching of metals from deep-sea manganese nodules-A critical review of fundamentals and applications. Miner. Eng. 2011, 24, 1379–1396. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.H.; Kristianová, E.; Dvořák, P.; Abramowski, T.; Dreiseitl, I.; Adrysheva, A. Modified Leach Residues from Processing Deep-Sea Nodules as Effective Heavy Metals Adsorbents. Metals 2019, 9, 472. [Google Scholar] [CrossRef]









| Point | O | F | Na | Mg | Al | Si | P | S | Cl | K | Ca | Ti | Mn | Fe | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 35.47 | 44.94 | 19.32 | |||||||||||||||
| 15 | 35.55 | 45.12 | 19.3 | |||||||||||||||
| 16 | 34.44 | 44.86 | 20.7 | |||||||||||||||
| 17 | 30.29 | 2.12 | 1.26 | 0.76 | 3.46 | 0.4 | 0.80 | 0.44 | 4.17 | 1.05 | 39.10 | 15.53 | 0.63 | |||||
| 18 | 34.37 | 2.02 | 2.09 | 1.72 | 0.58 | 1.37 | 0.71 | 0.17 | 0.77 | 1.83 | 0.18 | 45.25 | 3.11 | 0.14 | 3.26 | 1.96 | 0.46 | |
| 19 | 37.87 | 1.91 | 2.02 | 0.43 | 0.97 | 0.11 | 0.11 | 1.1 | 1.49 | 51.05 | 0.87 | 2.06 | ||||||
| 20 | 33.18 | 2.19 | 16.95 | 47.68 | ||||||||||||||
| 21 | 46.86 | 10.26 | 11.98 | 30.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczuk, P.B.; Bouzahzah, H.; Kleiv, R.A.; Aasly, K. Simultaneous Leaching of Seafloor Massive Sulfides and Polymetallic Nodules. Minerals 2019, 9, 482. https://doi.org/10.3390/min9080482
Kowalczuk PB, Bouzahzah H, Kleiv RA, Aasly K. Simultaneous Leaching of Seafloor Massive Sulfides and Polymetallic Nodules. Minerals. 2019; 9(8):482. https://doi.org/10.3390/min9080482
Chicago/Turabian StyleKowalczuk, Przemyslaw B., Hassan Bouzahzah, Rolf Arne Kleiv, and Kurt Aasly. 2019. "Simultaneous Leaching of Seafloor Massive Sulfides and Polymetallic Nodules" Minerals 9, no. 8: 482. https://doi.org/10.3390/min9080482
APA StyleKowalczuk, P. B., Bouzahzah, H., Kleiv, R. A., & Aasly, K. (2019). Simultaneous Leaching of Seafloor Massive Sulfides and Polymetallic Nodules. Minerals, 9(8), 482. https://doi.org/10.3390/min9080482