Personalized Dental Medicine: Impact of Intraoral and Extraoral Clinical Variables on the Precision and Efficiency of Intraoral Scanning
Abstract
:1. Introduction
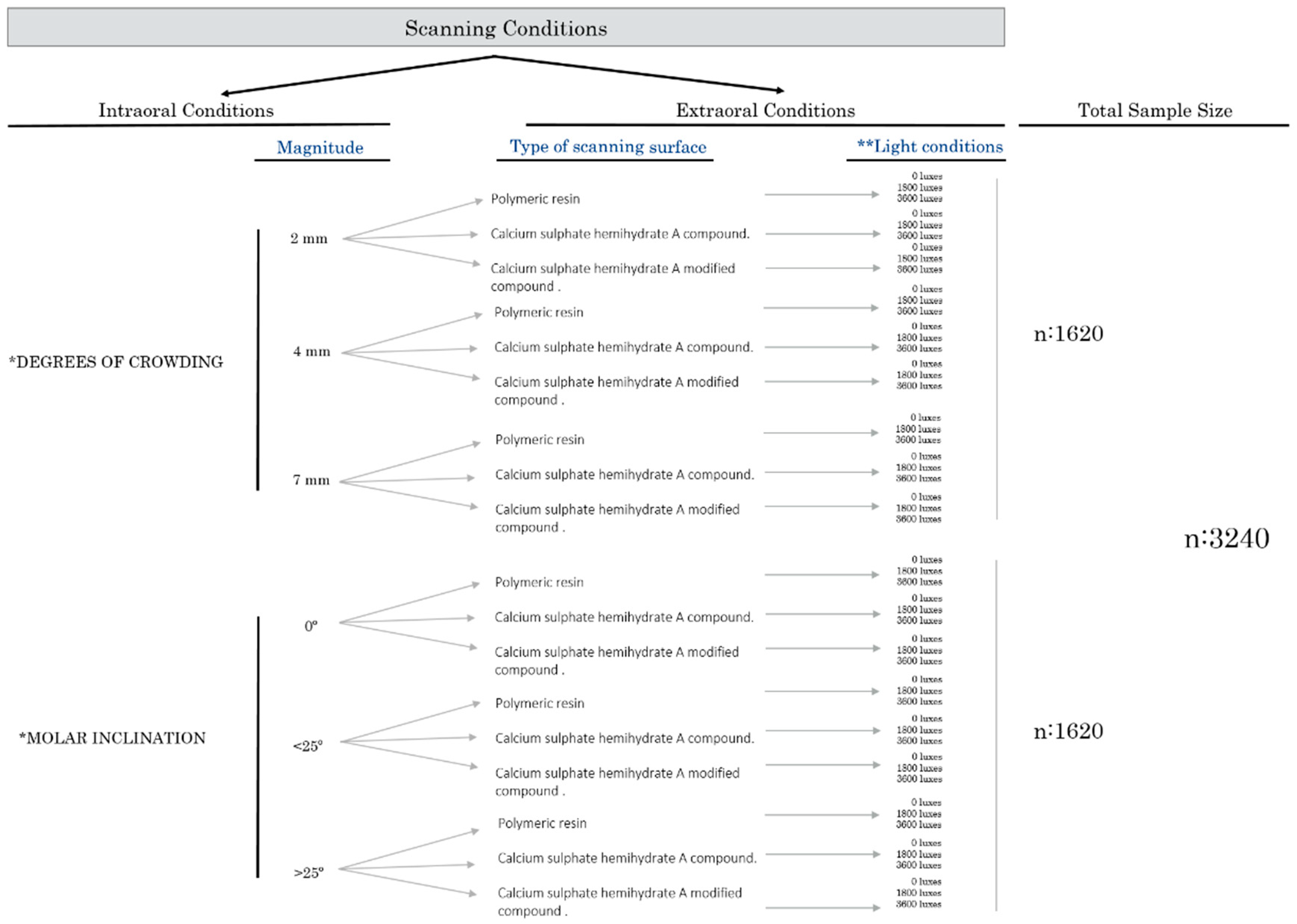
2. Material and Methods
2.1. Study Design
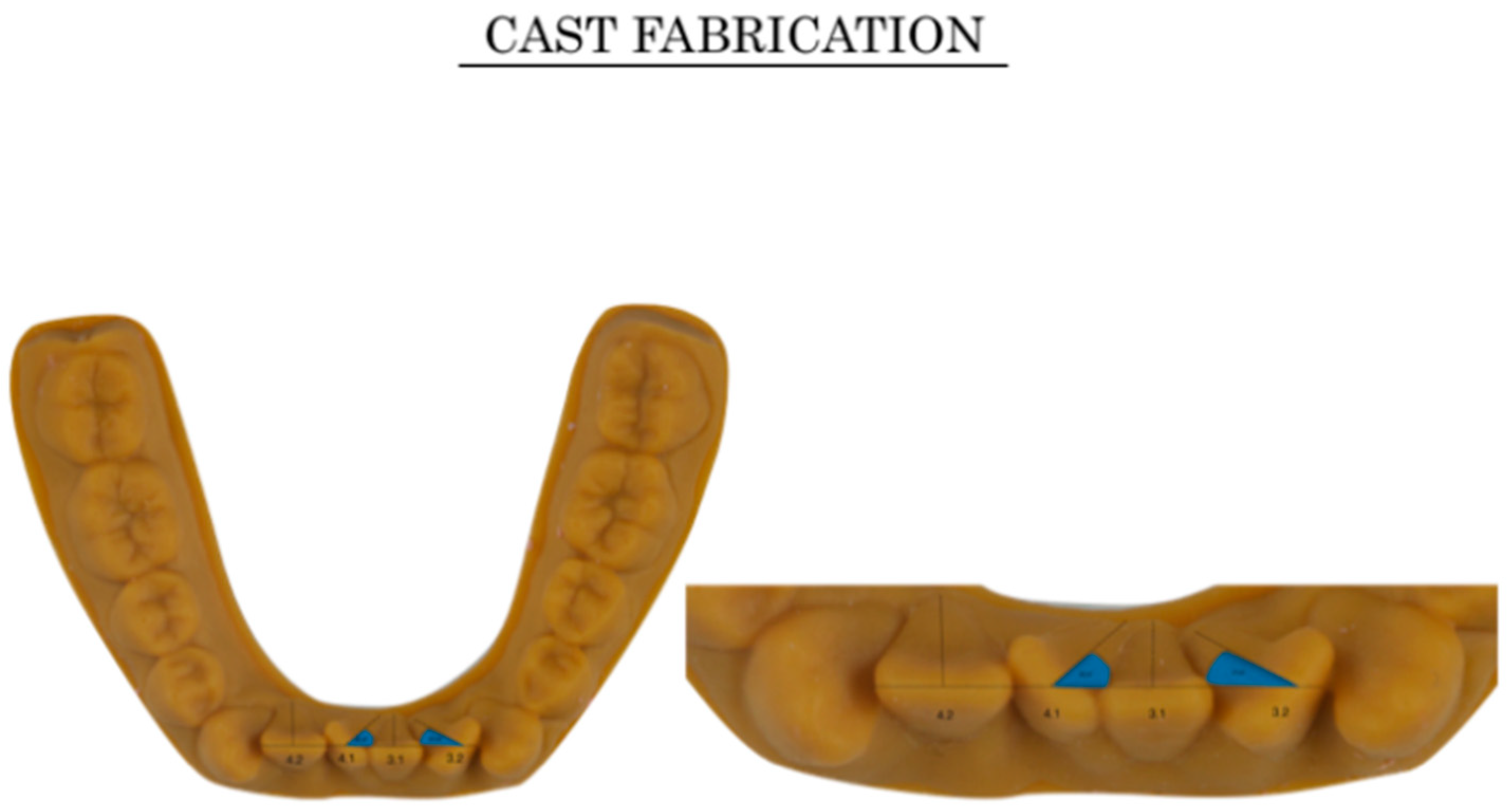
2.2. Ideal Cast, Virtual Set-Up Preparation, and Modified Cast Fabrication
2.2.1. Ideal Cast Obtention (Control)
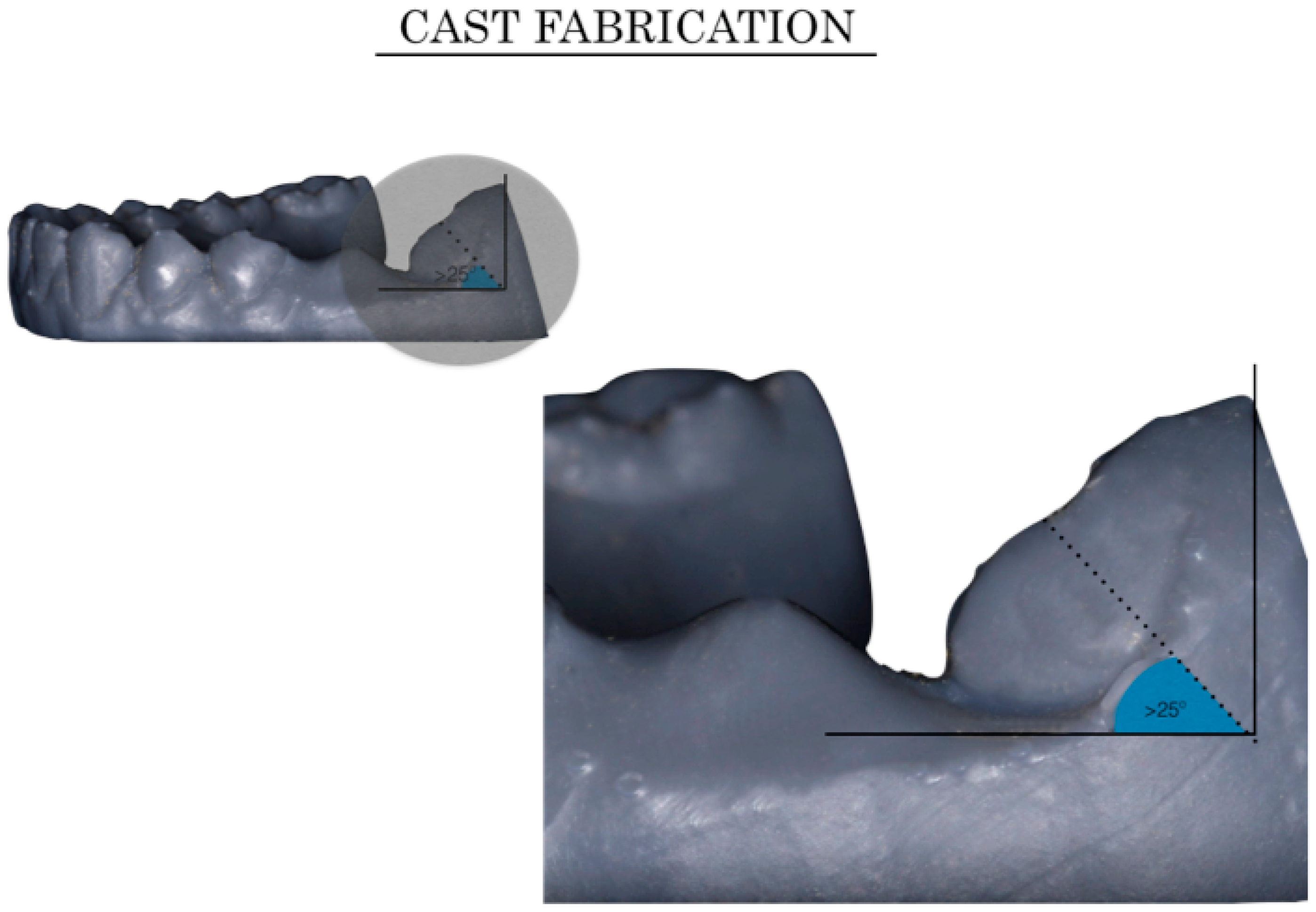
2.2.2. Virtual Set-Up Preparation: Intraoral Modifications (Crowding and Tipping)
2.2.3. Extraoral Conditions (Cast Material and Light Conditions)
Scanning Surface Material
External Light Source Intensity
2.3. Scanner and Scanning Method
2.4. Scanning Efficiency and Efficacy Evaluation
2.4.1. Scanning Efficiency Assessment
2.4.2. Scanning Efficacy Assessment: Virtual Superimpositions
2.5. Statistics
2.5.1. Intra-Interobserver Error and Reliability of the Method
2.5.2. Efficacy and Efficiency Assessments
3. Results
3.1. Intra-Interobserver Error and Reliability of the Method
3.2. Impact of Intraoral Modifications (Crowding and Molar Inclination) and Extraoral Variations (External Light Intensity and Type of Material) on Scanning Efficiency
3.3. Impact of Intraoral Modifications (Crowding and Molar Inclination) and Extraoral Variations (Light Intensity and Type of Material) on Scanning Efficacy
4. Discussion
5. Conclusions
- Clinical conditions such as tooth irregularities, molar inclination, cast material, and light conditions affect the scanning efficiency and can modify the digital acquisition, scanning chair-time, and scanning failures, taking into consideration that it is a technique-dependent procedure;
- There are more scanning failures in cases with severe crowding and greater molar inclination than in other internal conditions, necessitating more images and greater chair-time;
