1. Introduction
Infectious diseases have continually posed a significant threat to both human health and societal development. Thousands of fatalities are attributable to these diseases every year, hence the critical importance of studying their prevention and control mechanisms.
For disease control and prevention, the primary approach is proactive prevention, which underscores the importance of public health education. The latter enhances public awareness of disease prevention and control, thereby mitigating the prevalence of infectious diseases. The recent surge in using new media to disseminate epidemic prevention knowledge has proven efficient. This medium offers several benefits, including high dissemination efficiency, rapid transmission speed, and broad public acceptance [
1,
2,
3,
4]. By promoting infectious disease prevention knowledge among residents, the susceptibility of individuals can be mitigated, thereby reducing the risk of infectious diseases and achieving the objective of curbing disease spread. In 2018, Nyang’inja et al. [
5] introduced the educated compartment
, representing individuals educated about prevention strategies for tungiasis infection, and formulated a mathematical model for the dynamics of jigger infestation incorporating public health education using the systems of ordinary differential equations. Their findings affirmed the effectiveness of public health education as a control measure for eradicating jigger infestation in endemic communities.
Within the context of disease prevention and control, vaccinating susceptible populations has proven to be effective and plays a crucial role in curtailing the spread of epidemics. Vaccinations have been instrumental in controlling diseases such as measles and varicella, serving as a critical preventive measure for infected individuals [
6,
7,
8]. Furthermore, temporary immunity, occurring when an infected individual recovers or a susceptible one gets vaccinated, can also be a significant factor in disease spread. However, this immunity may fade over time, returning the individual to a susceptible state. Many researchers have introduced time delays into their epidemic models to account for this temporary immunity phenomenon [
9,
10,
11,
12,
13,
14,
15]. References [
9,
10,
11,
12] addressed the temporary immunity gained post-recovery. Kyrychko et al. [
11] conducted the analysis of a delayed susceptible–infective–recovered (SIR) model, incorporating temporary immunity, and illustrated the impact of the immunity period—on the long-term dynamics of solutions. In 2010, Xu and Ma [
12] researched a delayed susceptible-infective–recovered–susceptible (SIRS) epidemic model, which included saturation incidence and temporary immunity. They observed a correlation between the immunity period—and the amplitude of oscillations near the endemic equilibrium. Li et al. [
13] evaluated the temporary vaccination immunity in their epidemic models and provided conditions under which the disease would become extinct. Two cases of temporary immunity, recovery immunity and vaccination immunity, were also considered in the literature [
14,
15,
16]. In [
16], Liu and Chen analyzed a deterministic delay SIR epidemic model with vaccination and temporary immunity, suggesting that time-delayed models better mirror actual scenarios. The above study of the literature on temporary immunity shows that considering the effects of time-delay is essential for predicting the extinction and persistence of infectious diseases.
In real-world scenarios, the dynamics of infectious disease models may be impacted by environmental noise during disease transmission, leading to sudden and significant deviations. This has led to the introduction of perturbation into deterministic models. Thus, numerous researchers have explored stochastic infectious disease models [
17,
18,
19]. For instance, the authors of [
17] examined a stochastic delayed SIRS epidemic model with seasonal variation, defining the system’s stochastic threshold and observing that the periodic solution’s oscillation intensity is dependent on the noise intensity. In [
18], a stochastic cholera model with a saturated recovery rate was discussed, showing that high levels of noise could result in disease extinction. Major environmental disturbances such as floods, earthquakes, volcanoes, tsunamis, and hurricanes suggest the need to introduce Lévy jumps into infectious disease models to account for such abrupt fluctuations [
20,
21,
22,
23,
24,
25]. Zhang Xuegui et al. [
20] studied a stochastic two predator–one prey system with Lévy jumps and mixed functional responses, observing that Lévy jumps negatively impacted species’ existence. Liu and Chen [
23] proposed a general stochastic non-autonomous logistic model with delays and Lévy jumps, establishing sufficient criteria for extinction, non-persistence in the mean, and the weak persistence of the model. Their findings underscored the significant relationship between time-dependent delay, Lévy noise, and both persistence and extinction.
