Autism Spectrum Disorder and Childhood Apraxia of Speech: Early Language-Related Hallmarks across Structural MRI Study
Abstract
:1. Introduction
- (1)
- To test the hypothesis that the two clinical groups (ASD and CAS) display neurostructural differences in comparison with Typically Developing children (TD) through a morphometric MRI approach (ASD vs. TD; CAS vs. TD);
- (2)
- To investigate possible disease-specific brain structural patterns in the two clinical groups (ASD vs. CAS);
- (3)
- To evaluate the predictive power of machine-learning analysis in differentiating these three young populations (ASD, CAS, TD).
2. Participants and MRI Data Acquisition
3. MRI Acquisition and Processing
4. FreeSurfer Processing and Feature Extraction
5. Statistical Analysis
6. Results
6.1. Participants
6.2. Statistical Analysis
6.3. Comparison between ASD and TD
6.4. Comparison between CAS and TD
6.5. Comparison between ASD and CAS
6.6. Machine Learning Analysis
7. Discussion
7.1. Are ASD and CAS Brain Different from TD Brain?
7.1.1. ASD Versus TD
7.1.2. CAS Versus TD
7.2. Which Regions Directly Differentiate ASD vs. CAS?
7.3. Is Machine Learning Informative about Diagnosis Prediction?
7.4. Final Considerations
7.5. Strenghts and Weaknesses of the Study
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publication: Washington, DC, USA, 2013. [Google Scholar]
- Narzisi, A.; Posada, M.; Barbieri, F.; Chericoni, N.; Ciuffolini, D.; Pinzino, M.; Romano, R.; Scattoni, M.L.; Tancredi, R.; Calderoni, S.; et al. Prevalence of Autism Spectrum Disorder in a large Italian catchment area: A school-based population study within the ASDEU project. Epidemiol. Psychiatr. Sci. 2018, 29. [Google Scholar] [CrossRef] [PubMed]
- Loth, E.; Murphy, D.G.; Spooren, W. Defining precision medicine approaches to autism spectrum disorders: Concepts and challenges. Front. Psychiatry 2016, 7, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, M.V.; Lai, M.C.; Baron-Cohen, S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol. Psychiatry 2019, 24, 1435–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Speech-Language-Hearing Association. Childhood Apraxia of Speech [Technical Report]. 2007. Available online: www.asha.org/policy/ (accessed on 11 December 2020).
- Chilosi, A.M.; Lorenzini, I.; Fiori, S.; Graziosi, V.; Rossi, G.; Pasquariello, R.; Cipriani, P.; Cioni, G. Behavioral and neurobiological correlates of childhood apraxia of speech in Italian children. Brain Lang. 2015, 150, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Shriberg, L.D.; Aram, D.M.; Kwiatkowski, J. Developmental apraxia of speech: II. Toward a diagnostic marker. J. Speech Lang. Hear. Res. 1997, 40, 286–312. [Google Scholar] [CrossRef] [PubMed]
- Shriberg, L.D.; Strand, E.A.; Jakielski, K.J.; Mabie, H.L. Estimates of the prevalence of speech and motor speech disorders in persons with complex neurodevelopmental disorders. Clin. Linguist. Phon. 2019, 33, 707–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tierney, C.D.; Kurtz, M.; Souders, H. Clear as mud: Another look at autism, childhood apraxia of speech and auditory processing. Curr. Opin. Pediatr. 2012, 24, 394–399. [Google Scholar] [CrossRef]
- Shriberg, L.D.; Paul, R.; Black, L.M.; Van Santen, J.P. The hypothesis of apraxia of speech in children with autism spectrum disorder. J. Autism Dev. Disord. 2011, 41, 405–426. [Google Scholar] [CrossRef] [Green Version]
- Tager-Flusberg, H.; Kasari, C. Minimally verbal school-aged children with autism spectrum disorder: The neglected end of the spectrum. Autism Res. 2013, 6, 468–478. [Google Scholar] [CrossRef] [Green Version]