- Evaluation of the efficacy of scanning showed that the degree of inaccuracy influenced by intra and extraoral factors might have a negative impact on daily clinical practice;
- Future in vivo randomized clinical trials should be conducted in order to determine to what extent these and other clinical factors might be influencing the scanning process and its results.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Graf, S.; Vasudavan, S.; Wilmes, B. CAD-CAM design and 3-dimensional printing of mini-implant retained orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 877–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kihara, H.; Hatakeyama, W.; Komine, F.; Takafuji, K.; Takahashi, T.; Yokota, J.; Oriso, K.; Kondo, H. Accuracy and practicality of intraoral scanner in dentistry: A literature review. J. Prosthodont. Res. 2019, 64, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Suese, K. Progress in digital dentistry: The practical use of intraoral scanners. Dent. Mater. J. 2020, 39, 52–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treesh, J.C.; Liacouras, P.C.; Taft, R.M.; Brooks, D.I.; Raiciulesco, S.; Ellert, D.O.; Grant, G.T.; Ye, L. Complete-arch accuracy of intraoral scanners. J. Prosthet. Dent. 2018, 120, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Ender, A.; Zimmermann, M.; Mehl, A. Accuracy of complete-and partial arch impressions of actual intraoral scanning systems in vitro. Int. J. Comput. Dent. 2019, 22, 11–19. [Google Scholar]
- Keul, C.; Guth, J.F. Accuracy of full-arch digital impressions: An in vitro and in vivo comparison. Clin. Oral Investig. 2019, 27, 1. [Google Scholar] [CrossRef]
