A Prospective Study of CPAP Therapy in Relation to Cardiovascular Outcome in a Cohort of Romanian Obstructive Sleep Apnea Patients
Abstract
:1. Introduction
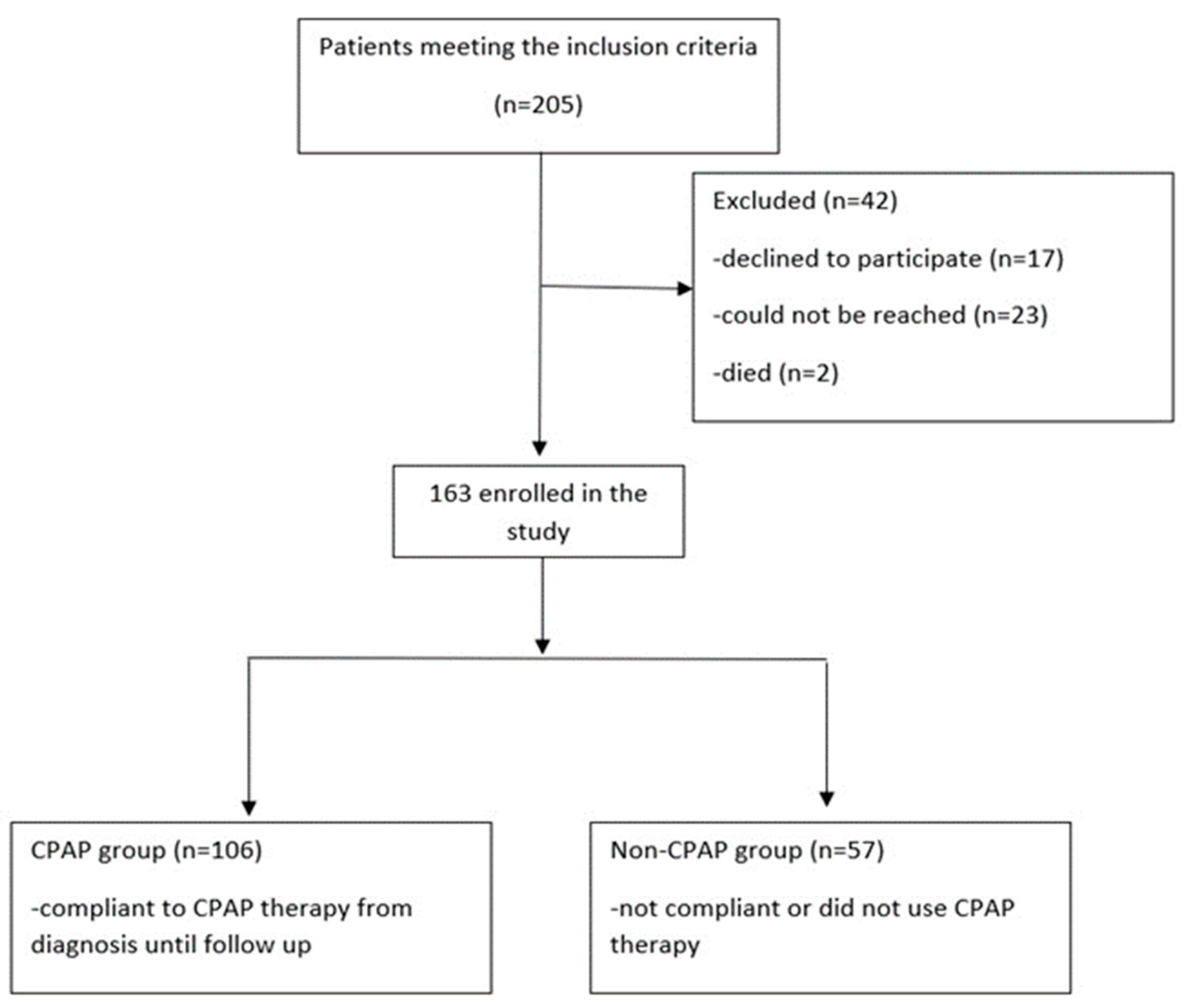
2. Materials and Methods
2.1. Study Population
2.2. Sleep Study
2.3. Echocardiography
2.4. Blood Pressure Assessment
2.5. Anthropometric and Biochemical Measurements
2.6. Outcome and Follow-Up
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Effect on Serum Lipids
4.2. CPAP and Markers of Inflamation
4.3. Effect on Blood Pressure
4.4. Effect on Ventricular Function
4.5. Lifestyle and CPAP Effect on BMI
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dong, R.; Dong, Z.; Liu, H.; Shi, F.; Du, J. Prevalence, risk factors, outcomes, and treatment of obstructive sleep apnea in patients with cerebrovascular disease: A systematic review. J. Stroke Cerebrovasc. Dis. 2018, 27, 1471–1480. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Pan, C.; Kong, D.; Li, Z.; Li, W.-J.; Gong, X.; Chen, H.; Zhao, W.; Wang, X.; Li, S.-Q.; et al. A novel method for sensitive determination of subclinical right ventricular systolic dysfunction in patients with obstructive sleep apnea. Clin. Respir. J. 2017, 11, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O’Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Bahammam, A.S.; Pandi-Perumal, S.R.; Spence, D.W.; Moscovitch, A.; Streiner, D.L. The save trial: Has the importance of CPAP for preventing cardiovascular events been discounted? Sleep Vigil. 2017, 1, 47–48. [Google Scholar] [CrossRef]
- Patt, B.T.; Jarjoura, D.; Haddad, D.N.; Sen, C.K.; Roy, S.; Flavahan, N.A.; Khayat, R.N. Endothelial dysfunction in the microcirculation of patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2010, 182, 1540–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, C.L.; McEwen, B.J.; Morel-Kopp, M.-C.; Yee, B.J.; Sullivan, D.R.; Ward, C.M.; Tofler, G.H.; Grunstein, R.R. Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea: A randomised, placebo-controlled crossover study. Thorax 2012, 67, 639–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gay, P.; Weaver, T.; Loube, D.; Iber, C. Positive airway pressure task force; standards of practice committee; american academy of sleep medicine. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 2006, 29, 381–401. [Google Scholar] [CrossRef]
- Rotenberg, B.W.; Murariu, D.; Pang, K.P. Trends in CPAP adherence over twenty years of data collection: A flattened curve. J. Otolaryngol. -Head Neck Surg. 2016, 45, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milleron, O.; Pillière, R.; Foucher, A.; De Roquefeuil, F.; Aegerter, P.; Jondeau, G.; Raffestin, B.G.; Dubourg, O. Benefits of obstructive sleep apnoea treatment in coronary artery disease: A long-term follow-up study. Eur. Heart J. 2004, 25, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.A.; Soler-Cataluña, J.J.; Ejarque-Martínez, L.; Soriano, Y.; Román-Sánchez, P.; Illa, F.B.; Canal, J.M.; Durán-Cantolla, J. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: A 5-year follow-up study. Am. J. Respir. Crit. Care Med. 2009, 180, 36–41. [Google Scholar] [CrossRef]
- Marti, S.; Sampol, G.; Muñoz, X.; Torres, F.; Roca, A.; Lloberes, P.; Sagalés, T.; Quesada, P.; Morell, F. Mortality in severe sleep ap-noea/hypopnoea syndrome patients: Impact of treatment. Eur. Respir. J. 2002, 20, 1511–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive sleep apnea in cardiovascular disease: A review of the literature and proposed multidisci-plinary clinical management strategy. J. Am. Heart Assoc. 2019, 8, e010440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cms.gov. National Coverage Analysis (NCA) for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). 2021. Available online: https://www.cms.gov/medicare-coverage-database/details/nca-details.aspx?NCAId=204&ver=24&NcaName=Continuous+Positive+Airway+Pressure+(CPAP)+Therapy+for+Obstructive+Sleep+Apnea+(OSA)&TAId=50 (accessed on 10 August 2021).