- Weiss, L.A.; Shen, Y.; Korn, J.M.; Arking, D.E.; Miller, D.T.; Fossdal, R.; Saemundsen, E.; Stefansson, H.; Ferreira, M.A.R.; Green, T.; et al. Association between Microdeletion and Microduplication at 16p11.2 and Autism. N. Engl. J. Med. 2008, 358, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.L. Toward epigenetic and gene regulation models of specific language impairment: Looking for links among growth, genes, and impairments. J. Neurodev. Disord. 2012, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregor, A.; Albrecht, B.; Bader, I.; Bijlsma, E.K.; Ekici, A.B.; Engels, H.; Hackmann, K.; Horn, D.; Hoyer, J.; Klapecki, J.; et al. Expanding the clinical spectrum associated with defects in CNTNAP2 and NRXN1. BMC Med. Genet. 2011, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Gong, J.; Li, L.; Chen, Y.; Liu, L.; Gu, H.T.; Luo, X.; Hou, F.; Zhang, J.; Song, R. Neurexin gene family variants as risk factors for autism spectrum disorder. Autism Res. 2018, 11, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.E.; Scharff, C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009, 25, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.M.; Hill, A.E.; Newman, A.C.; Hamilton, G.; Torrance, H.S.; Anderson, S.M.; Ogawa, F.; Derizioti, P.; Nicod, J.; Vernes, S.C.; et al. The DISC1 promoter: Characterization and regulation by FOXP2. Hum. Mol. Genet. 2012, 21, 2862–2872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Kuo, H.Y.; Bornschein, U.; Takahashi, H.; Chen, S.Y.; Lu, K.M.; Yang, H.Y.; Chen, G.M.; Lin, J.R.; Lee, Y.H.; et al. Foxp2 controls synaptic wiring of corticostriatal circuits and vocal communication by opposing Mef2c. Nat. Neurosci. 2016, 19, 1513–1522. [Google Scholar] [CrossRef]
- Mukame, Z.; Konopka, G.; Wexler, E.; Osborn, G.E.; Dong, H.; Bergman, M.Y.; Levitt, P.; Geschwind, D.H. Regulation of MET by FOXP2, genes implicated in higher cognitive dysfunction and autism risk. J. Neurosci. 2011, 31, 11437–11442. [Google Scholar] [CrossRef]
- Pagnozzi, A.M.; Conti, E.; Calderoni, S.; Fripp, J.; Rose, S.E. A systematic review of structural MRI biomarkers in autism spectrum disorder: A machine learning perspective. Int. J. Dev. Neurosci. 2018, 71, 68–82. [Google Scholar] [CrossRef]
- Fiori, S.; Guzzetta, A.; Mitra, J.; Pannek, K.; Pasquariello, R.; Cipriani, P.; Tosetti, M.; Cioni, G.; Rose, S.E.; Chilosi, A. Neuroanatomical correlates of childhood apraxia of speech: A connectomic approach. NeuroImage Clin. 2016, 12, 894–901. [Google Scholar] [CrossRef]
- Conti, E.; Calderoni, S.; Marchi, V.; Muratori, F.; Cioni, G.; Guzzetta, A. The first 1000 days of the autistic brain: A systematic review of diffusion imaging studies. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Conti, E.; Mitra, J.; Calderoni, S.; Pannek, K.; Shen, K.K.; Pagnozzi, A.; Rose, S.; Mazzotti, S.; Scelfo, D.; Tosetti, M.; et al. Network over-connectivity differentiates autism spectrum disorder from other developmental disorders in toddlers: A diffusion MRI study. Hum. Brain Mapp. 2017, 38, 2333–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadis, D.S.; Goshulak, D.; Namasivayam, A.; Pukonen, M.; Kroll, R.; De Nil, L.F.; Pang, E.W.; Lerch, J.P. Cortical thickness in children receiving intensive therapy for idiopathic apraxia of speech. Brain Topogr. 2014, 27, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, A.; Bonthrone, A.; Liegeois, F.J. Brain basis of childhood speech and language disorders: Are we closer to clinically meaningful MRI markers? Curr. Opin. Pediatr. 2016, 28, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Bloch, A.; Raznahan, A.; Bullmore, E.; Giedd, J. The convergence of maturational change and structural covariance in human cortical networks. J. Neurosci. 2013, 33, 2889–2899. [Google Scholar] [CrossRef]