- Ender, A. Accuracy of complete-arch dental impressions: A new method of measuring trueness and precision. J. Prosthet. 2013, 109, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Anh, J.W.; Park, J.M.; Chun, Y.S.; Kim, M.; Kim, M. A comparison of the precision of three-dimensional images acquired by 2 digital intraoral scanners: Effects of tooth irregularity and scanning direction. Korean J. Orthod. 2016, 46, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Revilla-León, M.; Subramanian, S.G.; Ozcan, M.; Krishnamurthy, V.R. Clinical study of the influence of ambient light scanning conditions on the accuracy (Trueness and precision) of an intraoral scanner. J. Prosthodont. 2020, 29, 107–113. [Google Scholar] [CrossRef]
- Mennito, A.S.; Evans, Z.P.; Lauer, A.W.; Patel, R.B.; Ludlow, M.E.; Renne, W.G. Evaluation of the effect scan pattern has on the trueness and precisión of six intraoral digital impression systems. J. Esthet. Restor. Dent. 2018, 30, 113–118. [Google Scholar] [CrossRef]
- Kang, S.Y.; Park, J.H.; Kim, J.H.; Kim, W.C. Three-dimensional trueness analysis of ceramic crowns fabricated using a chairside computer-aided design/manufacturing system: An in vitro study. J. Prosthodont. Res. 2020, 64, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kim, R.J.; Lee, K.W. Comparative reproducibility analysis of 6 intraoral scanners used on complex intracoronal comparations. J. Prosthet. Dent. 2020, 123, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, Y.K.; Huh, Y.H.; Cho, L.R.; Park, C.J. Comparison of intraoral scanning and conventional impression techniques using 3-dimensional superimposition. J. Adv. Prosthodont. 2015, 7, 460–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutwalli, H.; Braian, M.; Mahmood, D.; Larsson, C. Trueness and precision of three-dimensional digitizing intraoral devices. Int. J. Dent. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latham, J.; Ludlow, M.; Mennito, A.; Kelly, A.; Evans, Z.; Renne, W. Effect of scan pattern on complete-arch scans with 4 digital scanners. J. Prosthet. Dent. 2020, 123, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Yu, H.S.; Choi, Y.; Choi, T.H.; Choi, S.H.; Cha, J.Y. Model analysis of digital models in moderate to severe crowding: In vivo validation and clinical application. BioMed Res. Int. 2018, 2018, 8414605. [Google Scholar] [CrossRef]
- Abduo, J.; Elseyoufi, M. Accuracy of intraoral scanners: A systematic review of influencing factors. Eur. J. Prosthodont. Restor. Dent. 2018, 26, 101–121. [Google Scholar] [CrossRef]
- Kim, R.J.; Park, J.M.; Shim, J.S. Accuracy of 9 intraoral scanners for complete-arch image acquisition: A qualitative and quantitative evaluation. J. Prosthet. Dent. 2018, 120, 895–903. [Google Scholar] [CrossRef]
- Ting-Shu, S.; Jian, S. Intraoral digital impression technique: A review. J. Prosthodont. 2015, 24, 313–321. [Google Scholar] [CrossRef]
- Revilla-León, M.; Jiang, P.; Sadeghpour, M.; Piedra-Cascón, W.; Zandinejad, A.; Ozcan, M.; Krishnamurthy, V.R. Intraoral digital scans-Part 1: Influence of ambient scanning light conditions on the accuracy (trueness and precision) of different intraoral scanners. J. Prosthet. Dent. 2019. [Google Scholar] [CrossRef]
- Arakida, T.; Kanazawa, M.; Iwaki, M.; Suzuki, T.; Minakuchi, S. Evaluating the influence of ambient light on scanning trueness, precision, and time of intraoral scanner. J. Prosthodont. Res. 2018, 62, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Curtis, R.V.; Bartlett, D.W. Surface roughness of impression materials and dental stones scanned by non-contacting laser profilometry. Dent. Mater. 2009, 25, 500–505. [Google Scholar] [CrossRef] [PubMed]

| 3600 luxes (artificial light + natural light) | ||||||||
