Changes in the Gut Microbiome after Galacto-Oligosaccharide Administration in Loperamide-Induced Constipation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Experimental Design
2.3. Measurement of Fecal Parameters
2.4. Intestinal Transit Ratio
T: The intestinal tract ratio of charcoal
A: Total length of intestinal tract
B: Moving distance of the most distal end portion of the charcoal
2.5. Analysis of SCFAs
2.6. Histological Observation
2.7. Immunohistochemistry (IHC) Staining of Interstitial Cells of Cajal (ICC)
2.8. Analysis of Pro-Inflammatory Cytokine Levels
2.9. Analysis of 16S rRNA Gene Sequence and Microbial Community of Cecal Microbiota
2.10. Statistical Analysis
3. Results
3.1. Fecal Water Content and Number and Weight of Fecal Pellets
3.2. Effects of GOS on the Intestinal Transit Ratio
3.3. Production of SCFAs
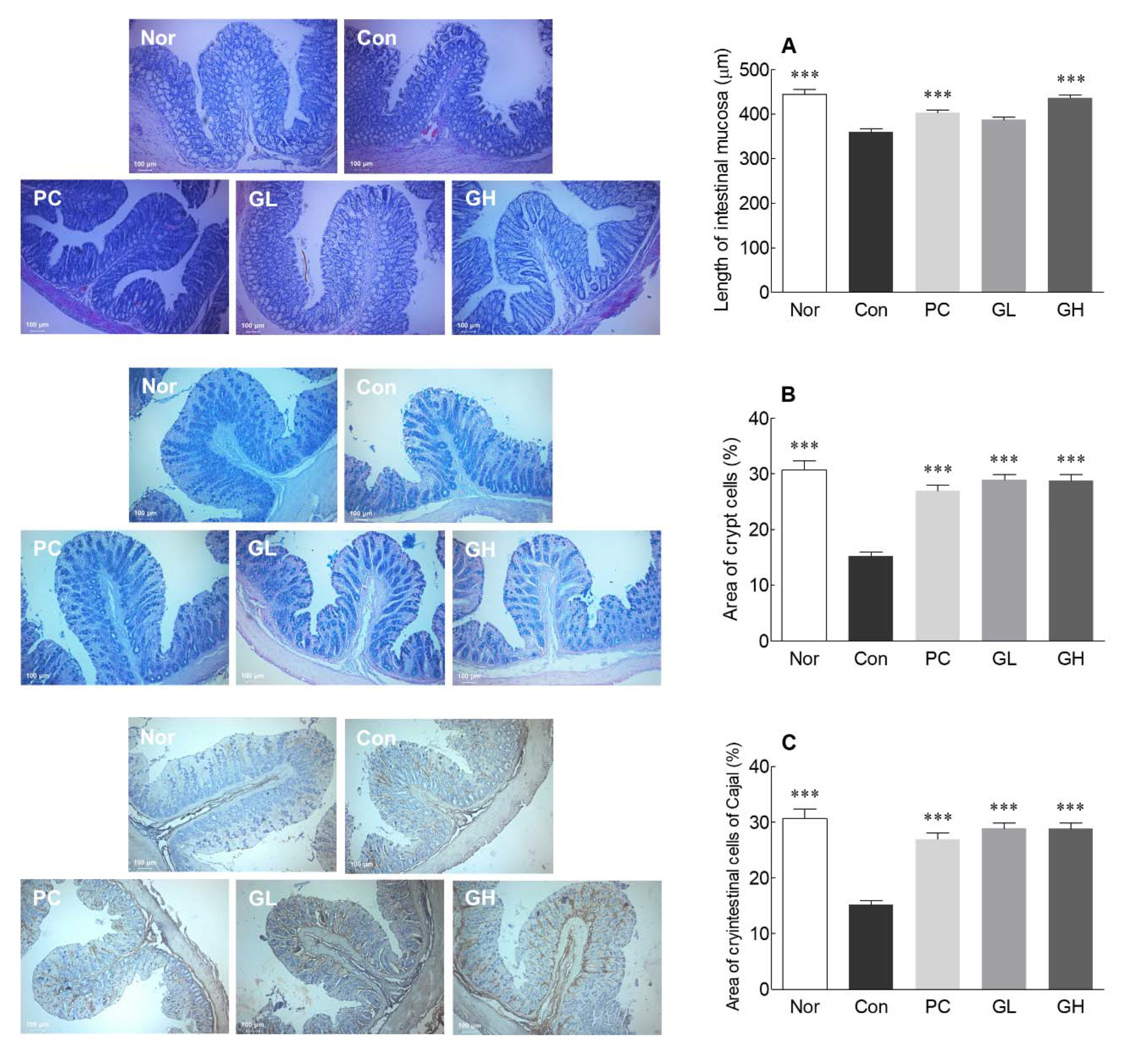
3.4. Histological Observations and IHC Staining of ICC
3.5. Effects of GOS on Levels of Pro-Inflammatory Cytokines
3.6. Effects of GOS on Cecal Microbiota Diversity and Taxonomic Composition of Cecal Microbiota at the Phylum Level
3.7. Effects of GOS on Taxonomic Composition of Cecal Microbiota at the Genus Level
3.8. Correlation between Intestinal Microbiota and Biological Indexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jun, D.W.; Park, H.Y.; Lee, O.Y.; Lee, H.L.; Yoon, B.C.; Choi, H.S.; Hahm, J.S.; Lee, M.H.; Lee, D.H.; Kee, C.S. A Population-Based Study on Bowel Habits in a Korean Community: Prevalence of Functional Constipation and Self-Reported Constipation. Dig. Dis. Sci. 2006, 51, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kwon, C.J.; Lim, S.H.; Kim, K.H.; Heo, N.K.; Jang, H.K.; Park, I.J.; Lee, K.J. Effects of Dietary Fiber Isolated from Synurus deltoides on Constipation in Loperamide-Induced Rats. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1715–1719. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.W.; Cho, H.R.; Kim, K.Y.; Lee, J.K.; Sohn, J.H.; Ku, S.K. Laxative effects of fermented rice extract in rats with loperamide-induced constipation. Exp. Ther. Med. 2014, 8, 1847–1854. [Google Scholar] [CrossRef]
- Han, S.H.; Park, K.; Kim, E.Y.; Ahn, S.H.; Lee, H.S.; Suh, H.J. Cactus (Opuntia humifusa) water extract ameliorates loperamide-induced constipation in rats. BMC Complement. Altern. Med. 2017, 17, 49. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.E.; Jung, H.K.; Lee, T.H.; Jo, Y.; Lee, H.; Song, K.H.; Hong, S.N.; Lim, H.C.; Lee, S.J.; Chung, S.S. Guidelines for the diagnosis and treatment of chronic functional constipation in Korea. J. Neurogastroenterol. Motil. 2016, 22, 383–411. [Google Scholar] [CrossRef]
- Cummings, J.H. Constipation, dietary fibre and the control of large bowel function. Postgrad. Med. J. 1984, 60, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.L.; Haack, V.S.; Janecky, C.W.; Vollendorf, N.W.; A Marlett, J. Mechanisms by which wheat bran and oat bran increase stool weight in humans. Am. J. Clin. Nutr. 1998, 68, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lembo, A.; Camilleri, M. Chronic constipation. N. Eng. J. Med. 2003, 349, 1360–1368. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Yan, S.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Effects of different oligosaccharides at various dosages on the composition of gut microbiota and short-chain fatty acids in mice with constipation. Food Funct. 2017, 8, 1966–1978. [Google Scholar] [CrossRef]