- Preston, J.L.; Molfese, P.J.; Mencl, W.E.; Frost, S.J.; Hoeft, F.; Fulbright, R.K.; Landi, N.; Grigorenko, E.L.; Seki, A.; Felsenfeld, S.; et al. Structural brain differences in school-age children with residual speech sound errors. Brain Lang. 2014, 128, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.T.; Su, M.; Reilly, S.; Conti-Ramsden, G.; Connelly, A.; Liégeois, F.J. A Brain Marker for Developmental Speech Disorders. J. Pediatr. 2018, 198, 234–239.e1. [Google Scholar] [CrossRef] [Green Version]
- Boedhoe, P.S.W.; van Rooij, D.; Hoogman, M.; Twisk, J.W.R.; Schmaal, L.; Abe, Y.; Alonso, P.; Ameis, S.H.; Anikin, A.; Anticevic, A.; et al. Subcortical brain volume, regional cortical thickness, and cortical surface area across disorders: Findings from the ENIGMA ADHD, ASD, and OCD working groups. Am. J. Psychiatry 2020, 177, 834–843. [Google Scholar] [CrossRef]
- Park, M.T.M.; Raznahan, A.; Shaw, P.; Gogtay, N.; Lerch, J.P.; Mallar Chakravarty, M. Neuroanatomical phenotypes in mental illness: Identifying convergent and divergent cortical phenotypes across autism, ADHD and schizophrenia. J. Psychiatry Neurosci. 2018, 43, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Swanson, M.R.; Shen, M.D.; Wolff, J.J.; Elison, J.T.; Emerson, R.W.; Styner, M.A.; Hazlett, H.C.; Truong, K.; Watson, L.R.; Paterson, S.; et al. Subcortical Brain and Behavior Phenotypes Differentiate Infants With Autism Versus Language Delay. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 664–672. [Google Scholar] [CrossRef]
- Carlisi, C.O.; Norman, L.J.; Lukito, S.S.; Radua, J.; Mataix-Cols, D.; Rubia, K. Comparative Multimodal Meta-analysis of Structural and Functional Brain Abnormalities in Autism Spectrum Disorder and Obsessive-Compulsive Disorder. Biol. Psychiatry 2017, 82, 83–102. [Google Scholar] [CrossRef] [Green Version]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. ADOS-2 Autism Diagnostic Observation Schedule, 2nd ed.; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Murray, E.; Mc Cabe, P.; Heard, R.; Ballard, K.J. Differential diagnosis of children with suspected childhood apraxia of speech. J. Speech Lang. Hear. Res. 2015, 58, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Retico, A.; Arezzini, S.; Bosco, P.; Calderoni, S.; Ciampa, A.; Coscetti, S.; Cuomo, S.; De Santis, L.; Fabiani, D.; Fantacci, M.E.; et al. ARIANNA: A research environment for neuroimaging studies in autism spectrum disorders. Comput. Biol. Med. 2017, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; Van Der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Casanova, M.F.; Buxhoeveden, D.P.; Switala, A.E.; Roy, E. Minicolumnar pathology in autism. Neurology 2002, 58, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Piven, J.; Elison, J.T.; Zylka, M.J. Toward a conceptual framework for early brain and behavior development in Autism. Mol. Psychiatry 2017, 22, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Carper, R.A.; Moses, P.; Tigue, Z.D.; Courchesne, E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage 2002, 16, 1038–1051. [Google Scholar] [CrossRef]
- Gori, I.; Giuliano, A.; Muratori, F.; Saviozzi, I.; Oliva, P.; Tancredi, R.; Cosenza, A.; Tosetti, M.; Calderoni, S.; Retico, A. Gray Matter Alterations in Young Children with Autism Spectrum Disorders: Comparing Morphometry at the Voxel and Regional Level. J. Neuroimaging 2015, 25, 866–874. [Google Scholar] [CrossRef]