| Group 1 | Group 2 | Group 3 | p value ¶ | Group 4 | Group 5 | Group 6 | p value ¶ | |
| Digital adquision | 407.61 ± 53.68 | 464.32 ± 56.23 | 469.06 ± 37.25 | 0.001 ** | 441.33 ± 42.41 | 475.90 ± 26.29 | 467.80 ± 38.34 | 0.034 |
| Scanning chair time (s) | 42.04 ± 4.19 | 48.54 ± 6.63 | 46.03 ± 3.86 | 0.001 ** | 50.56 ± 3.01 | 51.23 ± 3.14 | 50.68 ± 1.72 | 1.002 |
| Scanning failures | 0.64 ± 0.60 | 1.25 ± 0.99 | 0.96 ± 1.01 | 1.002 | 1.50 ± 1.10 | 1.45 ± 0.99 | 1.22 ± 0.88 | 0.011 |
| Undetected volume (µm3) | 0.02 ± 0.03 | 0.03 ± 0.07 | 0.08 ± 0.14 | 1.003 | 0.05 ± 0.08 | 0.05 ± 0.08 | 0.05 ± 0.08 | 1.001 |
| 1800 luxes (artificial light) | ||||||||
| Group 1 | Group 2 | Group 3 | p value ¶ | Group 4 | Group 5 | Group 6 | p value ¶ | |
| Digital adquision | 369.09 ± 69.03 | 418.03 ± 47.75 | 458.90 ± 88.73 | 0.022 | 416.90 ± 57.87 | 471.67 ± 108.06 | 449.12 ± 40.41 | 1.001 |
| Scanning chair time (s) | 36.58 ± 5.72 | 45.31 ± 6.47 | 47.89 ± 7.77 | 0.087 | 43.09 ± 7.34 | 46.68 ± 8.48 | 46.31 ± 5.91 | 1.001 |
| Scanning failures | 0.67 ± 0.70 | 1.06 ± 1.12 | 1.19 ± 0.87 | 1.001 | 1.13 ± 1.10 | 1.38 ± 0.88 | 1.48 ± 1.12 | 1.001 |
| Undetected volume (µm3) | 0.01 ± 0.01 | 0.03 ± 0.06 | 0.09 ± 0.19 | 1.002 | 0.09 ± 0.20 | 0.16 ± 0.20 | 0.11 ± 0.35 | 1.002 |
| 0 luxes (no light) | ||||||||
| Group 1 | Group 2 | Group 3 | p value ¶ | Group 4 | Group 5 | Group 6 | p value ¶ | |
| Digital adquision | 434.61 ± 59.85 | 432.96 ± 69.67 | 437.93 ± 85.53 | 1.001 | 428.16 ± 68.41 | 406.61 ± 65.43 | 439.67 ± 45.37 | 1.001 |
| Scanning chair time (s) | 38.70 ± 6.66 | 47.24 ± 7.48 | 48.63 ± 12.02 | 0.001 ** | 41.75 ± 8 | 41.82 ± 7.89 | 51.60 ± 5.8 | 0.001 ** |
| Scanning failures | 1.06 ± 0.99 | 1.70 ± 1.18 | 1.80 ± 1.30 | 0.426 | 1.43 ± 1.35 | 1.70 ± 1.10 | 1.51 ± 0.88 | 1.002 |