- Seo, Y.S.; Koh, J.H.; Kim, J.H. Properties of Galactooligosaccharide and Physiological Activities. Curr. Top. Lact. Acid Bact. Probiot. 2016, 4, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Tomomatsu, H. Health effects of oligosaccharides. Food Technol. 1994, 48, 61–65. [Google Scholar]
- Chonan, O.; Matsumoto, K.; Watanuki, M. Effect of Galactooligosaccharides on Calcium Absorption and Preventing Bone Loss in Ovariectomized Rats. Biosci. Biotechnol. Biochem. 1995, 59, 236–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, S.O.; Lee, Y.H.; Kim, Y.S.; Ryu, M.H.; Kim, H.K. Preparation of active fraction from radish water extracts for improving the intestinal functions and constipation activities. Appl. Biol. Chem. 2004, 47, 315–320. [Google Scholar]
- Demigné, C.; Rémésy, C. Stimulation of Absorption of Volatile Fatty Acids and Minerals in the Cecum of Rats Adapted to a Very High Fiber Diet. J. Nutr. 1985, 115, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, L.; Xu, Q.; Yin, B.; Fang, D.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis Exerts Strain-Specific Effects on Constipation Induced by Loperamide in BALB/c Mice. Int. J. Mol. Sci. 2017, 18, 318. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.; Lee, J.H.; Jung, Y.; Kim, M.; Kim, S.; Kim, B.K.; Lim, Y.W. EzTaxon: A web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2007, 57, 2259–2261. [Google Scholar] [CrossRef]
- Sin, H.J.; Kim, K.O.; Kim, S.H.; Kim, Y.A.; Lee, H.S. Effect of Resistant Starch on the Large Bowel Environment and Plasma Lipid in Rats with Loperamide-Induced Constipation. J. Korean Soc. Food Sci. Nutr. 2010, 39, 684–691. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2015, 65, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Kojima, R.; Doihara, H.; Nozawa, K.; Kawabata-Shoda, E.; Yokoyama, T.; Ito, H. Characterization of Two Models of Drug-Induced Constipation in Mice and Evaluation of Mustard Oil in These Models. Pharmacology 2009, 84, 227–233. [Google Scholar] [CrossRef]
- Hughes, S.; Higgs, N.B.; A Turnberg, L. Loperamide has antisecretory activity in the human jejunum in vivo. Gut 1984, 25, 931–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, K.; Onoda, Y. Comparison of the Effects of T-1815, Yohimbine and Naloxone on Mouse Colonic Propulsion. J. Smooth Muscle Res. 1993, 29, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takasaki, K.; Kishibayashi, N.; Ishii, A.; Karasawa, A. Effects of KW-5092, a Novel Gastroprokinetic Agent, on the Delayed Colonic Propulsion in Rats. Jpn. J. Pharmacol. 1994, 65, 67–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintola, O.A.; O Sunmonu, T.; Afolayan, A.J. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010, 10, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niittynen, L.; Kajander, K.; Korpela, R. Galacto-oligosaccharides and bowel function. Scand. J. Food Nutr. 2007, 51, 62–66. [Google Scholar] [CrossRef]