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masa, J.F.; Jiménez, A.; Durán, J.; Capote, F.; Monasterio, C.; Mayos, M.; Terán, J.; Hernández, L.; Barbé, F.; Maimó, A.; et al. Alternative methods of titrating continuous positive airway pressure: A large multicenter study. Am. J. Respir. Crit. Care Med. 2004, 170, 1218–1224. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of, Cardiovascular Imaging. Eur. Heart J. -Cardiovasc. Imaging 2016, 16, 233–271. [Google Scholar] [CrossRef]
- Xu, H.; Yi, H.; Guan, J.; Yin, S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: A meta-analysis of randomized controlled trials. Atherosclerosis 2014, 234, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Simon, B.; Gabor, B.; Barta, I.; Paska, C.; Nagy, G.B.; Vizi, E.; Antus, B. Effect of 5-year continuous positive airway pressure treatment on the lipid profile of patients with obstructive sleep apnea: A pilot study. J. Sleep Res. 2019, 29, e12874. [Google Scholar] [CrossRef]
- Sharma, S.K.; Agrawal, S.; Damodaran, D.; Sreenivas, V.; Kadhiravan, T.; Lakshmy, R.; Jagia, P.; Kumar, A. Retraction: CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N. Engl. J. Med. 2011, 365, 2277–2286. [Google Scholar] [CrossRef] [Green Version]
- Hoyos, C.M.; Killick, R.; Yee, B.J.; Phillips, C.; Grunstein, R.R.; Liu, P.Y. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: A randomised sham-controlled study. Thorax 2012, 67, 1081–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorkova, Z.; Petrasova, D.; Molcanyiova, A.; Popovnakova, M.; Tkacova, R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008, 134, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Steiropoulos, P.; Tsara, V.; Nena, E.; Fitili, C.; Kataropoulou, M.; Froudarakis, M.; Christaki, P.; Bouros, D. Effect of continuous positive airway pressure treatment on serum cardiovascular risk factors in patients with obstructive sleep apnea-hypopnea syndrome. Chest 2007, 132, 843–851. [Google Scholar] [CrossRef]
- Phillips, C.L.; Yee, B.J.; Marshall, N.S.; Liu, P.Y.; Sullivan, D.R.; Grunstein, R.R. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: A randomized, placebo-controlled crossover trial. Am. J. Respir. Crit. Care Med. 2011, 184, 355–361. [Google Scholar] [CrossRef]
- McArdle, N.; Hillman, D.; Beilin, L.; Watts, G. Metabolic risk factors for vascular disease in obstructive sleep apnea: A matched controlled study. Am. J. Respir. Crit. Care Med. 2007, 175, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumpawat, S.; Goel, A.; Banga, A.; Ramakrishnan, L.; Chaturvedi, P. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007, 8, 12–17. [Google Scholar] [CrossRef]
- Kono, M.; Tatsumi, K.; Saibara, T.; Nakamura, A.; Tanabe, N.; Takiguchi, Y.; Kuriyama, T. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest 2007, 131, 1387–1392. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Gurubhagavatula, I.; Teff, K.; Rader, D.J.; Wadden, T.A.; Townsend, R.; Foster, G.D.; Maislin, G.; Saif, H.; Broderick, P.; et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 2265–2275. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.T.; Lin, H.H.; Lee, P.L.; Weng, P.H.; Lee, C.C.; Lai, T.C.; Liu, W.; Chen, C.L. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: A meta-analysis. Sleep Breath. 2015, 19, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, X.-L.; Liu, H.; Wang, Z.-G.; Yin, K.-S. Association among plasma interleukin-18 levels, carotid intima- media thickness and severity of obstructive sleep apnea. Chin. Med. J. 2009, 122, 24–29. [Google Scholar]
