1. Introduction
Cardiovascular disease (CVD) is one of the main causes of death and disability globally and involves one of the largest public health expenditures [
1,
2,
3,
4]. Cardiovascular risk factors (CRFs) are factors associated with CVD and can be biological characteristics, habits, or lifestyles that increase both the risk and probability of death. CRFs are classified into non-modifiable (age, sex, genetic factors, and family history) and modifiable (high blood pressure, smoking, high cholesterol, diabetes mellitus, overweight/obesity, and physical inactivity).
In this regard, dyslipidemia is one of the most prevalent modifiable factors in the adult population and most important for cardiovascular diseases [
5], and it is caused by genetic factors and unhealthy lifestyles (e.g., diet and exercise) [
6]. Physical exercise can reduce cardiovascular risk by increasing HDL-Col and decreasing the other three components (LDL-Col, VLDL-Col, and triglycerides) while facilitating weight and body fat loss [
7]. In recent years, interventions have targeted modifiable risk factors to reduce the number of cardiovascular disease events [
8,
9,
10,
11]. According to the WHO, over 80% of premature deaths related to cardiovascular disease could be avoided if modifiable risk factors were minimized or prevented by healthy lifestyle habits, including a healthy diet, physical exercise, and smoking cessation [
12]. In this way, it is recommended to reduce sedentary behaviors and increase levels of physical activity (PA) to prevent risk factors associated with cardiovascular diseases [
13] and reduce the sedentary lifestyle that has been increasing in recent years [
14]. Moreover, physical inactivity (PI) is the fourth most important risk factor for mortality worldwide and leads to 6% of deaths worldwide, above overweight and obesity (5% of global mortality). PI rates are only exceeded by hypertension (13%), cigarette consumption (9%), and excess blood glucose (hyperglycemia) (6%) [
15].
Strategies applied by public health have been directed towards the implementation and development of programs focused on the elimination of sedentary behaviors, an increase in physical activity, and the inclusion of exercise as a lifestyle for the population in order to achieve improvements in health and reduce cardiovascular diseases [
16]. Exercise is effective in reducing blood pressure [
17], and increased adherence to physical exercise leads to improvement in healthy lifestyles [
18]. Therefore, exercise and physical activity are considered protective factors for CVD (Ozemek C, 2018). In this regard, community exercise is becoming one of the most prominent strategies [
19], improving the interrelationship of all elements that can affect behaviors related to physical activity or exercise such as personal factors (biological and psychological characteristics), social factors (family, affiliation group, and work factors), environmental factors (context for PA performance), and political factors [
20]. Reducing physical inactivity in the population can, therefore, be a key public health intervention and could potentially reduce health costs [
21,
22].
Community-based programs that include simple exercises to increase motivation and enjoyment have been designed to enhance autonomy [
23], and they could improve exercise adherence and lifestyle changes by reducing cardiovascular risk factors [
24]. In addition, training should be individualized and adapted to the characteristics of the participant [
25,
26]. All this could reduce the risk factors for people at risk of CVD and lead to a lifestyle change away from disease [
27]. However, the effects of community-based exercise programs on people with cardiovascular risk factors are unclear. Therefore, the objective of this study was to analyze the effect of two community-based exercise programs with different protocols and duration on adherence, body composition, and metabolic profile in participants with cardiovascular risk.
