Serum Sialylation Changes in Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Histopathological Examination
2.3. Laboratory Determinations
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, O.T.; Ranmuthu, C.K.I.; Hall, P.N.; Funston, G.; Walter, F.M. Recognising Skin Cancer in Primary Care. Adv. Ther. 2020, 37, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollison, D.E.; Viarisio, D.; Amorrortu, R.P.; Gheit, T.; Tommasino, M. An Emerging Issue in Oncogenic Virology: The Role of Beta Human Papillomavirus Types in the Development of Cutaneous Squamous Cell Carcinoma. J. Virol. 2019, 93, e01003-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Nicolae, I.; Dumitru, A.; Matei, C.; Manolescu, L.; Popa, G.L.; Caruntu, C.; Georgescu, S.R. The Role of Beta HPV Types and HPV-Associated Inflammatory Processes in Cutaneous Squamous Cell Carcinoma. J. Immunol. Res. 2020, 2020, 5701639. [Google Scholar] [CrossRef] [PubMed]
- Cela, E.M.; Paz, M.L.; Leoni, J.; Maglio, D.H.G. Immune System Modulation Produced by Ultraviolet Radiation. Immunoregul. Asp. Immunother. 2018, 103. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; He, Y.-Y. Ultraviolet Radiation-Induced Non-Melanoma Skin Cancer: Regulation of DNA Damage Repair and Inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Tufaro, A.P.; Chuang, J.C.-M.; Prasad, N.; Chuang, A.; Chuang, T.C.; Fischer, A.C. Molecular Markers in Cutaneous Squamous Cell Carcinoma. Int. J. Surg. Oncol. 2011, 2011, 231475. [Google Scholar] [CrossRef]
- Wang, J.; Aldabagh, B.; Yu, J.; Arron, S.T. Role of human papillomavirus in cutaneous squamous cell carcinoma: A meta-analysis. J. Am. Acad. Dermatol. 2014, 70, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Chahoud, J.; Semaan, A.; Chen, Y.; Cao, M.; Rieber, A.G.; Rady, P.; Tyring, S.K. Association between β-Genus Human Papillomavirus and Cutaneous Squamous Cell Carcinoma in Immunocompetent Individuals—A Meta-Analysis. JAMA Dermatol. 2016, 152, 1354–1364. [Google Scholar] [CrossRef] [Green Version]
- Hampras, S.S.; Reed, R.A.; Bezalel, S.; Cameron, M.; Cherpelis, B.; Fenske, N.; Sondak, V.K.; Messina, J.; Tommasino, M.; Gheit, T. Cutaneous Human Papillomavirus Infection and Development of Subsequent Squamous Cell Carcinoma of the Skin. J. Skin Cancer 2016, 2016, 1368103. [Google Scholar] [CrossRef]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Endo, Y.; Tanioka, M.; Miyachi, Y. Prognostic Factors in Cutaneous Squamous Cell Carcinoma: Is Patient Delay in Hospital Visit a Predictor of Survival? ISRN Dermatol. 