Clinical Management of Bile Duct Diseases: Role of Endoscopic Ultrasound in a Personalized Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search for Literature
2.2. Available Echoendoscopes and Technique of EUS Examination of the Biliary System
3. Clinical Application and Performance of EUS in Biliary Diseases
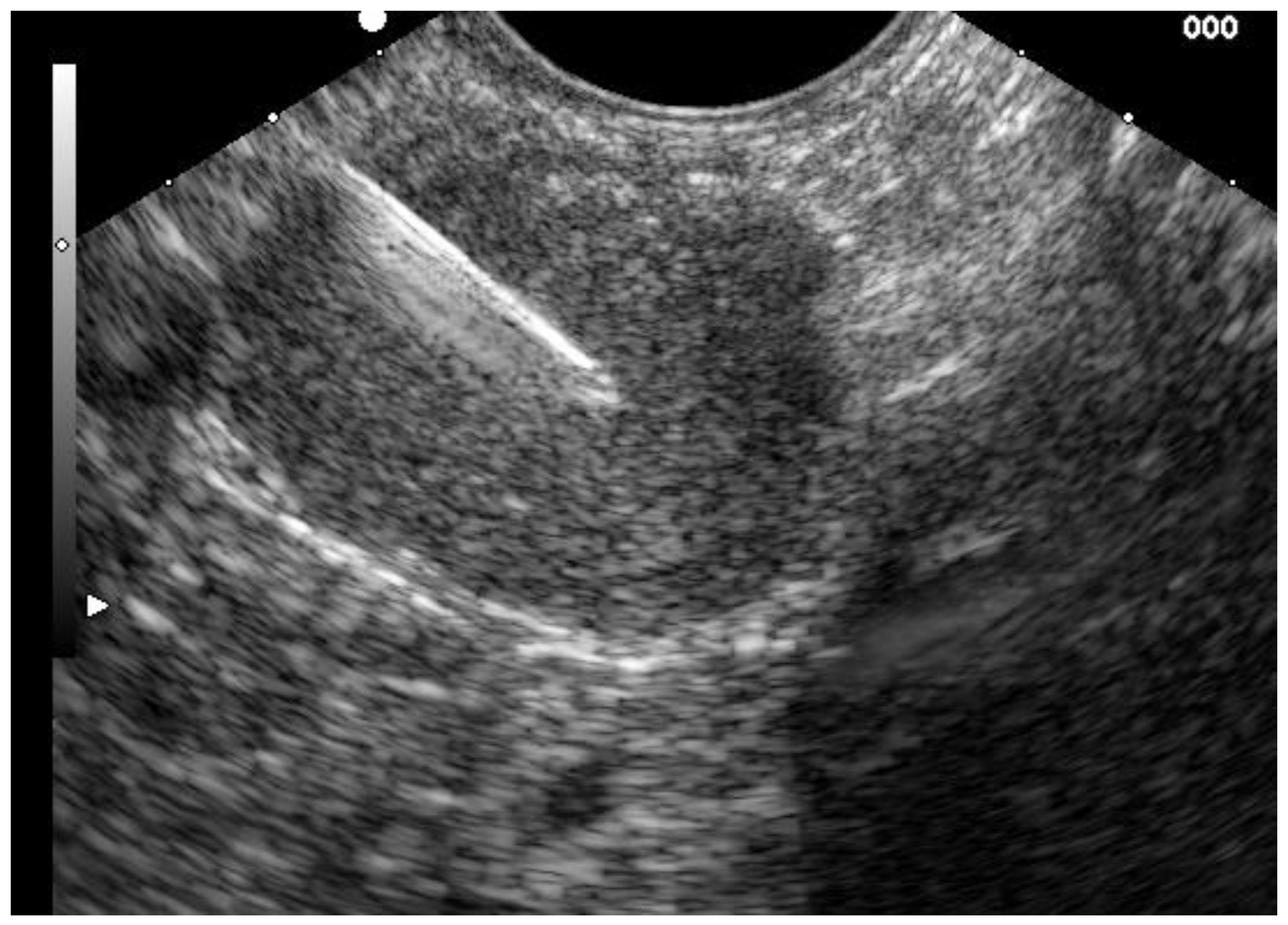
3.1. Biliary Stone Disease
3.2. Evaluation of Indeterminate Biliary Strictures
- Local EUS-staging of CCC includes evaluation of the tumor growth pattern and involvement of local lymph nodes. EUS provides high accuracy in terms of local tumor staging of 66–81% and of local lymph node staging of 64–81% and 88–100% in prediction of portal vein infiltration [22]. Intraductal ultrasound seems to provide a high overall accuracy for the local T-staging of cholangiocarcinoma of 92%, whereas its accuracy for staging of lymph node involvement is low with 43% [4].