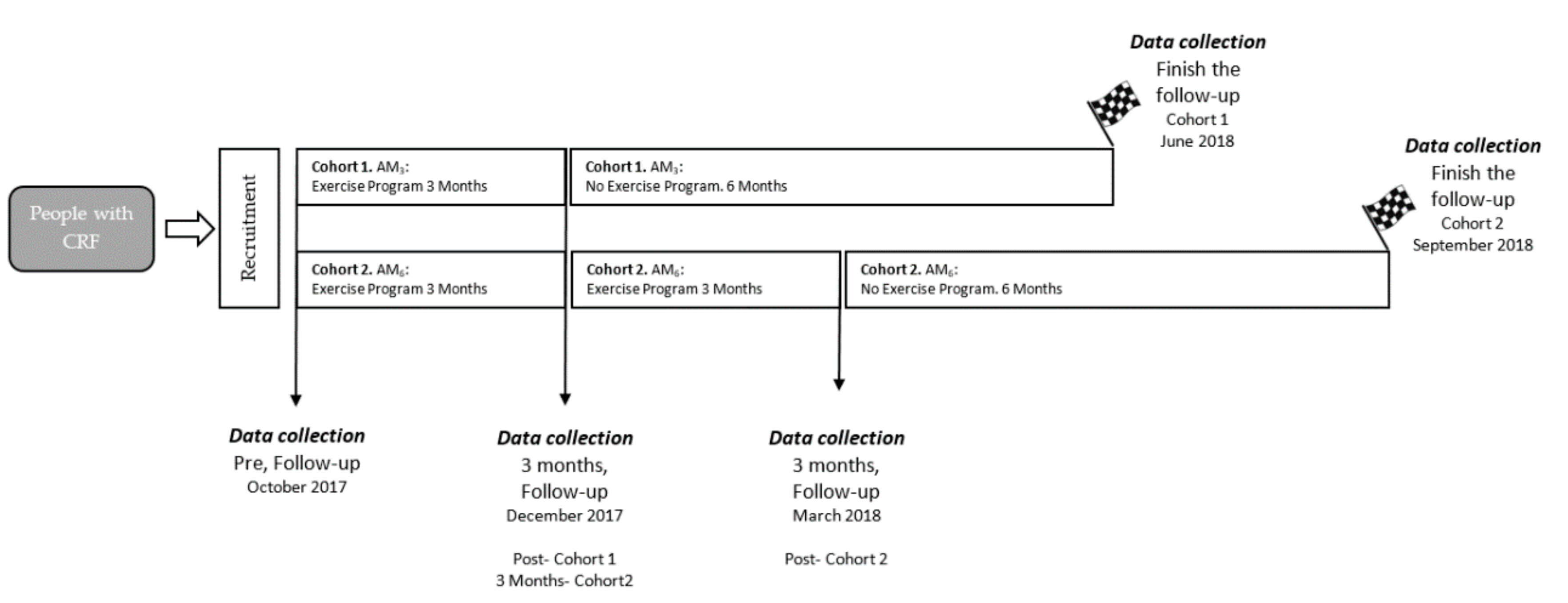
4. Discussion
This study was conducted to determine the effects of two community exercise programs on adherence (level of physical activity), cardiometabolic markers, and body composition in older people with cardiovascular risk factors. The main findings indicated that progressive, individualized, long-term exercise improved the amount of physical activity performed by participants six months after the intervention was completed. In addition, LDL and insulin levels decreased in both groups.
The current study found that the amount of physical activity 6 months after AM
6 (1649 METs) was higher than that of AM
3 (1031 METs). Therefore, the AM
6 group decreased their level of physical inactivity, which could lead to a change in lifestyle. A possible explanation for these results may be due to the long duration of the intervention. According to the transtheoretical model of health behavior change, a minimum of 6 months is recommended to achieve adherence to exercise [
39,
40].
Another possible explanation could be due to the differences in the characteristics of the physical exercise programs. Individualized and progressively loaded training programs could increase feelings of competence and enjoyment as well as increase motivation [
23,
25,
41], and the AM
6 program could have benefited from combining these features. In contrast, the AM
3 program with high-intensity and non-individualized training may have led to more negative affective responses, resulting in a lack of long-term adherence to exercise [
24,
26]. In this way, the improvements in health and feelings of wellness that exercise can provide are also needed to increase exercise adherence. For this reason, the training load must be properly adjusted and individualized for each participant to maximize health gains [
42,
43].
Contrary to expectations, this study did not find a significant difference in body composition after follow up. The lack of significant results could be due to the intensity of the training programs. Sultana et al., 2019 [
44] suggest that low-volume HIIT is inefficient at lowering total body fat mass or percentage of total body fat compared to a control without exercise and with continuous exercise of moderate intensity. Therefore, the results observed suggest that the intensity and load of training were not enough to modify the body composition of the participants. As a result, the recommendations for exercise to combat obesity suggest higher volumes (equivalent to ≥ 1000 MET-min/week) [
45] of exercise for significant weight loss [
46]. However, several studies have observed improvements in body composition with high-intensity and intervallic exercise. Nevertheless, training programs should incorporate a sufficient length of time to lead to a significant decrease in body fat [
47]. Thus, the focus of treatment should be on producing high metabolic stress rather than an energetic imbalance for adults who are overfat [
48].