- Robinson, G.V.; Pepperell, J.C.T.; Segal, H.C.; Davies, R.J.O.; Stradling, J.R. Circulating cardiovascular risk factors in obstructive sleep apnoea: Data from randomised controlled trials. Thorax 2004, 59, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Imadojemu, V.A.; Mawji, Z.; Kunselman, A.; Gray, K.S.; Hogeman, C.S.; Leuenberger, U.A. Sympathetic Chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest 2007, 131, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Comondore, V.R.; Cheema, R.; Fox, J.; Butt, A.; Mancini, G.B.J.; Fleetham, J.A.; Ryan, C.F.; Chan, S.; Ayas, N.T. The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: A pilot feasibility randomized crossover trial. Lung 2009, 187, 17–22. [Google Scholar] [CrossRef]
- Panjapornpon, K.; Sangsayunh, P.; Bangpattanasiri, K.; Jun-Kroot, S.; Pumpothong, W.; Pungtaway, S.; Tupwang, A. Effect of continuous positive airway pressure (CPAP) on high-sensitivity c-reactive protein levels (hs-CRP) in patients with obstructive sleep apnea (OSA). Eur. Respir. J. 2017, 50, PA2274. [Google Scholar]
- Sproston, N.R.; Ashworth, J.J. Role of c-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaer, M.H.; Shammas, N.W.; Lemke, J.H.; Kapalis, M.J.; Dippel, E.J.; Harb, H.; Reddy, G.; McKinney, D.; Mahadevia, A.K. CPAP does not reduce high-sensitivity c-reactive protein in patients with coronary artery disease and obstructive sleep apnea. Int. J. Angiol. 2005, 14, 129–132. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Asensio-Cruz, M.I.; Cordero-Guevara, J.; Jurado-Gamez, B.; Carmona-Bernal, C.; Gonzalez-Martinez, M.; Troncoso, M.F.; Sanchez-Lopez, V.; Arellano-Orden, E.; Garcia-Sanchez, M.I.; et al. Effect of continuous positive airway pressure on inflammatory, antioxidant, and depression biomarkers in women with obstructive sleep apnea: A randomized controlled trial. Sleep 2019, 42, zsz145. [Google Scholar] [CrossRef]
- Thunström, E.; Glantz, H.; Yucel-Lindberg, T.; Lindberg, K.; Saygin, M.; Peker, Y. CPAP does not reduce inflammatory biomarkers in patients with coronary artery disease and nonsleepy obstructive sleep apnea: A randomized controlled trial. Sleep 2017, 40, zsx157. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Pan, L.; Ren, D.; Xie, X. Impact of continuous positive airway pressure on C-reactive protein in patients with obstructive sleep apnea: A meta-analysis. Sleep Breath. 2013, 17, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Bortolotto, L.A.; Figueiredo, A.C.; Krieger, E.M.; Lorenzi-Filho, G. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2007, 176, 706–712. [Google Scholar] [CrossRef]
- Iesato, K.; Tatsumi, K.; Saibara, T.; Nakamura, A.; Terada, J.; Tada, Y.; Sakao, S.; Tanabe, N.; Takiguchi, Y.; Kuriyama, T. Decreased lipoprotein lipase in obstructive sleep apnea syndrome. Circ. J. 2007, 71, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003, 289, 2560–2571. [Google Scholar] [CrossRef]