2011, 2011, 285289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of Risk Factors Determining Prognosis of Cutaneous Squamous-Cell Carcinoma: A Prospective Study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Reinehr, C.P.H.; Bakos, R.M. Actinic Keratoses: Review of Clinical, Dermoscopic, and Therapeutic Aspects. Anais Brasileiros de Dermatologia 2019, 94, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Matei, C.; Tampa, M.; Ion, R.; Neagu, M.; Constantin, C. Photodynamic properties of aluminium sulphonated phthalocyanines in human displazic oral keratinocytes experimental model. Dig. J. Nanomater. Biostruct. 2012, 7, 1535–1547. [Google Scholar]
- Vajaria, B.N.; Patel, K.R.; Begum, R.; Patel, P.S. Sialylation: An Avenue to Target Cancer Cells. Pathol. Oncol. Res. 2016, 22, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, C.; Nicolae, I. Heterogeneity of Gangliosides in Melanocytic Tumors. Acta Endocrinol. 2012, 8, 17–26. [Google Scholar] [CrossRef]
- Möginger, U.; Grunewald, S.; Hennig, R.; Kuo, C.-W.; Schirmeister, F.; Voth, H.; Rapp, E.; Khoo, K.-H.; Seeberger, P.H.; Simon, J.C.; et al. Alterations of the Human Skin N- and O-Glycome in Basal Cell Carcinoma and Squamous Cell Carcinoma. Front. Oncol. 2018, 8, 70. [Google Scholar] [CrossRef]
- Dobie, C.; Skropeta, D. Insights into the Role of Sialylation in Cancer Progression and Metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Sialosignaling: Sialyltransferases as Engines of Self-Fueling Loops in Cancer Progression. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2752–2764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wuhrer, M.; Holst, S. Serum Sialylation Changes in Cancer. Glycoconj. J. 2018, 35, 139–160. [Google Scholar] [CrossRef] [Green Version]
- Hata, K.; Tochigi, T.; Sato, I.; Kawamura, S.; Shiozaki, K.; Wada, T.; Takahashi, K.; Moriya, S.; Yamaguchi, K.; Hosono, M.; et al. Increased Sialidase Activity in Serum of Cancer Patients: Identification of Sialidase and Inhibitor Activities in Human Serum. Cancer Sci. 2015, 106, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Ene, C.D.; Nicolae, I.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Caruntu, A.; Caruntu, C.; Georgescu, S.R. Antiganglioside Antibodies and Inflammatory Response in Cutaneous Melanoma. J. Immunol. Res. 2020, 2020, 2491265. [Google Scholar] [CrossRef]
- Kolasińska, E.; Przybyło, M.; Janik, M.; Lityńska, A. Towards Understanding the Role of Sialylation in Melanoma Progression. Acta Biochim. Pol. 2016, 63, 533–541. [Google Scholar] [CrossRef]
- Rodrigues, E.; Macauley, M.S. Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers 2018, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Pearce, O.M.; Läubli, H. Sialic Acids in Cancer Biology and Immunity. Glycobiology 2016, 26, 111–128. [Google Scholar] [CrossRef] [Green Version]