- Macfarlane, G.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef]
- Hijová, E.; Chmelarova, A. Short chain fatty acids and colonic health. Bratisl. Lek. List. 2007, 108, 354–358. [Google Scholar]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; De Boeck, G.; Becker, K. Dietary Roles of Non-Starch Polysachharides in Human Nutrition: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef]
- Hidaka, H.; Eida, T.; Takizawa, T.; Tokunaga, T.; Tashiro, Y. Effects of Fructooligosaccharides on Intestinal Flora and Human Health. Bifid. Microflora 1986, 5, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.I.; Park, Y.; Noh, D.O.; Suh, H.J.; Han, S.H. Complex-oligosaccharide composed of galacto-oligosaccharide and lactulose ameliorates loperamide-induced constipation in rats. Food Sci. Biotechnol. 2018, 27, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Besten, G.D.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okazaki, M.; Fujikawa, S.; Matsumoto, N. Effect of Xylooligosaccharide on the Growth of Bifidobacteria. Bifid. Microflora 1990, 9, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Paassen, N.B.; Vincent, A.; Puiman, P.J.; Van Der Sluis, M.; Bouma, J.; Boehm, G.; Van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.P.; Huang, Y.; Zhang, Y.; Wang, X.H.; Shao, C.H. Clinical trial of cellulose in treatment of functional constipation in children. Chin. J. Contemp. Pediatrics 2011, 13, 377–380. [Google Scholar]
- Shimotoyodome, A.; Meguro, S.; Hase, T.; Tokimitsu, I.; Sakata, T. Decreased colonic mucus in rats with loperamide-induced constipation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 126, 203–212. [Google Scholar] [CrossRef]
- Sanders, K.M.; Koh, S.D.; Ward, S.M. Interstitial Cells of Cajal as Pacemakers in the Gastrointestinal Tract. Annu. Rev. Physiol. 2006, 68, 307–343. [Google Scholar] [CrossRef]
- Huizinga, J.D. The Physiology and Pathophysiology of Interstitial Cells of Cajal: Pacemaking, Innervation, and Stretch Sensation. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–335. [Google Scholar]
- Lyford, G.L.; He, C.L.; Soffer, E.; Hull, T.L.; A Strong, S.; Senagore, A.J.; Burgart, L.J.; Young-Fadok, T.; Szurszewski, J.H.; Farrugia, G. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut 2002, 51, 496–501. [Google Scholar] [CrossRef]
- Wang, J.P.; Ding, G.F.; Wang, Q.Z. Interstitial cells of Cajal mediate excitatory sympathetic neurotransmission in guinea pig prostate. Cell Tissue Res. 2013, 352, 479–486. [Google Scholar] [CrossRef]
- Tong, W.D.; Liu, B.H.; Zhang, L.Y.; Zhang, S.B.; Lei, Y. Decreased interstitial cells of Cajal in the sigmoid colon of patients with slow transit constipation. Int. J. Color. Dis. 2004, 19, 467–473. [Google Scholar] [CrossRef]
- Khalif, I.L.; Quigley, E.M.M.; A Konovitch, E.; Maximova, I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef]