Building upon the foundation established by previous works, this study seeks to explore the dynamical properties of an innovative stochastic SEIRS model. The objective of this paper is to delve deeper into the factors influencing the spread of the infectious disease. We consider factors such as the effect of public health education, recovery and vaccination rates, contact rate, and time-delay, to analyze their impact on disease persistence and extinction. In particular, we will discuss the effects of discontinuous stochastic interference and its intensity on the average extinction and persistence of diseases.
This paper is structured as follows:
Section 2 details the establishment of the stochastic SEIRS model and provides interpretations of relevant parameters.
Section 3 demonstrates that the proposed system has a unique global positive solution. In
Section 4 and
Section 5, we established the sufficient conditions for extinction and persistence in the mean of the system.
Section 6 discusses numerical simulations and sensitivity analysis. We conclude the paper with a summary of key findings.
2. Modeling and Preparation
In this paper, we propose a stochastic time-delayed SEIRS epidemic model incorporating Lévy jumps and the influence of public health education. The new system can be written as
with the initial conditions
where
,
denotes the family of Lebesgue integrable functions from
into
and
.
Model (1) includes S, E, I, R, representing susceptible, educated, infected, and recovered populations, respectively. The educated population refers to those individuals informed about specific disease prevention strategies. The parameters are defined as follows: signifies the recruitment rate of the total population; represents the effective contact rate, with denoting the maximal effective contact rate between susceptible and infected individuals; is the efficient vaccination rate for susceptible individuals; symbolizes the recovery rate; is the natural death rate; indicates the disease-related death rate within the compartment I; is the proportion of the educated population; reflects the impact of public health education on infection reduction, with ; and , , , and are all positive constants, representing the coefficient of saturated incidence rate and the length of temporary immunity period, respectively. is a standard four-dimensional Brownian motion defined on a complete probability space with a filtration satisfying the usual conditions ( is right continuous and increasing while contains all -null sets), and satisfies . is the corresponding intensity of white noise. Let , N be a Poisson counting measure with a compensator and characteristic measure on a measurable subset of , which satisfies . are the left limits of , respectively. denotes the intensity of Lvy jumps.
In order to understand the meaning of solutions to stochastic differential system (1) and the Itô formula with Lvy jumps, we present the following definition and lemmas.
Definition 1 ([
26]).
Consider the following stochastic differential equation (SDE):with the initial condition . An -valued stochastic process is said to be the solution of SDE (3) if:- (i)
is continuous and -adapted;
- (ii)
, and ;
- (iii)
, for almost all with probability one.
Lemma 1 (The L
vy–Itô decomposition theorem [
27]).
If X is a Lvy process, then there exists , a Brownian motion B and independent Poisson random measure N which is independent of B, such that for each ,where Lemma 2 (Itô’s formula with L
vy jumps [
28]).
Let be a solution of the following stochastic differential equation with Lvy jumps:where and are measurable functions.Given , we define the operator whereThen, the generalized Itô formula with Lvy jumps is given by Notice that the first three equations in model (1) do not depend on the last equation, so it can be omitted without loss of generality. Thus, in the following text, we only discuss the simplified model
with the initial value
where
,
denotes the family of Lebesgue integrable functions from
into
and
. For the convenience of the subsequent proof, we make the following assumption in this paper.
Assumption 1. is a bounded function, and .
6. Numerical Simulations and Discussion
Model (1) typically applies to diseases found in remote or impoverished areas characterized by limited access to education, insufficient information, poor public health conditions, and extreme weather events. Such diseases include influenza, tungiasis, leptospirosis, and novel coronavirus pneumonia. Numerical simulations in this study use tungiasis as an illustrative example. Tungiasis is a parasitic skin disease caused by the invasion of female sand fleas into the human epidermis, commonly found in tropical, economically disadvantaged regions such as Latin America, the Caribbean, and sub-Saharan Africa. Detailed information about tungiasis can be found in reference [
31]. Now, the numerical simulations are performed using the Milstein method [
32] and Euler numerical approximation [
33]. Assume that the total number of the population is 800, and the initial value is
. Parameter values are derived from references [
5,
34,
35,
36,
37] and are presented in
Table 1.
