1. Introduction
The field of embodied cognition (EC) has provided a powerful theoretical framework amenable to bridge the gap between research probing our mental states and research investigating our physical actions [
1,
2,
3]. Indeed, within the framework of EC, the construct of agency conceived as a cognitive movement phenomenon [
4,
5,
6] may provide a way to finally connect the disparate fields of cognitive science and motor control. An important component of agency is action ownership [
6,
7,
8], i.e., the sense that sensory consequences of the actor’s action are intrinsically part of the actor’s inner sensations. When the actor owns the action, s/he has full control over those sensations that are internally self-generated and self-monitored by the actor’s brain and yet extrinsically modulated by external sensory goals. A critical aspect of this internal–external loop is the identification of the level of actor’s intent and its differential contribution to the action’s intended and unintended sensory consequences.
In recent years, a body of knowledge has increased our understanding on the sensory consequences derived from intentional actions, as such action components deliver an overall sense of agency [
9,
10] through elements of body-ownership closely interrelated with motor control [
11,
12]. Less explored, however, have been parts of the action that are unintended or that transpire spontaneously and largely beneath awareness. Such actions’ components exist at the involuntary (uncontrolled, random motions) and at the autonomic (pacemaker, periodic motions) levels of neuromotor control (
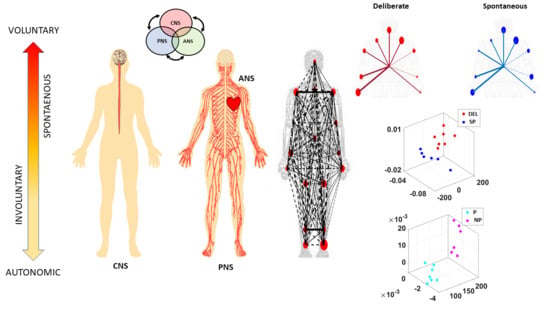
Figure 1). They do not require explicit instructions or precisely defined external goals, yet they too contribute to the differentiation of levels of intent in our actions [
13,
14]. More importantly, at the cognitive level of decision making, these unintended movements contribute to the acquisition of decision accuracy within the context of the type of motor learning that is induced by different cognitive loads [
15,
16].
At the motor control level, autonomous and spontaneous movements are important to develop a sense of action ownership in the face of motor redundancy [
17]. Spontaneous motions can be covert, as those subtle motions occurring in a coma patient [
18] or those occurring in a neonate [
19]; or overt, as when they coexist with deliberate/staged ones, embedded in complex sports routines [
13,
14] and/or ballet choreographies [
20]. Such complex overt movements require the coordination and control of many degrees of freedom (DoFs) across the body. Thus, as we produce fluid and timely goal-oriented actions, kinematic synergies self-emerge and dynamically recruit and release the bodily DoFs, according to task demands [
21,
22,
23]. Conscious decisions generating movements that attain external goals take place as the brain interweaves deliberate and spontaneous movement segments. Such segments in our complex actions gracefully build an ebb and flow of actions intended to a goal, and sensory consequences from those actions [
13]. Some of these sensations that voluntary movements give rise to [
24] return to the brain as feedback from the intentional part of the movements, thought to contribute to our internal models of action dynamics [
25,
26]. This form of volitionally controlled kinesthetic reafference cumulatively helps us build accurate predictions of those intended sensory consequences [
24], while other unintended movements return to the brain as spontaneous reafference in the precise sense that they do not follow from instructed acts. These spontaneous activities provide contextual cues that support motor learning, motor adaptation and action generalization across different situations [
13], including pathologies of the nervous systems [
27,
28].
One informative aspect of this ebb and flow of intent and spontaneity in our actions is the fundamental differences that emerge in the geometric features of the positional trajectories that the moving body describes [
29,
30,
31]. When the motions are intended, geometric measures related to path curvature and path length show invariance to changes in movement dynamics, i.e., these metrics remain robust to changes in speed, mass, etc. [
21,
31,
32,
33,
34]. In contrast, trajectories from unintended motions produce different signatures of motor variability bound to return to the brain as
spontaneous feedback. These internal sensations could help the brain differentiate contextual variations emerging from external environmental cues from sensory information that is internally self-generated by the nervous systems [
13,
14]. External information may include, for example, changes in visual and auditory inputs, such as shifts in lighting conditions, or modulations in sound and music [
20,
35].