- Garnham, R.; Scott, E.; Livermore, K.; Munkley, J. ST6GAL1: A Key Player in Cancer. Oncol. Lett. 2019, 18, 983–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashima, S.; Tsuji, S. Functional Diversity of Mammalian Sialyltransferases. Trends Glycosci. Glycotechnol. 2011, 23, 178–193. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.A.; Vasconcelos, J.L.A.; Silva, R.C.W.C.; Cavalcanti, C.L.B.; Bezerra, C.L.; Rêgo, M.J.B.M.; Beltrão, E.I.C. Expression Patterns of A2,3-Sialyltransferase I and A2,6-Sialyltransferase I in Human Cutaneous Epithelial Lesions. Eur. J. Histochem. 2013, 57, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsett, K.A.; Marciel, M.P.; Hwang, J.; Ankenbauer, K.E.; Bhalerao, N.; Bellis, S.L. Regulation of ST6GAL1 Sialyltransferase Expression in Cancer Cells. Glycobiology 2021, 31, 530–539. [Google Scholar] [CrossRef]
- Glanz, V.Y.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Sialidase Activity in Human Pathologies. Eur. J. Pharmacol. 2019, 842, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T. Aberrant Expression of Sialidase and Cancer Progression. Proc. Jpn. Acad. Ser. B 2008, 84, 407–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagi, T.; Takahashi, K.; Hata, K.; Shiozaki, K.; Yamaguchi, K. Sialidase Significance for Cancer Progression. Glycoconj. J. 2012, 29, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.D.; Penescu, M.N.; Georgescu, S.R.; Tampa, M.; Nicolae, I. Posttranslational Modifications Pattern in Clear Cell Renal Cell Carcinoma. Metabolites 2020, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.D.; Georgescu, S.R.; Tampa, M.; Matei, C.; Mitran, C.I.; Mitran, M.I.; Penescu, M.N.; Nicolae, I. Cellular Response against Oxidative Stress, a Novel Insight into Lupus Nephritis Pathogenesis. J. Pers. Med. 2021, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.D.; Penescu, M.; Anghel, A.; Neagu, M.; Budu, V.; Nicolae, I. Monitoring Diabetic Nephropathy by Circulating Gangliosides. J. Immunoass. Immunochem. 2016, 37, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.-D.; Penescu, M.N.; Nicolae, I. Sialoglyco-Conjugate Abnormalities, IL-6 Trans-Signaling and Anti-Ganglioside Immune Response—Potential Interferences in Lupus Nephritis Pathogenesis. Diagnostics 2021, 11, 1129. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.; Pandhi, N.; Patel, P. Clinical Evaluation of Sialic Acid In Head and Neck Squamous Cell Carcinoma Patients and Tobacco Chewers or Smokers with No Cancer. Biomed. Pharmacol. J. 2017, 10, 2027–2033. [Google Scholar] [CrossRef]
- Vural, P.; Canbaz, M.; Selcuki, D. Total and Lipid-Bound Sialic Acid Levels in Actinic Keratosis and Basal Cell Carcinoma. Turk. J. Med. Sci. 1999, 29, 419–424. [Google Scholar]