- Martínez-García, M.A.; Capote, F.; Campos-Rodríguez, F.; Lloberes, P.; de Atauri, M.J.D.; Somoza, M.; Masa, J.F.; González, M.; Sacristán, L.; Barbé, F.; et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: The HIPARCO randomized clinical trial. JAMA 2013, 310, 2407–2415. [Google Scholar] [CrossRef] [Green Version]
- Lozano, L.; Tovar, J.L.; Sampol, G.; Romero, O.; Jurado, M.J.; Segarra, A.; Espinel, E.; Ríos, J.; Untoria, M.D.; Lloberes, P. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: A randomized, controlled trial. J. Hypertens. 2010, 28, 2161–2168. [Google Scholar] [CrossRef]
- Claudia de Oliveira, A.; Martinez, D.; Massierer, D.; Gus, M.; Cadaval Gonçalves, S.; Ghizzoni, F.; Maria Steinhorst, A.; Beltrami Moreira, L.; Costa Fuchs, S.; Fuchs, F.D. The antihypertensive effect of positive airway pressure on resistant hypertension of patients with obstructive sleep apnea: A randomized, double-blind, clinical trial. Am. J. Respir. Crit. care Med. 2014, 190, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Mokros, Ł.; Kuczyński, W.; Franczak, Ł.; Białasiewicz, P. Morning diastolic blood pressure may be independently associated with severity of obstructive sleep apnea in non-hypertensive patients: A cross-sectional study. J. Clin. Sleep Med. 2017, 13, 905–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.; Vennelle, M.; Connell, M.; McKillop, G.; Newby, D.E.; Douglas, N.J.; Riha, R.L. Arterial stiffness and endothelial function in obstructive sleep apnoea/hypopnoea syndrome. Sleep Med. 2013, 14, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Rumantir, M.; Kaye, D.; Jennings, G.; Hastings, J.; Socratous, F.; Lambert, G. Sympathetic nerve biology in essential hy-pertension. Clin. Exp. Pharmacol. Physiol. 2001, 28, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.G.; Jeong, D.U. Obstructive sleep apnea syndrome is associated with higher diastolic blood pressure in men but not in women. Am. J. Hypertens. 2014, 27, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Romero-Corral, A.; Somers, V.K.; Pellikka, P.A.; Olson, E.J.; Bailey, K.R.; Korinek, J.; Orban, M.; Sierra-Johnson, J.; Kato, M.; Amin, R.S.; et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest 2007, 132, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Chetan, I.M.; Gergely, B.D.; Albu, A.; Tomoaia, R.; Todea, D.A. Understanding the role of echocardiography in patients with obstructive sleep apnea and right ventricular subclinical myocardial dysfunction—comparison with other conditions affecting RV deformation. Med. Ultrason. 2021, 23, 213–219. [Google Scholar] [PubMed]
- Kjaergaard, J.; Petersen, C.L.; Kjaer, A.; Schaadt, B.K.; Oh, J.K.; Hassager, C. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur. J. Echocardiogr. 2006, 7, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Gavina, C.; Pinho, T.; Drummon, M.; Azevedo, A.; Winck, J.; Macedo, F.; Marques, J.; Rocha-Gonçalves, F. 1051 Right ventricular function in patients with moderate to severe obstructive sleep apnea syndrome. Eur. J. Echocardiogr. 2006, 7, S177. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Szabo, M.A.; Krakower, G.; Woods, T. Continuous positive airway pressure (CPAP) consequences on right heart function and serum biomarkers in obstructive sleep apnea assessed by tricuspid annular plane systolic excursion (TAPSE) and color tissue doppler imaging. J. Transl. Sci. 2015, 1, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Kourouklis, S.P.; Vagiakis, E.; Paraskevaidis, I.A.; Farmakis, D.; Kostikas, K.; Parissis, J.T.; Katsivas, A.; Kremastinos, D.T.; Anastasiou-Nana, M.; Filippatos, G. Effective sleep apnoea treatment improves cardiac function in patients with chronic heart failure. Int. J. Cardiol. 2013, 168, 157–162. [Google Scholar] [CrossRef]
- Karamanzanis, G.; Panou, F.; Lazaros, G.; Oikonomou, E.; Nikolopoulos, I.; Mihaelidou, M.; Ntounis, G.; Lekakis, J. Impact of continuous positive airway pressure treatment on myocardial performance in patients with obstructive sleep apnea. A conventional and tissue Doppler echocardiographic study. Sleep Breath. 2014, 19, 343–350. [Google Scholar] [CrossRef]
- Alchanatis, M.; Tourkohoriti, G.; Kakouros, S.; Kosmas, E.; Podaras, S.; Jordanoglou, J.B. Daytime pulmonary hypertension in patients with obstructive sleep apnea: The effect of continuous positive airway pressure on pulmonary hemodynamics. Respiration 2001, 68, 566–572. [Google Scholar] [CrossRef]
- Javaheri, S.; Javaheri, S.; Javaheri, A. Sleep apnea, heart failure, and pulmonary hypertension. Curr. Heart Fail. Rep. 2013, 10, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Sajkov, D.; Wang, T.; Saunders, N.A.; Bune, A.J.; McEvoy, R.D. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2002, 165, 152–158. [Google Scholar] [CrossRef]
- Coss, P.; King, G.; Murphy, R.; Mc Laughlin, A.M. Early effect of continuous positive airway pressure therapy on right ventricular function in patients with newly diagnosed obstructive sleep apnoea. Eur. Respir. J. 2020, 56, 3785. [Google Scholar]
- Akbar, M.; Woods, T.; Szabo, A.; Franco, R. Impact of CPAP for OSA on right heart function as measured by TAPSE and RV contraction velocity by color doppler ECHO. Chest 2011, 140, 822A. [Google Scholar] [CrossRef] [Green Version]
- Hammerstingl, C.; Schueler, R.; Wiesen, M.; Momcilovic, D.; Pabst, S.; Nickenig, G.; Skowasch, D. Impact of untreated ob-structive sleep apnea on left and right ventricular myocardial function and effects Of CPAP therapy. PLoS ONE 2013, 8, e76352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.B.; Beanlands, R.S.; Yoshinaga, K.; Haddad, H.; Leech, J.; De Kemp, R.; Burwash, I.G. Acute and chronic effects of continuous positive airway pressure therapy on left ventricular systolic and diastolic function in patients with obstructive sleep apnea and congestive heart failure. Can. J. Cardiol. 2008, 24, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Walley, K.R. Left ventricular function: Time-varying elastance and left ventricular aortic coupling. Crit. Care 2016, 20, 270. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, Y.; Floras, J.S.; Usui, K.; Plante, J.; Tkacova, R.; Kubo, T.; Ando, S.-I.; Bradley, T.D. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N. Engl. J. Med. 2003, 348, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Egea, C.J.; Aizpuru, F.; Pinto, J.A.; Ayuela, J.M.; Ballester, E.; Zamarrón, C.; Sojo, A.; Montserrat, J.M.; Barbe, F.; Alonso-Gomez, A.M.; et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: A multicenter study. Sleep Med. 2008, 9, 660–666. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Greer, S.M.; Goldstein, A.N.; Walker, M.P. The impact of sleep deprivation on food desire in the human brain. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Schoeller, D.A.; Penev, P.D. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann. Intern. Med. 2010, 153, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Harsch, I.; Konturek, P.; Koebnick, C.; Kuehnlein, P.; Fuchs, F.; Pour Schahin, S.; Wiest, G.; Hahn, E.; Lohmann, T.; Ficker, J. Leptin and ghrelin levels in patients with obstructive sleep apnoea: Effect of CPAP treatment. Eur. Respir. J. 2003, 22, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppard, P.E.; Young, T. Exercise and Sleep-Disordered Breathing: An association independent of body habitus. Sleep 2004, 27, 480–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, S.F.; O’Connor, G.T.; Quan, J.S.; Redline, S.; Resnick, H.E.; Shahar, E.; Siscovick, D.; Sherrill, D.L. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007, 11, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lim, H.J. Lifestyle modification in patients with obstructive sleep apnea. Sleep Med. Res. 2018, 9, 63–72. [Google Scholar] [CrossRef] [Green Version]
| Variable | Non-CPAP Group (n = 57, 34.9%) | CPAP Group (n = 106, 65%) | p Value |
|---|---|---|---|
| Age years | 59 (52.6; 65.2) | 61 (53.7; 67) | 0.76 |
| Sex female, n, % | 26 (45.6%) | 38 (35.8%) | 0.22 |
| BMI kg-m2 | 38.1 (34.4; 40.7) | 37.5 (33.08; 41.9) | 0.44 |
| Additional cardiovascular risk factors: | |||
| -DM type 2, n, % | 18 (31.6%) | 39 (36.8%) | 0.5 |
| -Arterial hypertension, n, % | 39 (68.4%) | 83 (78.3%) | 0.16 |
| -Smoking, n, % | 37 (64.9%) | 57 (53.8%) | 0.17 |
| -Alcohol, n, % | 6 (10.5%) | 10 (9.4%) | 0.8 |
| Medication: | |||
| Lipid lowering drugs, n, % | 22 (38.6%) | 37 (34.9%) | 0.08 |
| Anti-hypertensive agents, n, % | 39 (68.4%) | 83 (78.3%) | 0.16 |
| SBP average, mmhg | 145 (135; 157.5) | 136 (130; 145) | <0.001 |
| DBP average, mmhg | 89 (80; 90) | 82 (75; 89) | 0.01 |
| Serum levels lipids/CRP: | |||
| Cholesterol levels mg/dl | 199 (177; 218) | 205 (166; 236) | 0.4 |
| Triglyceride levels mg/dl | 160 (119; 225) | 195 (118.5; 233.7) | 0.9 |
| CRP, mg/L | 5.6 (3.4; 14) | 5.8 (3.4; 11) | 0.26 |
| Results of the sleep study: | |||
| AHI h−1 | 44.3 (22.8; 82.1) | 45 (32.2; 65.9) | 0.93 |
| ODI h−1 | 43.2 (27; 80) | 44.9 (27.3; 62) | 0.55 |
| Max O2 desaturation, % | 72 (67; 78) | 68.5 (60.5; 79.2) | 0.61 |
| Apnea average duration, s | 73.5 (55.5; 98) | 75 (49; 95) | 0.4 |
| Hypopnea average duration, s | 84 (62.5; 101.5) | 79.5 (56; 98) | 0.14 |
| Apneas, n | 72 (56; 101.5) | 71 (45; 103) | 0.059 |
| Hypopneas, n | 98 (73.5; 120) | 94.5 (71; 111.7) | 0.7 |
| Echocardiographic parameters | |||
| RV, mm | 35 (31.5; 39) | 37 (35; 40) | 0.16 |
| TAPSE, mm | 20 (17.5; 21) | 19 (18; 21) | 0.31 |
| RA-RV Gradient, mmhg | 30 (20; 40) | 35 (24; 36.5) | 0.9 |
| LVEF, % | 50 (50; 51.5) | 50 (45; 55) | 0.24 |
| Without CPAP | With CPAP | ||||