- Katuwal, G.J.; Cahill, N.D.; Baum, S.A.; Michael, A.M. The Predictive Power of Structural MRI in Autism Diagnosis. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Milan, Italy, 25–29 August 2015; pp. 4270–4273. [Google Scholar]
- Hesling, I.; Dilharreguy, B.; Peppé, S.; Amirault, M.; Bouvard, M.; Allard, M. The integration of prosodic speech in high functioning Autism: A preliminary fMRI study. PLoS ONE 2010, 5, e11571. [Google Scholar] [CrossRef] [Green Version]
- Eigsti, I.M.; Stevens, M.C.; Schultz, R.T.; Barton, M.; Kelley, E.; Naigles, L.; Orinstein, A.; Troyb, E.; Fein, D.A. Language comprehension and brain function in individuals with an optimal outcome from autism. NeuroImage Clin. 2016, 10, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Groen, W.; Teluij, M.; Buitelaar, J.; Tendolkar, I. Amygdala and Hippocampus Enlargement During Adolescence in Autism. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Schumann, C.M.; Hamstra, J.; Goodlin-Jones, B.L.; Lotspeich, L.J.; Kwon, H.; Buonocore, M.H.; Lammers, C.R.; Reiss, A.L.; Amaral, D.G. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J. Neurosci. 2004, 24, 6392–6401. [Google Scholar] [CrossRef] [PubMed]
- Barnea-Goraly, N.; Frazier, T.W.; Piacenza, L.; Minshew, N.J.; Keshavan, M.S.; Reiss, A.L.; Hardan, A.Y. A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Aylward, E.H.; Minshew, N.J.; Goldstein, G.; Honeycutt, N.A.; Augustine, A.M.; Yates, K.O.; Barta, P.E.; Pearlson, G.D. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology 1999, 53, 2145–2150. [Google Scholar] [CrossRef]
- Sparks, B.F.; Friedman, S.D.; Shaw, D.W.; Aylward, E.H.; Echelard, D.; Artru, A.A.; Maravilla, K.R.; Giedd, J.N.; Munson, J.; Dawson, G.; et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002, 59, 184–192. [Google Scholar] [CrossRef]
- Nicolson, R.; DeVito, T.J.; Vidal, C.N.; Sui, Y.; Hayashi, K.M.; Drost, D.J.; Williamson, P.C.; Rajakumar, N.; Toga, A.W.; Thompson, P.M. Detection and mapping of hippocampal abnormalities in autism. Psychiatry Res. Neuroimaging 2006, 148, 11–21. [Google Scholar] [CrossRef]
- Langen, M.; Durston, S.; Staal, W.G.; Palmen, S.J.M.C.; van Engeland, H. Caudate Nucleus Is Enlarged in High-Functioning Medication-Naive Subjects with Autism. Biol. Psychiatry 2007, 62, 262–266. [Google Scholar] [CrossRef]
- Hollander, E.; Anagnostou, E.; Chaplin, W.; Esposito, K.; Haznedar, M.M.; Licalzi, E.; Wasserman, S.; Soorya, L.; Buchsbaum, M. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol. Psychiatry 2005, 58, 226–232. [Google Scholar] [CrossRef]
- Calderoni, S.; Bellani, M.; Hardan, A.Y.; Muratori, F.; Brambilla, P. Basal ganglia and restricted and repetitive behaviours in Autism Spectrum Disorders: Current status and future perspectives. Epidemiol. Psychiatr. Sci. 2014, 23, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Floresco, S.B. The nucleus accumbens: An interface between cognition, emotion, and action. Annu. Rev. Psychol. 2015, 66, 25–32. [Google Scholar] [CrossRef]
- Supekar, K.; Kochalka, J.; Schaer, M.; Wakeman, H.; Qin, S.; Padmanabhan, A.; Menon, V. Deficits in mesolimbic reward pathway underlie social interaction impairments in children with autism. Brain 2018, 141, 2795–2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohls, G.; Chevallier, C.; Troiani, V.; Schultz, R.T. Social “wanting” dysfunction in autism: Neurobiological underpinnings and treatment implications. J. Neurodev. Disord. 2012, 4, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauda, F.; Geda, E.; Sacco, K.; D’Agata, F.; Duca, S.; Geminiani, G.; Keller, R. Grey matter abnormality in autism spectrum disorder: An activation likelihood estimation meta-analysis study. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.E.V.; Doyle-Thomas, K.A.R.; Tryfon, A.; Ouimet, T.; Anagnostou, E.; Evans, A.C.; Zwaigenbaum, L.; Lerch, J.P.; Lewis, J.D.; Hyde, K.L. Structural Gray Matter Differences during Childhood Development in Autism Spectrum Disorder: A Multimetric Approach. Pediatr. Neurol. 2015, 53, 350–359. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage 2009, 44, 489–501. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Aldinger, K.A.; Ashwood, P.; Bauman, M.L.; Blaha, C.D.; Blatt, G.J.; Chauhan, A.; Chauhan, V.; Dager, S.R.; Dickson, P.E.; et al. Consensus paper: Pathological role of the cerebellum in Autism. Cerebellum 2012, 11, 777–807. [Google Scholar] [CrossRef] [Green Version]
- St George, M.; Kutas, M.; Martinez, A.; Sereno, M.I. Semantic integration in reading: Engagement of the right hemisphere during discourse processing. Brain 1999, 122, 1317–1325. [Google Scholar] [CrossRef]
- Buchanan, C.P. A Neuropsychological Investigation of the “Weak Central Coherence” Anomaly in Autism. Ph.D. Thesis, Rosalind Franklin University, North Chicago, IL, USA, 2002. [Google Scholar]
- Condouris, K.; Meyer, E.; Tager-Flusberg, H. The relationship between standardized measures of language and measures of spontaneous speech in children with autism. Am. J. Speech-Lang. Pathol. 2003, 12, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.T.; Lee, S.S.; Sigman, M.; Dapretto, M. Neural basis of irony comprehension in children with autism: The role of prosody and context. Brain 2006, 129, 932–943. [Google Scholar] [CrossRef] [Green Version]
- Eigsti, I.M.; Schuh, J.; Mencl, E.; Schultz, R.T.; Paul, R. The neural underpinnings of prosody in autism. Child Neuropsychol. 2012, 18, 600–617. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Qiu, T.; Ke, X.; Xiao, X.; Xiao, T.; Liang, F.; Zou, B.; Huang, H.; Fang, H.; Chu, K.; et al. Autism spectrum disorder as early neurodevelopmental disorder: Evidence from the brain imaging abnormalities in 2–3 years old toddlers. J. Autism Dev. Disord. 2014, 44, 1633–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retico, A.; Gori, I.; Giuliano, A.; Muratori, F.; Calderoni, S. One-class support vector machines identify the language and default mode regions as common patterns of structural alterations in young children with autism spectrum disorders. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Jou, R.J.; Minshew, N.J.; Keshavan, M.S.; Vitale, M.P.; Hardan, A.Y. Enlarged right superior temporal gyrus in children and adolescents with autism. Brain Res. 2010, 1360, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardan, A.Y.; Muddasani, S.; Vemulapalli, M.; Keshavan, M.S.; Minshew, N.J. An MRI study of increased cortical thickness in autism. Am. J. Psychiatry 2006, 163, 1290–1292. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.L.; Samson, F.; Evans, A.C.; Mottron, L. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum. Brain Mapp. 2010, 31, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Raznahan, A.; Lenroot, R.; Thurm, A.; Gozzi, M.; Hanley, A.; Spence, S.J.; Swedo, S.E.; Giedd, J.N. Mapping cortical anatomy in preschool aged children with autism using surface-based morphometry. NeuroImage Clin. 2013, 2, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Hazlett, H.C.; Poe, M.D.; Gerig, G.; Styner, M.; Chappell, C.; Smith, R.G.; Vachet, C.; Piven, J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry 2011, 68, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Hadjikhani, N.; Joseph, R.M.; Snyder, J.; Tager-Flusberg, H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb. Cortex 2006, 16, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Zielinski, B.A.; Prigge, M.B.D.; Nielsen, J.A.; Froehlich, A.L.; Abildskov, T.J.; Anderson, J.S.; Fletcher, P.T.; Zygmunt, K.M.; Travers, B.G.; Lange, N.; et al. Longitudinal changes in cortical thickness in autism and typical development. Brain 2014, 137, 1799–1812. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.; Thurm, A.; Greenstein, D.; Farmer, C.; Swedo, S.; Giedd, J.; Raznahan, A. Cortical thickness change in autism during early childhood. Hum. Brain Mapp. 2016, 37, 2616–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillis, A.E.; Work, M.; Barker, P.B.; Jacobs, M.A.; Breese, E.L.; Maurer, K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain 2004, 127, 1479–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dronkers, N.F. A new brain region for coordinating speech articulation. Nature 1996, 384, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.; Riecker, A. The contribution(s) of the insula to speech production: A review of the clinical and functional imaging literature. Brain Struct. Funct. 2010, 214, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Vallar, G.; Papagno, C.; Rusconi, M.L.; Bisiach, E. Vestibular Stimulation, Spatial Hemineglect and Dysphasia. Selective Effects? Cortex 1995, 31, 589–593. [Google Scholar] [CrossRef]
- Gow, D.W.; Caplan, D.N. New levels of language processing complexity and organization revealed by granger causation. Front. Psychol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Budisavljevic, S.; Dell’Acqua, F.; Rijsdijk, F.V.; Kane, F.; Picchioni, M.; McGuire, P.; Toulopoulou, T.; Georgiades, A.; Kalidindi, S.; Kravariti, E.; et al. Age-related differences and heritability of the perisylvian language networks. J. Neurosci. 2015, 35, 12625–12634. [Google Scholar] [CrossRef] [Green Version]
- Rushworth, M.F.S.; Taylor, P.C.J. TMS in the parietal cortex: Updating representations for attention and action. Neuropsychologia 2006, 44, 2700–2716. [Google Scholar] [CrossRef]
- Price, C.J. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010, 1191, 62–88. [Google Scholar] [CrossRef]
- Nakamichi, N.; Takamoto, K.; Nishimaru, H.; Fujiwara, K.; Takamura, Y.; Matsumoto, J.; Noguchi, M.; Nishijo, H. Cerebral hemodynamics in speech-related cortical areas: Articulation learning involves the inferior frontal gyrus, ventral sensory-motor cortex, and parietal-temporal sylvian area. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Price, C.J. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 2012, 62, 816–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rüschemeyer, S.A.; Fiebach, C.J.; Kempe, V.; Friederici, A.D. Processing lexical semantic and syntactic information in first and second language: FMRI evidence from German and Russian. Hum. Brain Mapp. 2005, 25, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D. The Neural Basis of Language Development and Its Impairment. Neuron 2006, 52, 941–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornkessel-Schlesewsky, I.; Schlesewsky, M. Reconciling time, space and function: A new dorsal-ventral stream model of sentence comprehension. Brain Lang. 2013, 125, 60–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friederici, A.D.; Gierhan, S.M.E. The language network. Curr. Opin. Neurobiol. 2013, 23, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Elmer, S. Broca pars triangularis constitutes a “hub” of the language-control network during simultaneous language translation. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, S.; Watkins, K.E.; Bishop, D.V.M. Neurobiological Basis of Language Learning Difficulties. Trends Cogn. Sci. 2016, 20, 701–714. [Google Scholar] [CrossRef] [Green Version]