- The sensitivity and specificity of EUS-based tissue acquisition for the diagnosis of CCC in patients with indeterminate extrahepatic biliary strictures in two recent meta-analyses of 20 and 6 studies, were 66–80% and 97–100%, respectively [21,27]. Another meta-analysis of 10 studies illustrated that EUS was able to improve the detection rate of malignancies in those patients who were investigated for extrahepatic biliary strictures and received a primary non-malignant diagnosis in ERCP by 14% [28]. However, a proximal position of the stricture close to the hilum and indwell of biliary stents may impede the efficacy of EUS-TA in indeterminate extrahepatic biliary strictures. Where possible, EUS-TA should be accomplished directly before ERCP to improve diagnostic yield and staging accuracy in suspected biliary neoplasms. EUS-directed FNA is generally considered safe with low overall rates of adverse events and severe adverse events of 1% and 0.3% [21]. Some authors reported a risk of Needle-track seeding associated with EUS-FNA in hilar CCC whereas this may be of less relevance in distal biliary tumors, as the needle track within the duodenal wall following transmural EUS-FNA is entirely resected during pancreaticoduodenectomy [21].
3.3. Endoscopic Ultrasound Guided Biliary Drainage
3.3.1. Techniques of EUS-Guided Biliary Drainage (EUS-BD)
3.3.2. Efficacy and Safety of EUS-BD
- (a)
- Different techniques and routes to access the bile ducts, including hepatogastrostomy (EUS-HG), cholecystostomy, choledochoduodenostomy (EUS-CDD), and other techniques;
- (b)
- Use of different modalities of drainage, such as plastic stents, SEMS, LAMS, nasobiliary drainage tubes, and a combination of these;
- (c)
- The long learning curve with the use of EUS-BD with accumulating experience. A retrospective multicenter trial could show a higher success rate for EUS-BD procedures performed by endoscopists having done more than 500 procedures [40].
3.3.3. EUS-Guided Drainage of the Gallbladder
3.3.4. EUS-Guided Biliary Decompression Ready for Prime Time?
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dumonceau, J.M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manes, G.; Paspatis, G.; Aabakken, L.; Anderloni, A.; Arvanitakis, M.; Ah-Soune, P.; Barthet, M.; Domagk, D.; Dumonceau, J.-M.; Gigot, J.-F.; et al. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2019, 51, 472–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumonceau, J.-M.; Deprez, P.H.; Jenssen, C.; Iglesias-Garcia, J.; Larghi, A.; Vanbiervliet, G.; Aithal, G.P.; Arcidiacono, P.G.; Bastos, P.; Carrara, S.; et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated January 2017. Endoscopy 2017, 49, 695–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tringali, A.; Lemmers, A.; Meves, V.; Terheggen, G.; Pohl, J.; Manfredi, G.; Häfner, M.M.; Costamagna, G.; Devière, J.; Neuhaus, H.; et al. Intraductal biliopancreatic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy 2015, 47, 739–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polkowski, M.; Jenssen, C.; Kaye, P.; Carrara, S.; Deprez, P.; Ginès, A.; Fernández-Esparrach, G.G.; Eisendrath, P.; Aithal, G.P.; Arcidiacono, P.P.; et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline—March 2017. Endoscopy 2017, 49, 989–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; García-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testoni, P.A.; Mariani, A.; Aabakken, L.; Arvanitakis, M.; Bories, E.; Costamagna, G.; Devière, J.; Dinis-Ribeiro, M.J.M.; Dumonceau, J.-M.; Giovannini, M.; et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016, 48, 657–683. [Google Scholar] [CrossRef]