- Shiga, K.; Takahashi, K.; Sato, I.; Kato, K.; Saijo, S.; Moriya, S.; Hosono, M.; Miyagi, T. Upregulation of Sialidase NEU 3 in Head and Neck Squamous Cell Carcinoma Associated with Lymph Node Metastasis. Cancer Sci. 2015, 106, 1544–1553. [Google Scholar] [CrossRef] [Green Version]
- Manualul AJCC de Stadializare a Cancerului. 2018. Available online: https://www.clb.ro/manualul-ajcc-de-stadializare-a-cancerului-2018-0000176379--p343005.html (accessed on 10 July 2021).
- Menon, S.S.; Guruvayoorappan, C.; Sakthivel, K.M.; Rasmi, R.R. Ki-67 Protein as a Tumour Proliferation Marker. Clin. Chim. Acta 2019, 491, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.R.; Tampa, M.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour Microenvironment in Skin Carcinogenesis. Adv. Exp. Med. Biol. 2020, 1226, 123–142. [Google Scholar] [CrossRef]
- Ratushny, V.; Gober, M.D.; Hick, R.; Ridky, T.W.; Seykora, J.T. From Keratinocyte to Cancer: The Pathogenesis and Modeling of Cutaneous Squamous Cell Carcinoma. J. Clin. Investig. 2012, 122, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampa, M.; Matei, C.; Popescu, S.; Georgescu, S.-R.; Neagu, M.; Constantin, C.; Ion, R.-M. Zinc Trisulphonated Phthalocyanine Used in Photodynamic Therapy of Dysplastic Oral Keratinocytes. Rev. Chim. 2013, 64, 639–645. [Google Scholar]
- Peters, J.M.; Gonzalez, F.J. The Evolution of Carcinogenesis. Toxicol. Sci. 2018, 165, 272–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Tocut, S.M.; Popa, M.I.; Tampa, M. New Insights in the Pathogenesis of HPV Infection and the Associated Carcinogenic Processes: The Role of Chronic Inflammation and Oxidative Stress. J. Immunol. Res. 2018, 2018, 5315816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, L.R.A.; Bezerra, M.F.; Almeida, S.M.V.; Silva, L.P.B.G.; Beltrão, E.I.C.; Carvalho Júnior, L.B. Glycophenotype Evaluation in Cutaneous Tumors Using Lectins Labeled with Acridinium Ester. Dis. Markers 2013, 35, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raval, G.; Patel, D.; Parekh, L.; Patel, J.; Shah, M.; Patel, P. Evaluation of Serum Sialic Acid, Sialyltransferase and Sialoproteins in Oral Cavity Cancer: Sialic Acid, Sialyltransferase and Sialoproteins in Oral Cavity Cancer. Oral Dis. 2003, 9, 119–128. [Google Scholar] [CrossRef]
- Pietrobono, S.; Anichini, G.; Sala, C.; Manetti, F.; Almada, L.L.; Pepe, S.; Carr, R.M.; Paradise, B.D.; Sarkaria, J.N.; Davila, J.I.; et al. ST3GAL1 Is a Target of the SOX2-GLI1 Transcriptional Complex and Promotes Melanoma Metastasis through AXL. Nat. Commun. 2020, 11, 5865. [Google Scholar] [CrossRef]
- Ghosh, S. Sialic Acids and Sialoglycoconjugates in the Biology of Life, Health and Disease; Academic Press: Cambridge, MA, USA, 2020; ISBN 0-12-816127-2. [Google Scholar]