- Semendeferi, K.; Armstrong, E.; Schleicher, A.; Zilles, K.; Van Hoesen, G.W. Prefrontal cortex in humans and apes: A comparative study of area 10. Am. J. Phys. Anthropol. 2001, 114, 224–241. [Google Scholar] [CrossRef]
- Allegri, R.F. Prefrontal cortex in memory and attention. Rev. Neurol. 2001, 32, 449–453. [Google Scholar] [CrossRef]
- Middleton, F.A.; Strick, P.L. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res. Rev. 2000, 31, 236–250. [Google Scholar] [CrossRef]
- Packard, M.G.; Knowlton, B.J. Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci. 2002, 25, 563–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, A.; Shaw, D.W.W.; Sparks, B.F.; Friedman, S.; Giedd, J.N.; Dawson, G.; Bryan, M.; Dager, S.R. Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Res. 2011, 4, 212–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayate, A.; Bradshaw, J.L.; Rinehart, N.J. Autism and Asperger’s disorder: Are they movement disorders involving the cerebellum and/or basal ganglia? Brain Res. Bull. 2005, 67, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Belton, E.; Salmond, C.H.; Watkins, K.E.; Vargha-Khadem, F.; Gadian, D.G. Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum. Brain Mapp. 2003, 18, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Liégeois, F.J.; Hildebrand, M.S.; Bonthrone, A.; Turner, S.J.; Scheffer, I.E.; Bahlo, M.; Connelly, A.; Morgan, A.T. Early neuroimaging markers of FOXP2 intragenic deletion. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsotti, J.; Mangani, G.; Nencioli, R.; Pfanner, L.; Tancredi, R.; Cosenza, A.; Sesso, G.; Narzisi, A.; Muratori, F.; Cipriani, P.; et al. Grammatical comprehension in italian children with autism spectrum disorder. Brain Sci. 2020, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Retico, A.; Tosetti, M.; Muratori, F.; Calderoni, S. Neuroimaging-based methods for autism identification: A possible translational application? Funct. Neurol. 2014, 29, 231–239. [Google Scholar] [CrossRef]
- Frangou, S.; Modabbernia, A.; Doucet, G.; Papachristou, E.; Williams, S.C.; Agartz, I.; Aghajani, M.; Akudjedu, T.; Albajes-Eizagirre, A.; Alnæs, D.; et al. Cortical Thickness Trajectories across the Lifespan: Data from 17,075 healthy individuals aged 3–90 years. bioRxiv 2020. [Google Scholar] [CrossRef]
- Szucs, D.; Ioannidis, J.P. Sample size evolution in neuroimaging research: An evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. Neuroimage 2020, 221, 117164. [Google Scholar] [CrossRef]

| Age in Months (Mean ± std [Range]) by Subjects’ Category | |||||
|---|---|---|---|---|---|
| ASD (n = 26) | CAS (n = 24) | TD (n = 18) | |||
| 56 ± 11 (34–72) | 57 ± 10 (34–71) | 55 ± 13 (34–74) | |||
| Males | Females | Males | Females | Males | Females |
| (n = 20, 77%) | (n = 6, 23%) | (n = 18, 75%) | (n = 6, 25%) | (n = 13, 72%) | (n = 5, 28%) |
| 57 ± 11 [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] | 54 ± 12 [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] | 56 ± 10 [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] | 57 ± 12 [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] | 58 ± 12 [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74] | 47 ± 13 [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] |
| Comparison among ASD, CAS and TD Groups | Statistical Test § | Cohen’s d in the Between-Group Comparisons | |||||
|---|---|---|---|---|---|---|---|