- Enochsson, L.; Swahn, F.; Arnelo, U.; Nilsson, M.; Löhr, M.; Persson, G. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest. Endosc. 2010, 72, 1175–1184.e3. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Khan, M.A.; Kamal, F.; Tyberg, A.; Tombazzi, C.R.; Ali, B.; Kahaleh, M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 904–914. [Google Scholar] [CrossRef]
- Giovannini, M.; Moutardier, V.; Pesenti, C.; Bories, E.; Lelong, B.; Delpero, J.R. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001, 33, 898–900. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Dhir, V.; Kida, M.; Yasuda, I.; Jin, Z.D.; Seo, D.W.; Almadi, M.; Ang, T.L.; Hara, K.; Hilmi, I.; et al. Consensus guidelines on the optimal management in interventional EUS procedures: Results from the Asian EUS group RAND/UCLA expert panel. Gut 2018, 67, 1209–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishay, K.; Boyne, D.; Yaghoobi, M.; Khashab, M.A.; Shorr, R.; Ichkhanian, Y.; Forbes, N. Endoscopic ultrasound-guided transmural approach versus ERCP-guided transpapillary approach for primary decompression of malignant biliary obstruction: A meta-analysis. Endoscopy 2019, 51, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, B.S.; Mashiana, H.S.; Dhaliwal, A.; Mohan, B.P.; Jayaraj, M.; Sayles, H.; Singh, S.; Ohning, G.; Bhat, I.; Adler, D.G. EUS-guided biliary drainage: A systematic review and meta-analysis. Endosc. Ultrasound 2020, 9, 101–109. [Google Scholar] [PubMed]
- Baars, J.E.; Kaffes, A.J.; Saxena, P. EUS-guided biliary drainage: A comprehensive review of the literature. Endosc. Ultrasound 2018, 7, 4–9. [Google Scholar]
- Li, D.F.; Zhou, C.H.; Wang, L.S.; Yao, J.; Zou, D.W. Is ERCP-BD or EUS-BD the preferred decompression modality for malignant distal biliary obstruction? A meta-analysis of randomized controlled trials. Rev. Esp. Enferm. Dig. 2019, 111, 953–960. [Google Scholar] [CrossRef]
- Shabanzadeh, D.M.; Sørensen, L.T.; Jørgensen, T. Determinants for gallstone formation—A new data cohort study and a systematic review with meta-analysis. Scand. J. Gastroenterol. 2016, 51, 1239–1248. [Google Scholar] [CrossRef]
- Ko, C.W.; Lee, S.P. Epidemiology and natural history of common bile duct stones and prediction of disease. Gastrointest. Endosc. 2002, 56 (Suppl. 6), S165–S169. [Google Scholar] [CrossRef]
- Meeralam, Y.; Al-Shammari, K.; Yaghoobi, M. Diagnostic accuracy of EUS compared with MRCP in detecting choledocholithiasis: A meta-analysis of diagnostic test accuracy in head-to-head studies. Gastrointest. Endosc. 2017, 86, 986–993. [Google Scholar] [CrossRef]
- Giljaca, V.; Gurusamy, K.S.; Takwoingi, Y.; Higgie, D.; Poropat, G.; Štimac, D.; Davidson, B.R. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for common bile duct stones. Cochrane Database Syst. Rev. 2015, 2015, Cd011549. [Google Scholar] [CrossRef]
- Buxbaum, J.L.; Abbas Fehmi, S.M.; Sultan, S.; Fishman, D.S.; Qumseya, B.J.; Cortessis, V.K.; Schilperoort, H.; Kysh, L.; Matsuoka, L.; Yachimski, P.; et al. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest. Endosc. 2019, 89, 1075–1105.e15. [Google Scholar] [CrossRef]