- Munkley, J.; Oltean, S.; Vodák, D.; Wilson, B.T.; Livermore, K.E.; Zhou, Y.; Star, E.; Floros, V.I.; Johannessen, B.; Knight, B. The Androgen Receptor Controls Expression of the Cancer-Associated STn Antigen and Cell Adhesion through Induction of ST6GalNAc1 in Prostate Cancer. Oncotarget 2015, 6, 34358. [Google Scholar] [CrossRef] [Green Version]
- Munkley, J. The Role of Sialyl-Tn in Cancer. Int. J. Mol. Sci. 2016, 17, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, K.; Balasundaram, S. Evaluation of Total and Lipid Bound Sialic Acid in Serum in Oral Leukoplakia. J. Clin. Diagn Res. 2017, 11, ZC25–ZC27. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Yan, Y.; Yuan, B.; Dasgupta, A.; Sun, J.; Mu, H.; Do, K.-A.; Ueno, N.T.; Andreeff, M.; Battula, V.L. ST8SIA1 Regulates Tumor Growth and Metastasis in TNBC by Activating the FAK-AKT-MTOR Signaling Pathway. Mol. Cancer 2018, 17, 2689–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vajaria, B.N.; Patel, K.A.; Patel, P.S. Role of Aberrant Glycosylation Enzymes in Oral Cancer Progression. J. Carcinog. 2018, 17, 5. [Google Scholar] [CrossRef]
- Wang, P.-H.; Lee, W.-L.; Lee, Y.-R.; Juang, C.-M.; Chen, Y.-J.; Chao, H.-T.; Tsai, Y.-C.; Yuan, C.-C. Enhanced Expression of α 2,6-Sialyltransferase ST6Gal I in Cervical Squamous Cell Carcinoma. Gynecol. Oncol. 2003, 89, 395–401. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Ho, S.B.; Schoeppner, H.L.; Kim, Y.S.; Sleisenger, M.H.; Brodt, P.; Byrd, J.C. Enhanced Sialylation of Mucin-Associated Carbohydrate Structures in Human Colon Cancer Metastasis. Gastroenterology 1996, 110, 1354–1367. [Google Scholar] [CrossRef]
- Recchi, M.-A.; Hebbar, M.; Hornez, L.; Harduin-Lepers, A.; Peyrat, J.-P.; Delannoy, P. Multiplex Reverse Transcription Polymerase Chain Reaction Assessment of Sialyltransferase Expression in Human Breast Cancer. Cancer Res. 1998, 58, 4066–4070. [Google Scholar]
- Fialka, F.; Gruber, R.M.; Hitt, R.; Opitz, L.; Brunner, E.; Schliephake, H.; Kramer, F.-J. CPA6, FMO2, LGI1, SIAT1 and TNC Are Differentially Expressed in Early- and Late-Stage Oral Squamous Cell Carcinoma—A Pilot Study. Oral Oncol. 2008, 44, 941–948. [Google Scholar] [CrossRef]
- Mehta, K.A.; Patel, K.A.; Pandya, S.J.; Patel, P.S. Aberrant Sialylation Plays a Significant Role in Oral Squamous Cell Carcinoma Progression. J. Oral Pathol. Med. 2020, 49, 253–259. [Google Scholar] [CrossRef]
- Boukamp, P. Non-Melanoma Skin Cancer: What Drives Tumor Development and Progression? Carcinogenesis 2005, 26, 1657–1667. [Google Scholar] [CrossRef]