| Undetected volume (µm3) | 0.096 ± 0.90 | 0.14 ± 0.14 | 0.10 ± 0.18 | 1.002 | 0.07 ± 0.08 | 0.16 ± 0.20 | 0.11 ± 0.10 | 1.001 |
| 3600 luxes (artificial light + natural light) | ||||||||
| Group 1 | Group 2 | Group 3 | p value ¶ | Group 4 | Group 5 | Group 6 | p value ¶ | |
| Digital adquision | 378.03 ± 36.87 | 427.77 ± 43.89 | 438.12 ± 55.07 | 0.001 ** | 345.51 ± 32.56 | 437.48 ± 36.17 | 387.16 ± 34.70 | 0.001 ** |
| Scanning chair time (s) | 37.87 ± 3.43 | 42.02 ± 3.81 | 42.29 ± 4.95 | 0.001 ** | 34.51 ± 2.95 | 43.70 ± 3.34 | 39.34 ± 2.76 | 0.001 ** |
| Scanning failures | 1.77 ± 0.99 | 1.80 ± 1.01 | 2.29 ± 0.97 | 1 | 1.51 ± 0.96 | 2.09 ± 0.87 | 1.45 ± 0.50 | 0.183 |
| Undetected volume (µm3) | 0.08 ± 0.08 | 0.11 ± 0.10 | 0.16 ± 0.20 | 1 | 0.16 ± 0.25 | 0.17 ± 0.24 | 0.25 ± 0.25 | 0.023 |
| 1800 luxes (artificial light) | ||||||||
| Group 1 | Group 2 | Group 3 | p value ¶ | Group 4 | Group 5 | Group 6 | p value ¶ | |
| Digital adquision | 318.70 ± 25.02 | 358.70 ± 38.25 | 364.32 ± 23.12 | 0.001 ** | 341.19 ± 24.60 | 439.03 ± 33.80 | 412.58 ± 29.62 | 0.002 * |
| Scanning chair time (s) | 32.20 ± 1.68 | 36.00 ± 1.69 | 36.00 ± 1.69 | 0.001 ** | 33.72 ± 1.90 | 44.33 ± 4.17 | 41.63 ± 4.59 | 0.001 ** |
| Scanning failures | 0.61 ± 0.66 | 1.35 ± 0.55 | 1.58 ± 0.67 | 0.006 | 1.06 ± 0.72 | 2.16 ± 0.87 | 1.38 ± 1.11 | 0.003 * |
| Undetected volume (µm3) | 0.08 ± 0.06 | 0.13 ± 0.09 | 0.19 ± 0.20 | 1.002 | 0.033 ± 0.10 | 0.06 ± 0.20 | 0.052 ± 0.099 | 1.001 |
| 0 luxes (no light) | ||||||||
| Group 1 | Group 2 | Group 3 | p value ¶ | Group 4 | Group 5 | Group 6 | p value ¶ | |
| Digital adquision | 348.45 ± 28.33 | 373.77 ± 24.12 | 466.45 ± 53.20 | 0.001 ** | 327.03 ± 23.58 | 393.96 ± 40.74 | 428.74 ± 38.93 | 0.003 * |