- Sadeghi, A.; Mohamadnejad, M.; Islami, F.; Keshtkar, A.; Biglari, M.; Malekzadeh, R.; Eloubeidi, M.A. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: A systematic review and meta-analysis. Gastrointest. Endosc. 2016, 83, 290–298.e1. [Google Scholar] [CrossRef] [PubMed]
- Khashab, M.A.; Fockens, P.; Al-Haddad, M.A. Utility of EUS in patients with indeterminate biliary strictures and suspected extrahepatic cholangiocarcinoma (with videos). Gastrointest. Endosc. 2012, 76, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.M.; Cheng, H.Y.; Jiang, W.B.; Chen, D.; Jia, Y.C.; Wu, M.C. Multi-slice three-dimensional spiral CT cholangiography: A new technique for diagnosis of biliary diseases. Hepatobiliary Pancreat. Dis. Int. 2002, 1, 595–603. [Google Scholar]
- Taylor, A.C.; Little, A.F.; Hennessy, O.F.; Banting, S.W.; Smith, P.J.; Desmond, P.V. Prospective assessment of magnetic resonance cholangiopancreatography for noninvasive imaging of the biliary tree. Gastrointest. Endosc. 2002, 55, 17–22. [Google Scholar] [CrossRef]
- Rösch, T.; Meining, A.; Frühmorgen, S.; Zillinger, C.; Schusdziarra, V.; Hellerhoff, K.; Classen, M.; Helmberger, H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest. Endosc. 2002, 55, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Gerges, C.; Beyna, T.; Tang, R.S.Y.; Bahin, F.; Lau, J.Y.W.; Van Geenen, E.; Neuhaus, H.; Reddy, D.N.; Ramchandani, M. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: A prospective, randomized, multicenter trial (with video). Gastrointest. Endosc. 2020, 9, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Njei, B.; Venkatesh, P.G.; Lourdusamy, V.; Sanaka, M.R. Endoscopic ultrasound in the diagnosis of cholangiocarcinoma as the etiology of biliary strictures: A systematic review and meta-analysis. Gastroenterol. Rep. 2015, 3, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.; Theriault, M.; Salim, M.; James, P.D. The incremental benefit of EUS for the identification of malignancy in indeterminate extrahepatic biliary strictures: A systematic review and meta-analysis. Endosc. Ultrasound 2019, 8, 310–317. [Google Scholar]
- Domagk, D.; Oppong, K.W.; Aabakken, L.; Czakó, L.; Gyökeres, T.; Manes, G.; Meier, P.; Poley, J.-W.; Ponchon, T.; Tringali, A.; et al. Performance measures for ERCP and endoscopic ultrasound: A European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2018, 50, 1116–1127. [Google Scholar] [CrossRef] [Green Version]
- Donnan, E.; Bentrem, D.J.; Komanduri, S.; Mahvi, D.M.; Keswani, R.N. ERCP in potentially resectable malignant biliary obstruction is frequently unsuccessful when performed outside of a comprehensive pancreaticobiliary center. J. Surg. Oncol. 2016, 113, 647–651. [Google Scholar] [CrossRef]
- Nennstiel, S.; Weber, A.; Frick, G.; Haller, B.; Meining, A.; Schmid, R.M.; Neu, B. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis Over 10 Years. J. Clin. Gastroenterol. 2015, 49, 764–770. [Google Scholar] [CrossRef]
- Fabbri, C.; Luigiano, C.; Lisotti, A.; Cennamo, V.; Virgilio, C.; Caletti, G.; Fusaroli, P. Endoscopic ultrasound-guided treatments: Are we getting evidence based--a systematic review. World J. Gastroenterol. 2014, 20, 8424–8448. [Google Scholar] [CrossRef]
- Hindryckx, P.; DeGroote, H.; Tate, D.J.; Deprez, P.H. Endoscopic ultrasound-guided drainage of the biliary system: Techniques, indications and future perspectives. World J. Gastrointest. Endosc. 2019, 11, 103–114. [Google Scholar] [CrossRef]