After the two types of programs were completed, there was a non-significant decrease in blood pressure (AM
6 = −3.1 mmHg, AM
3 = 3.8 mmHg). However, DBP showed a statistically significant decrease of 8 mmHg in the AM
6 group and a non-significant decrease in the AM
3 group. These results are not consistent with previous studies showing that exercise leads to a decrease in systolic and diastolic blood pressure [
49,
50,
51,
52]. Oliver et al. [
17] performed a meta-analysis and observed that moderate-strength training (60–80% 1RM) with a frequency of 2 or 3 weeks and a length of at least 10 weeks is effective in reducing systolic and diastolic blood pressure. In addition, several studies have also found that high-intensity interval training, moderate-intensity continuous training, and aerobic training reduce blood pressure [
49,
50,
51,
52]. However, Cornelissen et al. [
53] observed no change in blood pressure after a 10 week program at a frequency of three sessions per week, 1 h each session, and with an intensity of 33% or 66% of HRR. Therefore, the lack of significant results could be due to the duration of the training programs, with a minimum of 10 weeks at a moderate intensity being necessary to find significant changes [
17]. However, the duration of the program could be shorter when working at higher intensities [
54].
Nevertheless, the training protocols carried out in the Activa Murcia program are designed to change lifestyles and increase the population’s adherence to exercise. Hence, the intensity of AM6 and the duration of AM3 do not seem to be adequate enough to achieve the desired BP changes. Another possible explanation for the lack of significant results could be due to the heterogeneity of the sample and the initial blood pressure values. Several studies have shown decreases in blood pressure in people with hypertension [
49,
50,
52,
55]. In these studies, it is easier to observe improvements in blood pressure because all the people included were hypertensive. People with different CRFs were included in our study; therefore, it is more difficult to observe improvements in blood pressure because all the participants were not hypertensive.
In relation to lipid profile, our results suggest that they significantly decreased LDL and insulin resistance. This improvement was greater in the AM
6 program than in the AM
3 program for LDL. According to the European Society of Cardiology and European Society of Atherosclerosis, LDL is the most important health marker of the lipid profile [
56,
57]. However, no other changes were observed in any other marker of the lipid profile. In this regard, several studies show that a progressive increase in exercise intensity and the practice of regular physical activity are key factors in improving the lipid profile [
7,
58,
59]. Mann et al. confirmed the effects of regular physical activity on cholesterol levels in their review. In addition, individualization of exercise as well as the duration of the physical exercise program could be key factors in improving physical activity levels [
7,
59]. Motalebi et al. [
60] analyzed 24 weeks of walking for 5 days a week and saw significant cholesterol improvements compared the control group in older women. Therefore, the differences observed between programs could be due to differences in the follow-up program (3 months vs. 6 months).
Diet is essential in controlling and reducing cardiovascular risk factors [
61] Furthermore, previous studies have shown that diet combined with exercise could be an effective method of preventing and improving cardiovascular risk factors [
62], enhancements in body composition, as well as the glycemic profile [
7,
63]. Nevertheless, the diet in our study was not altered during the follow-up period, therefore, we assumed that diet did not interfere with the effects of exercise. Thus, future studies should prescribe community-based physical exercise programs and individualize diet to the characteristics of the participants.
However, although the exercise was adapted for each participant, the lack of significant results regarding adherence could be due to the absence of more individualized treatment during the study progression by health staff in the health centers. Future studies should follow the recommendations proposed by Ciccone et al. [
64], who have suggested individualized care between caregivers and patients, recommending lifestyle changes, monitoring the evolution of the conditions and providing them with the information and advice needed to promote patient empowerment, improve self-management skills and achieve better compliance with care recommendations.
The main strengths of the present study were that the participants were prescribed physical exercise from a public health center and that exercise was considered the means to decrease cardiovascular risk factors. On the other hand, two exercise programs of different duration and type of exercise were compared with the aim of optimising the results for future studies. However, there are several limitations to be considered. This study was an observational cohort study and no modifications and/or adaptations of previously planned exercise programs could be made. Nevertheless, family history was considered when participants were prescribed the exercise intervention but not for statistical analysis. However, there was no follow-up communication between medical staff, sport professionals and patients. In addition, there was a low number of participants included in each observation group, and there was high inter- and intra-cohort heterogeneity.