The geometry of the uninstructed spontaneous movements’ trajectories dramatically changes with fluctuations in the movements’ dynamics. Changes in speed [
21,
30,
31,
32] or mass [
14] affect their motor variability in fundamentally different ways (if we compare the signatures of variability derived from the spontaneous samples to those derived from deliberately staging the same movement trajectories [
14,
32].) More importantly, the fluctuations in the motor variability of these spontaneous motions can forecast symptoms of Parkinson’s disease before the onset of high severity [
27,
36]. They have also aided in evoking the sense of action ownership and agency in young pre-verbal children [
28]. For these reasons, here we posit that deliberate and spontaneous segments of complex covert actions ought to differentially contribute to our physical sense of action ownership and to our overall sense of agency. To examine this proposition, we follow a phylogenetically orderly taxonomy of the nervous systems’ maturation (
Figure 1B) and examine all levels of neuromotor control—from autonomic to deliberate—necessary to coordinate voluntary motions (
Figure 1A).
More specifically, since autonomic systems are vital to our survival and wellbeing, they may remain impervious to subtle distinctions between deliberate and spontaneous motions that take place across the body, as the end effector completes goal-directed actions. Here we explore the interplay between autonomic signals and voluntary motor control in actions that integrate deliberate and spontaneous motions across the body. We use a new unifying statistical framework for individualized behavioral analyses and network connectivity analyses and offer a quantitative account of how these movement classes contribute to the overall embodied sense of agency.
3. Results
3.1. Higher Motor Intent Results in Higher NSR in Spatial Parameters
Motor intent in the context of our experimental assay specifically refers to the level of deliberateness (or spontaneity) of the movement segment in route to an external target (away from it). An instructed pointing action to touch the target is a goal-directed reach with high level of intent. In contrast, the uninstructed spontaneous retraction away from the target carries lower motor intent than the goal-directed one.
As a first set of analysis, the MMS extracted from the angular acceleration data from each body part were aggregated across all trials and conditions and arranged by different movement segments (forward–deliberate vs. backward–spontaneous) and different sides (performing vs. non-performing). The same was also done on the MMS extracted from the absolute difference in angular acceleration from all pairs of body parts. The NSR was found to be significantly higher when the motions were deliberate and on the performing side (
Figure 6).
Specifically, NSRs of the kinematics time series from each body part shown was highest when an individual exerted higher motor control under higher level of motor intent, such as on the performing side of the body and during a forward–deliberate motion. Conversely, when an individual did not deliberately intend to move the arm, as exhibited on the non-performing side of the body and during a backward–spontaneous motion, the NSR was at its lowest. The NSRs for all pairs of body parts’ absolute difference in angular acceleration (i.e., change in distance between the pairs of body parts), on the other hand, was higher on the performing side (vs. non-performing side) but did not show such a consistent pattern when comparing between the two motion segments (forward vs. backward). Details of the 95% confidence interval of the fitted Gamma scale parameter (i.e., the NSR) for all participants, all body parts (
Figure A3), and all pairs of body parts (
Figure A4) can be found in the
Appendix A.
3.2. Higher Motor Intent Results in Higher Cross-Correlations and Clustering of Temporal Parameters
We used the MATLAB Network Connectivity toolbox [
49] and examined the adjacency matrix derived from the pairwise maximal cross-correlation coefficient based on the time series of linear speed values. The clustering coefficient (CC) was obtained for each body part as a metric of functional segregation. For analysis, we examined the median cross-correlation values as a function of the CC values. Here we found that higher level of motor intent (i.e., during forward–deliberate motion performed with the performing hand) resulted in a tendency of increased CC and increased median cross-correlation values (
Figure 7).
When we compared between different motion segments, median cross-correlations were higher for forward motions than for backward ones for all but two participants. When we compared between different sides, all participants showed higher correlation on the performing side than the non-performing one. The median CC was higher for forward motions than for backward segments for all participants and higher for the performing side than the non-performing side for all but two participants. For all participants, both measures showed statistical significance in their difference (see
Table A1 of
Appendix A for detailed statistical results).
The distinctions that we observed from these findings, on how different levels of motor intent had separable network connectivity patterns based on temporal aspects of the kinematics data, are consistent with the patterns uncovered using spatial aspects of the kinematics data. Specifically, when we exerted higher intent on our body, regardless of the physical trajectory of the motion, there was a stronger connectivity across our body parts. However, we noted that this pattern was not as uniform across all participants as we had found in the spatial aspect of the network analysis.
3.3. Kinematics and EKG (Heart) Signals Show Larger Stochastic Differences for Higher Motor Intent and Control
To assess patterns of connectivity between biophysical signals derived from voluntary and autonomic levels of motor control we examined the kinematics (generated by the CNS–PNS) and the heart activity (generated by the ANS). The patterns of MMS stochasticity and temporal correlation across these systems distinguished levels of motor intent and control.