For the assumed parameters, we set
, the intensity of stochastic disturbance as follows:
It is easy to calculate that
, and the condition of Theorem 2 is satisfied. The numerical simulation results are given in
Figure 1. It can be found that the infected population
is extinct in both the deterministic model and the stochastic model. In fact, all values of
which are less than 0.01498 lead to obtaining a value less than one of the basic reproduction numbers
of the system (1), so the disease will always be extinct.
The coefficient
can likewise increase if we increase the contact between the infected population and the susceptible population. Similarly, if we reduce the effective vaccination rate among the infected population, the coefficient
will decrease. For this analysis, we retain the parameter values used in
Table 1, except for
and
, which are chosen to be 0.114 and 0.01, respectively. The values of stochastic noise intensity are as follows:
In this case,
, satisfying the conditions of Theorem 3, and the numerical simulations are shown in
Figure 2. We can find that the trend of persistence in the mean of the infected population
is consistent with the deterministic model after adding stochastic perturbations. Furthermore, we can calculate
, which shows the minimum level of infected population in the average sense. By comparing
Figure 1 and
Figure 2, it becomes evident that the disease contact rate
and vaccination rate
significantly influence the extinction and persistence of the disease.
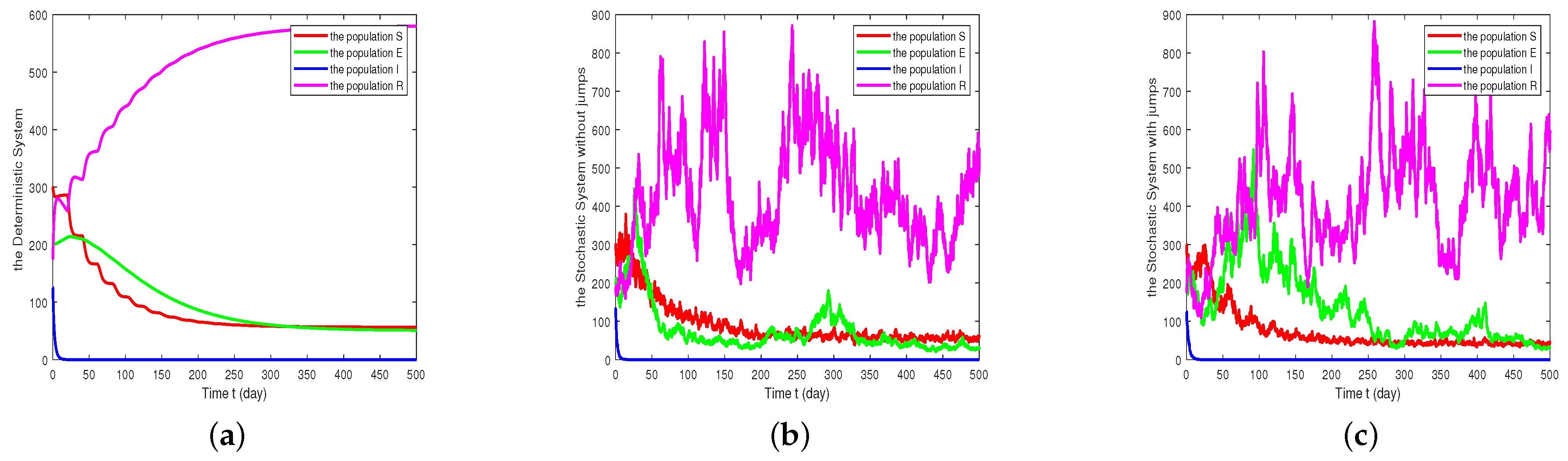
Next, we also explore the effect of stochastic disturbance intensity on disease extinction. Parameters
and
are set at 0.02 and 0.03, respectively, with other parameter values in
Table 1 remain unchanged.