- Plzák, J.; Smetana, K., Jr.; Chovanec, M.; Betka, J. Glycobiology of Head and Neck Squamous Epithelia and Carcinomas. ORL 2005, 67, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Holíková, Z.; Hrdlicková-Cela, E.; Plzák, J.; Smetana, K., Jr.; Betka, J.; Dvoránková, B.; Esner, M.; Wasano, K.; André, S.; Kaltner, H.; et al. Defining the Glycophenotype of Squamous Epithelia Using Plant and Mammalian Lectins. Differentiation-dependent Expression of A2, 6-and A2, 3-linked N-acetylneuraminic Acid in Squamous Epithelia and Carcinomas, and Its Differential Effect on Binding of the Endogenous Lectins Galectins-1 And-3. Apmis 2002, 110, 845–856. [Google Scholar]
- Caruntu, C.; Mirica, A.; Roşca, A.E.; Mirica, R.; Caruntu, A.; Tampa, M.; Matei, C.; Constantin, C.; Neagu, M.; Badarau, A.I.; et al. The role of estrogens and estrogen receptors in melanoma development and progression. Acta Endocrinol. 2016, 12, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kakugawa, Y.; Wada, T.; Yamaguchi, K.; Yamanami, H.; Ouchi, K.; Sato, I.; Miyagi, T. Up-Regulation of Plasma Membrane-Associated Ganglioside Sialidase (Neu3) in Human Colon Cancer and Its Involvement in Apoptosis Suppression. Proc. Natl. Acad. Sci. USA 2002, 99, 10718–10723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, S.; Saito, S.; Wada, T.; Yamaguchi, K.; Satoh, M.; Arai, Y.; Miyagi, T. Plasma Membrane-Associated Sialidase Is up-Regulated in Renal Cell Carcinoma and Promotes Interleukin-6-Induced Apoptosis Suppression and Cell Motility. J. Biol. Chem. 2006, 281, 7756–7764. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, S.; Sato, I.; Wada, T.; Yamaguchi, K.; Li, Y.; Li, D.; Zhao, X.; Ueno, S.; Aoki, H.; Tochigi, T. Plasma Membrane-Associated Sialidase (NEU3) Regulates Progression of Prostate Cancer to Androgen-Independent Growth through Modulation of Androgen Receptor Signaling. Cell Death Differ. 2012, 19, 170–179. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takahashi, K.; Shiozaki, K.; Yamaguchi, K.; Moriya, S.; Hosono, M.; Shima, H.; Miyagi, T. Potentiation of Epidermal Growth Factor-Mediated Oncogenic Transformation by Sialidase NEU3 Leading to Src Activation. PLoS ONE 2015, 10, e0120578. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Shiga, K.; Yamaguchi, K.; Hata, K.; Kobayashi, T.; Miyazaki, K.; Saijo, S.; Miyagi, T. Plasma-Membrane-Associated Sialidase (NEU3) Differentially Regulates Integrin-Mediated Cell Proliferation through Laminin-and Fibronectin-Derived Signalling. Biochem. J. 2006, 394, 647–656. [Google Scholar] [CrossRef]