The analyses involving EKG and kinematics revealed larger stochastic differences in MMS data when higher motor intent and control were exerted. More precisely, the pairwise EMD showed higher differentiation between these two signals in all but one participant when forward motion was made, but only on the performing side of the body. Furthermore, all but two participants showed higher EMD on the performing side of the body, but only during forward motions. On the other hand, however, when backward motion was made, we found an opposite pattern, where all participants showed higher EMD on the non-performing side. We inferred that there may have been a modulating factor that underlied the stochastic relation between kinematics and heart signals.
When we examined the temporal relations between the two signals, by computing pair-wise cross-correlations, we saw higher cross-correlations when there was lower motor intent across all participants, that is, during backward motions and on the non-performing side. Here, we noted the low range of the correlation coefficient values of around 0.1. However, we saw a similar trend when this was based on the non-filtered raw EKG data, with a higher range around 0.6.
3.4. EKG Leads Kinematics under Higher Motor Intent, but Opposite Pattern Emerges in Spontaneous Motions Requiring Less Motor Intent
We also examined the lag values to assess which signal leads the other. We found that with motions under higher motor intent (i.e., during forward–deliberate motions performed with the performing side of the arm), EKG signals tended to lead the kinematics signal. On the other hand, in movements performed under lower intent (i.e., during backward–spontaneous motion, and on the non-performing side of the arm), kinematics signals tended to lead the EKG signals. This is depicted in
Figure 8.
We caveat that because the EKG device and motion capture system were not exactly synchronized, the absolute lag value may not be as meaningful. Nevertheless, as we analyzed these data in terms of the difference (i.e., the delta lag values between forward and backward motions, and between performing and non-performing sides), it was indeed meaningful to find such patterns uniformly across all participants.
Table 1 summarizes the results that we showed in the sections above. We emphasize that although we examined a small number (eight) of participants, each individual’s data were composed of a significant amount of data points with unique non-Gaussian stochastic characteristics. For that reason, instead of presenting the results with NHST (null hypothesis significant tests), we presented the results by comparing the median difference between data points, from different levels of intent, for each individual.
4. Discussion
This paper examined elements of the construct of agency from the embodied cognition framework and dissected several layers of neuromotor control contributing to the sense of action ownership. These layers, defined along a phylogenetically orderly taxonomy of maturation, follow a higher-to-lower gradient of intent, from voluntary, to involuntary, to autonomic signals. At the voluntary level, we followed the instructed–deliberate and the uninstructed–spontaneous segments of the target-directed pointing act, positing that they could differentiate between levels of intent and as such, delineate (from the fluctuations in their biorhythmic activity) when a given movement segment was deliberately performed with intent vs. when the segment happened spontaneously without instruction. This differentiation is important to distinguish the sensory consequences of voluntary acts from those of acts that are not intended or that occur autonomically. The sensory consequences of the latter have not been currently studied, yet they seem important to complement von Holst’s and Mittelstaedt’s principle of reafference as we know it today [
24].
Our initial thought was that autonomic systems contributing to our brain’s autonomy over the body and to our overall embodied sense of agency would remain impervious to stochastic shifts at the voluntary levels. We reasoned that given the vital role of these systems for survival, their robust signal would not reflect subtle changes in levels of intent, motor awareness, and voluntary control. As such, our guess was that if during voluntary movements, there were stochastic differences between instructed–deliberate and uninstructed–spontaneous segments of the reach, or between performing and non-performing sides of the body, such shifts in patterns of variability would not be appreciable in the heart signals’ fluctuations. Our guess was altogether wrong. Not only were the heart signal differences quantifiable at the level of micro fluctuations in signal amplitude, these differences were appreciable as well in the inter-dynamics of the kinematics and cardiac signals.
4.1. The Autonomic Nervous System Differentiates across Levels of Motor Intent: Implications for Computational Models and Basic Cognitive Neuroscience
We found that when movements are intended and deliberately performed to attain the goal defined by an external (visual) target, the heart signal leads the movement kinematics signal. Yet, when these overt movements are spontaneous in nature, i.e., uninstructed and not pursuing the completion of a specific externally defined task goal, the heart signal lags the movement kinematics signal. Across spatial and temporal parameters, we found consistent trends and confirmed the trends through different parameters. Indeed, deliberate motions, executed with the performing effector, carry higher levels of NSR, denoting higher fluctuations away from the empirically estimated mean.