| Scanning chair time (s) | 34.52 ± 2.39 | 37.62 ± 2.36 | 45.06 ± 3.33 | 0.001 ** | 32.61 ± 1.63 | 39.92 ± 3.39 | 42.14 ± 2.60 | 0.002 * |
| Scanning failures | 0.93 ± 0.77 | 1.64 ± 0.66 | 1.96 ± 0.83 | 0.001 ** | 0.96 ± 0.70 | 2.58 ± 1.08 | 1.83 ± 0.58 | 0.002 * |
| Undetected volume (µm3) | 0.07 ± 0.07 | 0.09 ± 0.092 | 0.13 ± 0.14 | 1.002 | 0.16 ± 0.21 | 0.19 ± 0.27 | 0.18 ± 0.25 | 1.001 |
| 3600 luxes (artificial light + natural light) | ||||||||
| Group 1 | Group 2 | Group 3 | p value ¶ | Group 4 | Group 5 | Group 6 | p value ¶ | |
| Digital adquision | 417.38 ± 53.36 | 499.45 ± 67.49 | 484.19 ± 70.17 | 0.001 ** | 456.43 ± 60.61 | 487.25 ± 29.51 | 481.06 ± 42.42 | 0.219 |
| Scanning chair time (s) | 42.23 ± 3.56 | 51.91 ± 6.20 | 49.97 ± 5.28 | 0.001 ** | 52.24 ± 3.63 | 52.66 ± 4.18 | 52.76 ± 2.67 | 1.005 |
| Scanning failures | 1 ± 0.68 | 1.90 ± 1.39 | 1.22 ± 1.17 | 0.831 | 1.80 ± 1.15 | 1.58 ± 0.99 | 1.61 ± 1.11 | 0.367 |
| Undetected volume (µm3) | 0.076 ± 0.084 | 0.091 ± 0.10 | 0.19 ± 0.22 | 0.212 | 0.15 ± 0.14 | 0.20 ± 0.24 | 0.21 ± 0.24 | 1.001 |
| 1800 luxes (artificial light) | ||||||||
| Group 1 | Group 2 | Group 3 | p value ¶ | Group 4 | Group 5 | Group 6 | p value ¶ | |
| Digital adquision | 497.89 ± 95.46 | 560.70 ± 105.13 | 561.33 ± 124.53 | 0.002 * | 420.36 ± 65.71 | 428.16 ± 86.98 | 419.45 ± 71.73 | 0.847 |
| Scanning chair time (s) | 56.31 ± 10.48 | 62.24 ± 14.04 | 60.36 ± 12.84 | 0.016 | 46.68 ± 12.31 | 48.59 ± 7.63 | 46.60 ± 10.40 | 1.003 |
| Scanning failures | 2.10 ± 1.37 | 2.23 ± 1.27 | 2.40 ± 1.32 | 1.001 | 2.06 ± 1.87 | 1.83 ± 1.34 | 2.25 ± 1.56 | 0.688 |
| Undetected volume (µm3) | 0.06 ± 0.07 | 0.11 ± 0.09 | 0.26 ± 0.30 | 0.002 * | 0.15 ± 0.12 | 0.23 ± 0.20 | 0.21 ± 0.26 | 1.001 |
| 0 luxes (no light) | ||||||||
| Group 1 | Group 2 | Group 3 | p value ¶ | Group 4 | Group 5 | Group 6 | p value ¶ | |