The numerical simulations are presented in
Figure 3. When we select the intensities of disturbance are small, such as
,
, it is easy to calculate that
, the infected population
is not extinct in both the deterministic model and stochastic models. When we select larger disturbance intensities,
,
, it is easy to calculate that
, so the condition of Theorem 2 is satisfied. The infected population
is extinct in the stochastic models with and without jumps (see
Figure 3d,e), which indicates that the large intensities of environmental disturbance have a positive effect on the disease extinction. In addition, by comparing
Figure 3b,d and
Figure 3c,e, we find that the results of stochastic disturbance with jump are consistent with those of stochastic disturbance without jump. Discontinuous environmental shocks described by L
vy jumps can also suppress the spread of the disease in our model.
To further analyze the specific effects of different parameters on disease extinction and persistence, parameter sensitivity analysis is as follows:
For disease extinction and persistence, the effects of parameters
and
on
are considered. At the same time, according to the sensitivity analysis formula in reference [
38], we obtain
The results of numerical simulations are shown in
Figure 4: (a) select
, respectively, so we can see that the smaller
is, the faster population
will go extinct; (b) Select
, respectively, so we can see that
decreases as
decreases; (c) Select
, respectively, we can see that the smaller
is, the faster population
will go extinct; and (d) select
, respectively, so we can see that
decreases as
decreases.
The decrease in parameter and will lead to the decrease in , and it can be concluded that parameters and have a positive correlation with the basic reproduction number. That is to say, the closer is to 0, the better the effect of education on disease infection prevention, as well as the smaller is, and the less contact susceptible people have with infected people, the fewer infected people there are. This indicates that public health education and disease contact rate have a certain role in promoting disease extinction.
For disease extinction and persistence, the effects of parameters
and
on
are also considered. According to the sensitivity analysis formulas, we obtain
The results of numerical simulations are shown in
Figure 5: (a) select
, respectively, so we can see that the smaller
is, the more time the population
will take to go extinct; (b) select
, respectively, we can see that
increases as
decreases; (c) select
, respectively, we can see that the smaller
is, the more time the population
will take to go extinct; (d) Select
, respectively, we can see that
increases as
decreases.
The decrease in parameters and will lead to the increase in , and it can be concluded that parameter and have a negative correlation with the basic reproduction number. In other words, the smaller is, the fewer the number of infected people will recover, as well as the smaller is, the fewer the number of susceptible people will be vaccinated, such that a greater number of infected people indicates that the disease recovery rate and the vaccination rate have a certain promoting effect on disease extinction.
7. Conclusions
In this paper, we propose a stochastic delayed SEIRS epidemic model considering the impacts of public health education and temporary immunity. We prove the global existence and uniqueness of the positive solution of the system (4), providing sufficient conditions for the extinction and persistence of the disease. We perform numerical simulations to corroborate our results and analyze each parameter’s influence on the disease. In conclusion, factors such as public health education, disease contact rate, disease recovery rate, vaccination rate, and high stochastic noises can all contribute to disease extinction. The higher the levels of education, recovery and vaccination rates, and the level of noise, coupled with a lower rate of contact, the more likely it is for the disease to become extinct. Notably, the basic reproduction numbers and are independent of time delay, suggesting that time delay might not significantly affect the disease’s average persistence and extinction.
The study in reference [
5] considered the deterministic susceptible–educated–infective–susceptible (SEIS) model impacted by public health education. The disease extinction threshold
obtained in reference [
5] shows a strong relationship between disease extinction and the contact rate, recovery rate, and mortality rate. This paper expands on that by also considering the intensity of stochastic disturbance, thus demonstrating that the average persistence and extinction of infectious diseases are influenced by a combination of these factors. Furthermore, large-intensity noise disturbances can lead to disease extinction, as illustrated in
Figure 3. Similarly, both continuous interference described by white Gaussian noise and discontinuous environmental shocks represented by L
vy jumps can suppress the spread of the disease in our model.
This work provides a fundamental stochastic delayed dynamical model, which can be instrumental in understanding the impact of public health education and stochastic noises on infectious disease prevention and control. In terms of real-world disease prevention and control efforts, the findings suggest potential measures, such as increasing education awareness, heightening environmental disturbance, minimizing patient contact with others, and encouraging advance vaccination.