We interpret these findings considering the principle of reafference, treating micro-movements as a form of re-entrant sensory feedback [
24]. Furthermore, we discuss the possible contributions of these self-generated signals to the self-emergence of cognitive agency from motor agency, namely the sense that one can physically realize what one mentally intends to do, confirm the consequences (both intended and unintended), and, as such, mentally own the physical action.
Von Holst and Mittelstaedt studied the complexities of reafference across the nervous systems in the 1950s. They tried to capture the inherent recursiveness that relates movements and their sensations as they flow within closed feedback loops between the external and the internal environments of the organism. They wrote, “Voluntary movements show themselves to be dependent on the returning stream of afference which they themselves cause.” Undeniably, feedback from voluntary movements currently play an important role in theoretical motor control, particularly within the framework of internal models for action [
25,
26] and more recent models of stochastic feedback control [
50]. Central to all these conceptualizations of the motor control problem has been the notion of anticipating the sensory consequences of impending intended actions. Nevertheless, nothing has been said about the consequences of action segments that bear a lower level of intent, which occur spontaneously, or that are altogether occurring autonomously.
The implications of our results are manifold: Modelers and experimenters in motor control do not seem to be aware of self-emergent, uninstructed, spontaneous motions. These motions are rather assumed to be far removed from cognitive processes, perhaps because they transpire largely beneath awareness (although see [
13,
51] more recently). Yet, unintended consequences from the uninstructed–spontaneous segments of the voluntary action seem as important as those sensory consequences that result from the instructed–deliberate segments. They may serve to inform learning new tasks, adapting to new environmental conditions or situations, and more generally, they may play a role as a surprise factor to aid propel curiosity and/or to stimulate creative, exploratory thinking. They may help make our “invisible” automatic movements visible to the conscious brain planning and controlling them, and/or to the external observer tracking our behaviors.
Neither these models, nor Von Holst’s work considered the contributions of unintended consequences from spontaneous acts quantifiable at different anatomical and physiological layers of the nervous systems, while trying to model the basic problem that the organism faces, i.e., the paradox of understanding the “self”, which entails parsing out external from internal reafference. Without a unifying framework to quantify these multilayered interactions and their contributions to the emergence of the notion of self, it becomes rather challenging to bridge the cognitive sense of agency, and more basically of action ownership, “I can do this!; It’s me who’s doing this!”, with the type of autonomous motor control that enables successful self-initiation and completion of the intended act. We argue that inclusion of the unintended consequences from overt spontaneous motions and autonomic signals in our models of motor control will help define embodied agency and provide a new framework to objectively quantify it.
The present work provides empirical evidence that (1) different levels of cognitive intent, awareness, and control are indeed embodied and quantifiable in natural, unconstrained movements, and (2) there are important contributions to central cognitive control quantifiable at the periphery in spontaneous segments of our motions
and their consequences, but also in motions from supporting (non-performing) body parts. Importantly, such differentiating contributions are also present in patterns from signals generated by the autonomic nervous systems. Recent work from our lab has examined cognitive load in relation to autonomic signals and found systematic changes bound to impact the type of feedback that these signals mediated by cardiac muscles generate within the nervous systems [
16,
52]. These aspects of the motor control problem are not considered at present in any of the mathematical and computational frameworks used to model the human brain, despite a body of empirical data differentiating classes of movements that are less sensitive to changes in dynamics [
14,
21,
30,
31,
32,
33,
34] from those which are dynamic dependent [
14].
Our work augments Von Holst’s and Mittelstaedt’s principle of reafference nontrivially by including reafferent contributions from other layers of the nervous systems (
Figure 1A) and highlighting the need to update our conceptualization of internal models for action. In the past, the literature has focused on voluntary control and goal-directed behavior to define and to characterize agency [
5,
6,
8,
11]. However, if new generations of AI models aim to attain artificial autonomous agents with real agency, it may be necessary to reformulate our models and reconceptualize our experiments in embodied cognition to encompass these multiple layers of intent, awareness, and motor function. These results provide a way to distinguish levels of intent in the stochastic feedback from a robust (autonomic signal), as an important addition to prior work distinguishing levels in more variable speed and acceleration signals [
14].