| Digital adquision | 385.48 ± 63.14 | 431.20 ± 48.98 | 473.77 ± 84.37 | 1.001 | 438.60 ± 59.00 | 478.67 ± 88.32 | 471.32 ± 43.48 | 1.002 |
| Scanning chair time (s) | 38.63 ± 5.59 | 46.75 ± 6.52 | 49.24 ± 7.83 | 0.002 * | 45.57 ± 7.06 | 48.47 ± 8.30 | 48.05 ± 6.77 | 0.005 * |
| Scanning failures | 0.77 ± 0.99 | 1.41 ± 1.28 | 1.67 ± 1.30 | 0.606 | 1.23 ± 1.25 | 1.70 ± 1.07 | 1.61 ± 1.20 | 1.002 |
| Undetected volume (µm3) | 0.09 ± 0.06 | 0.11 ± 0.92 | 0.18 ± 0.22 | 0.612 | 0.070 ± 0.0733 | 0.21 ± 0.25 | 0.22 ± 0.311 | 0.072 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rodríguez, C.; Patricia, J.-P.; Ricardo, O.-A.; Alejandro, I.-L. Personalized Dental Medicine: Impact of Intraoral and Extraoral Clinical Variables on the Precision and Efficiency of Intraoral Scanning. J. Pers. Med. 2020, 10, 92. https://doi.org/10.3390/jpm10030092
Martínez-Rodríguez C, Patricia J-P, Ricardo O-A, Alejandro I-L. Personalized Dental Medicine: Impact of Intraoral and Extraoral Clinical Variables on the Precision and Efficiency of Intraoral Scanning. Journal of Personalized Medicine. 2020; 10(3):92. https://doi.org/10.3390/jpm10030092
Chicago/Turabian StyleMartínez-Rodríguez, César, Junco-Plana Patricia, Ortega-Aranegui Ricardo, and Iglesias-Linares Alejandro. 2020. "Personalized Dental Medicine: Impact of Intraoral and Extraoral Clinical Variables on the Precision and Efficiency of Intraoral Scanning" Journal of Personalized Medicine 10, no. 3: 92. https://doi.org/10.3390/jpm10030092
APA StyleMartínez-Rodríguez, C., Patricia, J.-P., Ricardo, O.-A., & Alejandro, I.-L. (2020). Personalized Dental Medicine: Impact of Intraoral and Extraoral Clinical Variables on the Precision and Efficiency of Intraoral Scanning. Journal of Personalized Medicine, 10(3), 92. https://doi.org/10.3390/jpm10030092