- Jeurink, P.V.; Van Esch, B.C.; Rijnierse, A.; Garssen, J.; Knippels, L.M. Mechanisms underlying immune effects of dietary oligosaccharides. Am. J. Clin. Nutr. 2013, 98, 572S–577S. [Google Scholar] [CrossRef] [Green Version]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex Interactions Among Diet, Gastrointestinal Transit, and Gut Microbiota in Humanized Mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Li, M.; Mei, L.; Cong, L.; Liu, Y.; Zhang, B.; He, C.; Zheng, P.; Yuan, J. Manipulation of intestinal dysbiosis by a bacterial mixture ameliorates loperamide-induced constipation in rats. Benef. Microbes 2018, 9, 453–464. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.T.; Wen, P.; Fu, H.Z.; Lin, G.Y.; Liao, S.T.; Zou, Y.X. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019, 10, 1513–1528. [Google Scholar] [CrossRef]
- Liang, Y.X.; Wen, P.; Wang, Y.; Ouyang, D.M.; Wang, D.; Chen, Y.Z.; Song, Y.; Deng, J.; Sun, Y.M.; Wang, H.; et al. The Constipation-Relieving Property of d-Tagatose by Modulating the Composition of Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5721. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Feng, W.; Zhang, S.; Chen, L.; Tang, F.; Sheng, Y.; Ao, H.; Peng, C. Gut microbial modulation in the treatment of chemotherapy-induced diarrhea with Shenzhu Capsule. BMC Complement. Altern. Med. 2019, 19, 126. [Google Scholar] [CrossRef]
- Eor, J.Y.; Tan, P.L.; Lim, S.M.; Choi, D.H.; Yoon, S.M.; Yang, S.Y.; Kim, S.H. Laxative effect of probiotic chocolate on loperamide-induced constipation in rats. Food Res. Int. 2019, 116, 1173–1182. [Google Scholar] [CrossRef]
- Hong, K.B.; Kim, J.H.; Kwon, H.K.; Han, S.H.; Park, Y.; Suh, H.J. Evaluation of Prebiotic Effects of High-Purity Galactooligosaccharides in vitro and in vivo. Food Technol. Biotechnol. 2016, 54, 156–163. [Google Scholar] [CrossRef]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; Ritter, A.J.; Savaiano, D.; Monteagudo-Mera, A.; Anderson, C.; Magness, S.T.; Klaenhammer, T.R. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc. Natl. Acad. Sci. USA 2017, 114, E367–E375. [Google Scholar] [CrossRef] [Green Version]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic Galactooligosaccharides Reduce Adherence of Enteropathogenic Escherichia coli to Tissue Culture Cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.G.; Jo, K.; Chang, Y.B.; Suh, H.J.; Hong, K.-B. Changes in the Gut Microbiome after Galacto-Oligosaccharide Administration in Loperamide-Induced Constipation. J. Pers. Med. 2020, 10, 161. https://doi.org/10.3390/jpm10040161
Kim MG, Jo K, Chang YB, Suh HJ, Hong K-B. Changes in the Gut Microbiome after Galacto-Oligosaccharide Administration in Loperamide-Induced Constipation. Journal of Personalized Medicine. 2020; 10(4):161. https://doi.org/10.3390/jpm10040161
Chicago/Turabian StyleKim, Min Guk, Kyungae Jo, Yeok Boo Chang, Hyung Joo Suh, and Ki-Bae Hong. 2020. "Changes in the Gut Microbiome after Galacto-Oligosaccharide Administration in Loperamide-Induced Constipation" Journal of Personalized Medicine 10, no. 4: 161. https://doi.org/10.3390/jpm10040161
APA StyleKim, M. G., Jo, K., Chang, Y. B., Suh, H. J., & Hong, K.-B. (2020). Changes in the Gut Microbiome after Galacto-Oligosaccharide Administration in Loperamide-Induced Constipation. Journal of Personalized Medicine, 10(4), 161. https://doi.org/10.3390/jpm10040161