4.2. Distinguishing Performing vs. Non-Performing End Effector
Another aspect of this work explored the differentiation between the performing end effector and the non-performing one, within the context of connectivity network analyses and levels of NSR. There we found that the micro fluctuations in kinematics activity taken as a weighted directed graph representing an interconnected network of nodes (body parts), can automatically reveal which side of the body is performing the goal-directed task with intent vs. which side of the body is performing the uninstructed spontaneous segment. The importance of this result is several-fold: First, it demonstrates that we can gain information by considering arm movements within a broader context of bodily motions, treated as a fully interconnected network, rather than examining the end effector in isolation. The network connectivity analyses presented here adapt and extend similar methods used in brain analyses to full bodily motions. This is important to connect data from the CNS and the PNS within a full network (see here [
52]) and infer the contributions of different bodily sides on the planning, execution, and coordination of the many DoFs of the body in motion. We need these empirical data to improve our multi-layered generative analytical models of neuromotor control involving different spaces of joints and end effectors [
21,
29,
32,
33]. Secondly, these results underscore the importance of not eliminating gross data through grand averaging methods that assume a priori a probability distribution and take theoretical means across all data. Here we personalize the analyses and for each participant, we examine the micro fluctuations (away from empirically estimated distribution moments) contributed by both the performing and the non-performing limbs. We do so within the context of full body macro- and micro-motions, thus considering the value of the NSR derived from the gross data that is often thrown away as superfluous noise. The importance of these new methods is that we can examine possible asymmetries in neurological disorders like Parkinson’s disease, where, e.g., tremor may emerge at the performing hand and yet be forecasted in the NSR of activity recorded in the non-performing limbs. Differentiating performing from non-performing NSR in the context of voluntary motions is now possible using these new statistical methods and network connectivity analyses adapted to full body motions. This type of data is rarely examined in clinical work. Lastly, we offer new ways to examine kinematics synergies and possible patterns of co-articulation across the body, while examining the outcomes from the traditional pointing task now extended to also include the spontaneously retracting segments of the full pointing loop.
4.3. Implications of the Results for Translational Cognitive Science
An area where these results could be relevant is smart health and AI, connecting digital biomarkers with clinical observational criteria (e.g., [
53]). In the clinical world, there are many problems that will require us to be mindful of this intended vs. unintended dichotomy, as there are phenomena that occur spontaneously and largely beneath awareness. It is difficult to model these phenomena within the voluntary reafference framework. The type of reafference that we need to model those problems belongs in the realm of self-emerging aspects of naturalistic behaviors. Among those which are disrupted due to pathologies of the nervous systems are sudden freezing of gait in Parkinson’s disease, leading to the loss of balance and occasional falls; seizures across a broad range of disorders; heart attacks; a subset of repetitive behaviors and self-injurious or aggressive episodes in autism; among others. All these episodes have in common the element of surprise connected to their spontaneity. Several new emerging areas in basic research with a focus on the relationships between property and agency in neurological disorders can also be incorporated in new AI concepts for smart health [
15,
19,
28,
53,
54,
55].
No algorithm relying exclusively on intentional control signals can appropriately capture the essence of these phenomena. To properly characterize it, forecast it, and quickly detect it, we need veridical generative models that understand the differences between the consequences of something that was intended and under voluntary control, something that spontaneously happened, and something that happens autonomically, with high accuracy. We do not have autonomous robots with embodied agency yet, because their staged motions are mostly pre-programmed. These programs may only mimic the predictive consequences of voluntary actions. Self-correcting robotic systems, where such behaviors spontaneously self-emerge, are less common. It is perhaps self-emerging awareness derived from the consequences of spontaneous and autonomic phenomena that makes our embodied agency a special human trait contributing to intelligent control. This type of control, combining deliberate and spontaneous acts, may produce solutions that are capable of generalizing from a small set of specific situations; transfer the learning from one context to another (using contextual variations); and retain robustness to potential interference from new situations in unknown contexts. In future research, it will be important to understand how the type of differentiation that we discovered here, paired with externally vs. internally generated rewards, may contribute to the fast or slow acquisition of memories from transient acts vs. memories from systematic, periodic repetitions of those acts.
Here we offer a unifying framework with a taxonomy of function and differentiable levels of intent, awareness, and control paired with a new statistical platform for personalized analyses of natural behaviors. This new model aims to capture and characterize the micro-fluctuations in the gross data of our biorhythms that traditional approaches throw away as noise through grand averaging and “one size fits all” methods. Our approach allows integration of multilayered hierarchical signals and provides the means to differentiate re-entrant contributions from multilayered exo- and endo-afference. This can help our self-realization of embodied agency as the spontaneous transformation of mental intent into physical volition. We invite the reader to consider this new model for embodied cognition and offer novel avenues to bridge the currently disconnected fields of motor control and cognitive phenomena.