|---|---|---|---|---|---|
| Variable | Baseline | Follow Up | Baseline | Follow Up | p Value |
| AHI h−1 | 44.3 (22.8; 82.1) | 32 (16.5; 38.1) | 44.3 (22.8; 82.1) | 5.1 (2.1; 9.1) | <0.001 |
| ODI h−1 | 43.2 (27; 80) | 31.2 (16.8; 42.7) | 44.9 (27.3; 62) | 4.7 (2; 8.6) | <0.001 |
| Apnea average duration, s | 73.5 (55.5; 98) | 31 (12; 24) | 75 (49; 95) | 12 (8; 32) | 0.01 |
| Hypopnea average duration, s | 84 (62.5; 101.5) | 52 (25; 68) | 79.5(56; 98) | 25 (15.7; 49.2) | 0.03 |
| Apneas, n | 72 (56; 101.5) | 23 (5; 63.5) | 71 (45; 103) | 8.5 (2; 23) | <0.001 |
| Hypopneas, n | 98 (73.5; 120) | 56 (13; 79) | 94.5 (71; 111.7) | 15 (12; 36) | 0.001 |
| Max O2 desaturation, % | 72 (67; 78) | 84 (75.5; 87) | 68.5 (60.5; 79.2) | 90 (83; 94) | 0.03 |
| BMI, kg-m2 | 38.1 (34.4; 40.7) | 37.2 (32.6; 39.7) | 37.5 (33.08; 41.9) | 35.2 (31.09; 40.3) | 0.001 |
| Cholesterol levels, mg/dL | 199 (177;218) | 190 (180; 222) | 205 (166; 236) | 175.5 (145; 210) | 0.001 |
| Triglyceride levels, mg/dL | 160 (119; 225) | 159 (119; 193) | 195 (118.5; 233.7) | 150 (104.5; 186.75) | 0.2 |
| CRP, mg/L | 5.6 (3.4; 14) | 7 (4.3; 8.7) | 5.8 (3.4; 11) | 3.1 (2.1; 9.4) | 0.1 |
| SBP average, mmHg | 145 (135; 157.5) | 135 (129; 145) | 136 (130; 145) | 125 (120; 130) | 0.5 |
| DBP average, mmHg | 89 (80; 90) | 80 (75; 90) | 82 (75; 89) | 75 (70; 80) | 0.001 |
| RV, mm | 35 (31.5; 39) | 36 (32; 39) | 37 (35; 40) | 37 (35; 40) | 0.4 |
| TAPSE, mm | 20 (17.5; 21) | 19 (18; 21) | 19 (18; 21) | 20 (19; 21) | 0.03 |
| RA-RV Gradient, mmhg | 30 (20; 40) | 28 (20; 39) | 35 (24; 36.5) | 34 (25; 38.5) | 0.01 |
| LVEF, % | 50 (50; 51.5) | 50 (50; 55) | 50 (45; 55) | 50 (45; 55) | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chetan, I.M.; Maierean, A.D.; Domokos Gergely, B.; Cabau, G.; Tomoaia, R.; Chis, A.F.; Albu, A.; Stoia, M.A.; Vesa, S.C.; Blendea, D.; et al. A Prospective Study of CPAP Therapy in Relation to Cardiovascular Outcome in a Cohort of Romanian Obstructive Sleep Apnea Patients. J. Pers. Med. 2021, 11, 1001. https://doi.org/10.3390/jpm11101001
Chetan IM, Maierean AD, Domokos Gergely B, Cabau G, Tomoaia R, Chis AF, Albu A, Stoia MA, Vesa SC, Blendea D, et al. A Prospective Study of CPAP Therapy in Relation to Cardiovascular Outcome in a Cohort of Romanian Obstructive Sleep Apnea Patients. Journal of Personalized Medicine. 2021; 11(10):1001. https://doi.org/10.3390/jpm11101001
Chicago/Turabian StyleChetan, Ioana Maria, Anca Diana Maierean, Bianca Domokos Gergely, Georgiana Cabau, Raluca Tomoaia, Ana Florica Chis, Adriana Albu, Mirela Anca Stoia, Stefan Cristian Vesa, Dan Blendea, and et al. 2021. "A Prospective Study of CPAP Therapy in Relation to Cardiovascular Outcome in a Cohort of Romanian Obstructive Sleep Apnea Patients" Journal of Personalized Medicine 11, no. 10: 1001. https://doi.org/10.3390/jpm11101001
APA StyleChetan, I. M., Maierean, A. D., Domokos Gergely, B., Cabau, G., Tomoaia, R., Chis, A. F., Albu, A., Stoia, M. A., Vesa, S. C., Blendea, D., & Todea, D. A. (2021). A Prospective Study of CPAP Therapy in Relation to Cardiovascular Outcome in a Cohort of Romanian Obstructive Sleep Apnea Patients. Journal of Personalized Medicine, 11(10), 1001. https://doi.org/10.3390/jpm11101001