- Mallery, S.; Matlock, J.; Freeman, M.L. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest. Endosc. 2004, 59, 100–107. [Google Scholar] [CrossRef]
- Wiersema, M.J.; Sandusky, D.; Carr, R.; Wiersema, L.M.; Erdel, W.C.; Frederick, P.K. Endosonography-guided cholangiopancreatography. Gastrointest. Endosc. 1996, 43 Pt 1, 102–106. [Google Scholar] [CrossRef]
- Khashab, M.A.; Levy, M.J.; Itoi, T.; Artifon, E.L. EUS-guided biliary drainage. Gastrointest. Endosc. 2015, 82, 993–1001. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, J.; Xing, L.; Wang, Y.; Jin, Z.; Li, Z. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest. Endosc. 2016, 83, 1218–1227. [Google Scholar] [CrossRef]
- Mohan, B.P.; Shakhatreh, M.; Garg, R.; Ponnada, S.; Navaneethan, U.; Adler, D.G. Efficacy and Safety of Endoscopic Ultrasound-guided Choledochoduodenostomy: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2019, 53, 243–250. [Google Scholar] [CrossRef]
- Anderloni, A.; Fugazza, A.; Troncone, E.; Auriemma, F.; Carrara, S.; Semeraro, R.; Maselli, R.; Di Leo, M.; D’Amico, F.; Sethi, A.; et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest. Endosc. 2019, 89, 69–76. [Google Scholar] [CrossRef]
- Vila, J.J.; Pérez-Miranda, M.; Vazquez-Sequeiros, E.; Abadia, M.A.; Pérez-Millán, A.; González-Huix, F.; Gornals, J.; Iglesias-Garcia, J.; De La Serna, C.; Aparicio, J.R.; et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest. Endosc. 2012, 76, 1133–1141. [Google Scholar] [CrossRef]
- Anderloni, A.; Buda, A.; Vieceli, F.; Khashab, M.A.; Hassan, C.; Repici, A. Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: A systematic review and pooled analysis. Surg. Endosc. 2016, 30, 5200–5208. [Google Scholar] [CrossRef] [PubMed]
- Podboy, A.; Yuan, J.; Stave, C.D.; Chan, S.M.; Hwang, J.; Teoh, A.Y.B. Comparison of EUS-guided endoscopic transpapillary and percutaneous gallbladder drainage for acute cholecystitis: A systematic review with network meta-analysis. Gastrointest. Endosc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Donatelli, G.; Cereatti, F.; Spota, A.; Danan, D.; Tuszynski, T.; Dumont, J.L.; Derhy, S. Long-term placement of lumen-apposing metal stent after endoscopic ultrasound-guided duodeno- and jejunojejunal anastomosis for direct access to excluded jejunal limb. Endoscopy 2020. [Google Scholar] [CrossRef]
- Ichkhanian, Y.; Yang, J.; James, T.W.; Baron, T.H.; Irani, S.; Nasr, J.; Sharaiha, R.Z.; Law, R.; Wannhoff, A.; Khashab, M.A. EUS-directed transenteric ERCP in non-Roux-en-Y gastric bypass surgical anatomy patients (with video). Gastrointest. Endosc. 2020, 91, 1188–1194.e2. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyna, T.; Gerges, C. Clinical Management of Bile Duct Diseases: Role of Endoscopic Ultrasound in a Personalized Approach. J. Pers. Med. 2021, 11, 1. https://doi.org/10.3390/jpm11010001
Beyna T, Gerges C. Clinical Management of Bile Duct Diseases: Role of Endoscopic Ultrasound in a Personalized Approach. Journal of Personalized Medicine. 2021; 11(1):1. https://doi.org/10.3390/jpm11010001
Chicago/Turabian StyleBeyna, Torsten, and Christian Gerges. 2021. "Clinical Management of Bile Duct Diseases: Role of Endoscopic Ultrasound in a Personalized Approach" Journal of Personalized Medicine 11, no. 1: 1. https://doi.org/10.3390/jpm11010001