| (a) | (b) | (c) | (d) | ||||
| F/X2 | p Value | ASD > TD | CAS > TD | CAS < TD | ASD > CAS | ||
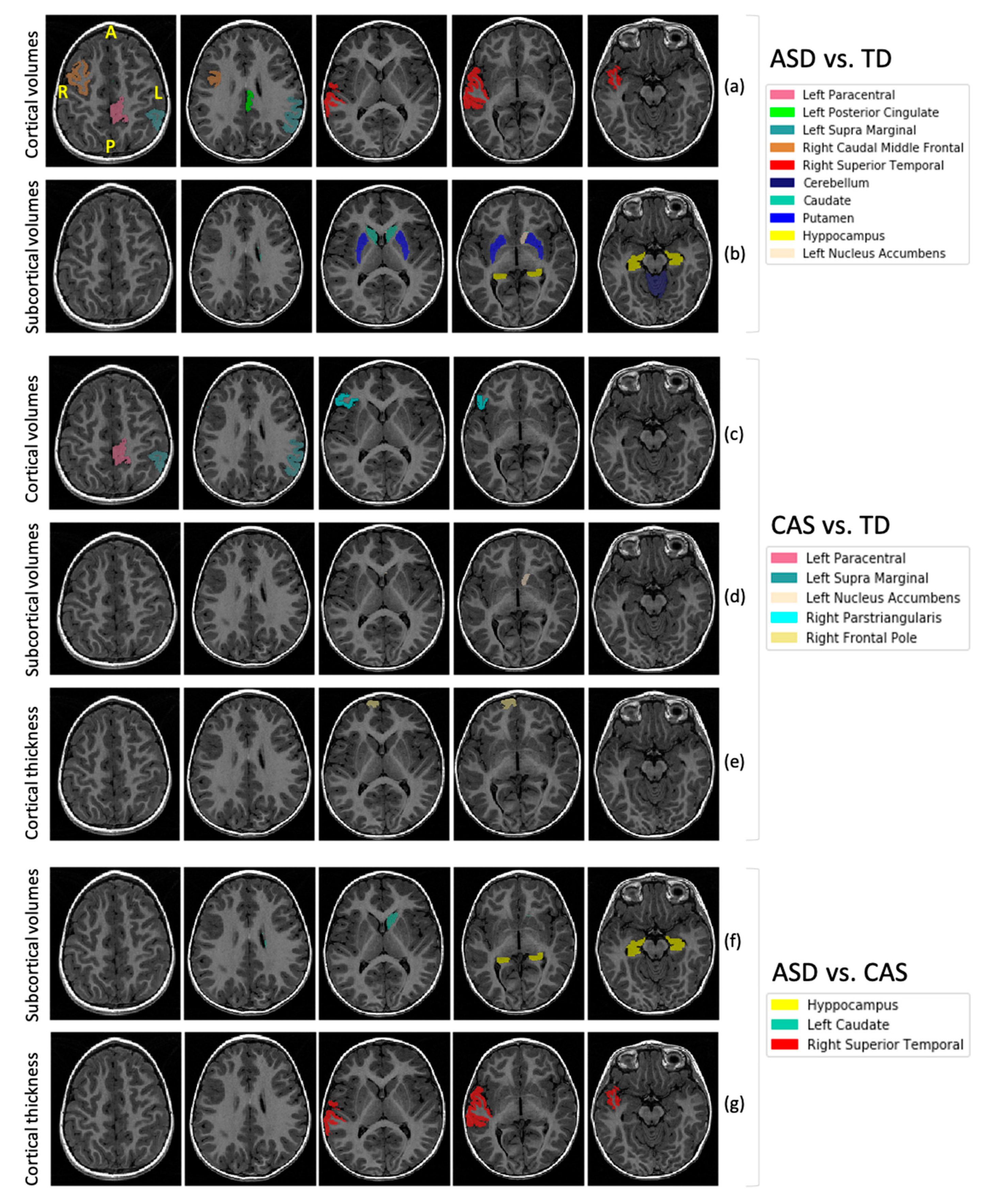
| Cortical volumes | Left Paracentral volume | 4.1 | 0.02 | 0.83 | 0.79 | / | / |
| Left Posterior Cingulate volume | 4.0 | 0.02 | 0.73 | / | / | / | |
| Left Supra Marginal volume § | 7.7 | 0.02 | 0.58 | 0.48 | / | / | |
| Right Caudal Middle Frontal volume § | 5.9 | 0.05 | 0.77 | / | / | / | |
| Right Pars Triangularis volume § | 8.3 | 0.01 | / | 0.53 | / | / | |
| Right Superior Temporal volume | 5.9 | 0.004 | 0.95 | / | / | / | |
| Cortical Thickness | Right Superior Temporal thickness | 4.1 | 0.02 | / | / | / | 0.79 |
| Right Frontal Pole thickness | 4.1 | 0.02 | / | / | 0.97 | / | |
| Subcortical structures, cerebellum and global measures | Left Caudate volume | 5.8 | 0.005 | 1.04 | / | / | 0.68 |
| Left Cerebellum Cortex volume | 4.1 | 0.02 | 0.97 | / | / | / | |
| Left Hippocampus volume § | 12 | 0.002 | 1.15 | / | / | 0.57 | |
| Left Nucleus Accumbens § | 11 | 0.004 | 0.92 | 0.97 | / | / | |
| Left Putamen volume § | 7.7 | 0.02 | 0.89 | / | / | / | |
| Right Caudate volume § | 8.0 | 0.02 | 0.89 | / | / | / | |
| Right Cerebellum Cortex volume | 4.5 | 0.01 | 1.02 | / | / | / | |
| Right Hippocampus volume § | 12 | 0.002 | 1.19 | / | / | 0.56 | |
| Right Putamen volume § | 9.3 | 0.01 | 0.88 | / | / | / | |
| SubCortical Gray matter volume | 5.3 | 0.008 | 0.97 | / | / | / | |
| Total Gray matter volume | 3.1 | 0.05 | 0.71 | / | / | / | |
| Features | AUC (Mean ± SD) | |||
|---|---|---|---|---|
| ASD vs. TD | CAS vs. TD | ASD vs. CAS | ||
| (n = 44) | (n = 42) | (n = 50) | ||
| Subcortical volumes + cerebellum | m = 34 | 0.75 ± 0.16 | 0.48 ± 0.17 | 0.42 ± 0.14 |
| Subcortical volumes and global measures | m = 38 | 0.76 ± 0.14 | 0.54 ± 0.18 | 0.45 ± 0.12 |
| Cortical volumes | m = 68 | 0.53 ± 0.17 | 0.52 ± 0.18 | 0.45 ± 0.17 |
| Cortical thicknesses | m = 70 | 0.52 ± 0.19 | 0.62 ± 0.21 | 0.64 ± 0.17 |
| All cortical features (volumes and thicknesses) | m = 138 | 0.63 ± 0.18 | 0.59 ± 0.17 | 0.50 ± 0.15 |
| All structural features and global measures | m = 176 | 0.73 ± 0.19 | 0.61 ± 0.17 | 0.45 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, E.; Retico, A.; Palumbo, L.; Spera, G.; Bosco, P.; Biagi, L.; Fiori, S.; Tosetti, M.; Cipriani, P.; Cioni, G.; et al. Autism Spectrum Disorder and Childhood Apraxia of Speech: Early Language-Related Hallmarks across Structural MRI Study. J. Pers. Med. 2020, 10, 275. https://doi.org/10.3390/jpm10040275
Conti E, Retico A, Palumbo L, Spera G, Bosco P, Biagi L, Fiori S, Tosetti M, Cipriani P, Cioni G, et al. Autism Spectrum Disorder and Childhood Apraxia of Speech: Early Language-Related Hallmarks across Structural MRI Study. Journal of Personalized Medicine. 2020; 10(4):275. https://doi.org/10.3390/jpm10040275
Chicago/Turabian StyleConti, Eugenia, Alessandra Retico, Letizia Palumbo, Giovanna Spera, Paolo Bosco, Laura Biagi, Simona Fiori, Michela Tosetti, Paola Cipriani, Giovanni Cioni, and et al. 2020. "Autism Spectrum Disorder and Childhood Apraxia of Speech: Early Language-Related Hallmarks across Structural MRI Study" Journal of Personalized Medicine 10, no. 4: 275. https://doi.org/10.3390/jpm10040275
APA StyleConti, E., Retico, A., Palumbo, L., Spera, G., Bosco, P., Biagi, L., Fiori, S., Tosetti, M., Cipriani, P., Cioni, G., Muratori, F., Chilosi, A., & Calderoni, S. (2020). Autism Spectrum Disorder and Childhood Apraxia of Speech: Early Language-Related Hallmarks across Structural MRI Study. Journal of Personalized Medicine, 10(4), 275. https://doi.org/10.3390/jpm10040275