- Fanzani, A.; Zanola, A.; Faggi, F.; Papini, N.; Venerando, B.; Tettamanti, G.; Sampaolesi, M.; Monti, E. Implications for the Mammalian Sialidases in the Physiopathology of Skeletal Muscle. Skelet. Muscle 2012, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Tringali, C.; Silvestri, I.; Testa, F.; Baldassari, P.; Anastasia, L.; Mortarini, R.; Anichini, A.; López-Requena, A.; Tettamanti, G.; Venerando, B. Molecular Subtyping of Metastatic Melanoma Based on Cell Ganglioside Metabolism Profiles. BMC Cancer 2014, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kappagantula, S.; Andrews, M.R.; Cheah, M.; Abad-Rodriguez, J.; Dotti, C.G.; Fawcett, J.W. Neu3 Sialidase-Mediated Ganglioside Conversion Is Necessary for Axon Regeneration and Is Blocked in CNS Axons. J. Neurosci. 2014, 34, 2477–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampa, M.; Mitran, M.I.; Mitran, C.I.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Caruntu, A.; Tocut, S.M.; Popa, M.I.; Caruntu, C.; et al. Mediators of Inflammation—A Potential Source of Biomarkers in Oral Squamous Cell Carcinoma. J. Immunol. Res. 2018, 2018, 1061780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inal, E.; Laçin, M.; Asal, K.; Ceylan, A.; Köybaşioğlu, A.; Ileri, F.; Uslu, S.S. The Significance of Ferritin, Lipid-Associated Sialic Acid, CEA, Squamous Cell Carcinoma (SCC) Antigen, and CYFRA 21-1 Levels in SCC of the Head and Neck. Kulak Burun Bogaz Ihtisas Dergisi 2004, 12, 23–30. [Google Scholar] [PubMed]
- O’Shea, L.K.; Abdulkhalek, S.; Allison, S.; Neufeld, R.J.; Szewczuk, M.R. Therapeutic Targeting of Neu1 Sialidase with Oseltamivir Phosphate (Tamiflu®) Disables Cancer Cell Survival in Human Pancreatic Cancer with Acquired Chemoresistance. OncoTargets Ther. 2014, 7, 117. [Google Scholar]
- Haxho, F.; Allison, S.; Alghamdi, F.; Brodhagen, L.; Kuta, V.E.; Abdulkhalek, S.; Neufeld, R.J.; Szewczuk, M.R. Oseltamivir Phosphate Monotherapy Ablates Tumor Neovascularization, Growth, and Metastasis in Mouse Model of Human Triple-Negative Breast Adenocarcinoma. Breast Cancer Targets Ther. 2014, 6, 191. [Google Scholar]
- Haxho, F.; Neufeld, R.J.; Szewczuk, M.R. Neuraminidase-1: A Novel Therapeutic Target in Multistage Tumorigenesis. Oncotarget 2016, 7, 40860–40881. [Google Scholar] [CrossRef] [Green Version]

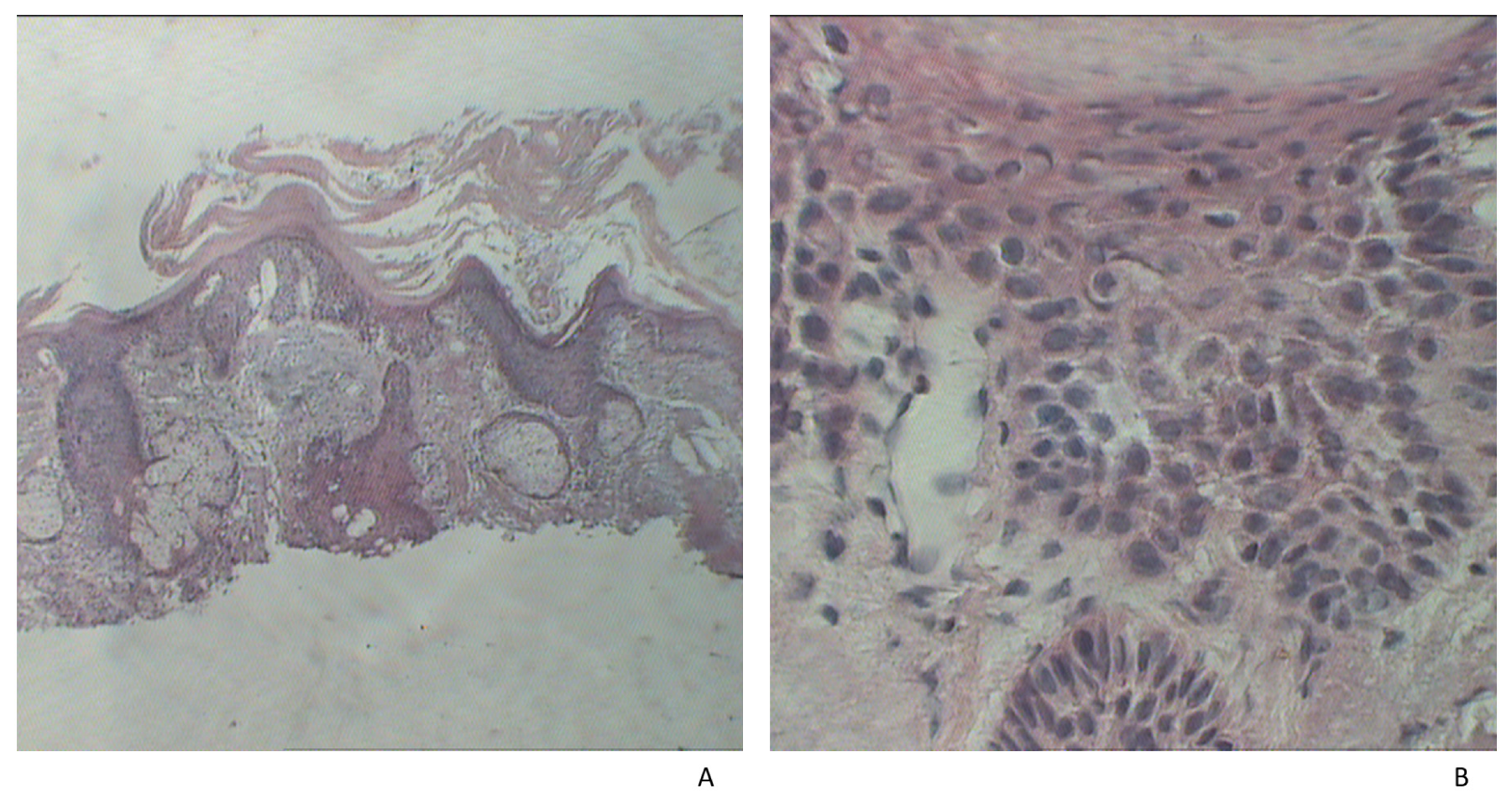
| cSCC Group (n = 40) | AK Group (n = 28) | Control Group (n = 40) | |
|---|---|---|---|
| Patient characteristics | |||
| Female/male | 16/24 | 12/16 | 18/22 |
| Age (years) | 56.6 ± 10.8 | 55.8 ± 8.5 | 54.6 ± 9.3 |
| BMI (kg/m2) | 23.6 ± 1.7 | 22.4 ± 1.4 | 22.1 ± 1.9 |
| Skin phototypes I-II/III-IV | 16/24 | 15/13 | 22/18 |
| Exposure/non-exposure to UV | 34/6 | 24/4 | 32/8 |
| Tumor characteristics | |||
| Tumor diameter <2 cm/>2 cm | 28/12 | 28/0 | - |
| Depth of invasion <4 mm/>4 mm | 23/17 | - | - |
| Ki67 index: <25%/25–75%/>75% | 15/14/11 | - | - |
| Lesion ulceration - Present/absent | 23/17 | 1/27 | - |
| Parameter | cSCC Group (n = 40, A) | AK Group (n = 28, B) | Control Group (n = 40, C) | p * | p ** |
|---|---|---|---|---|---|
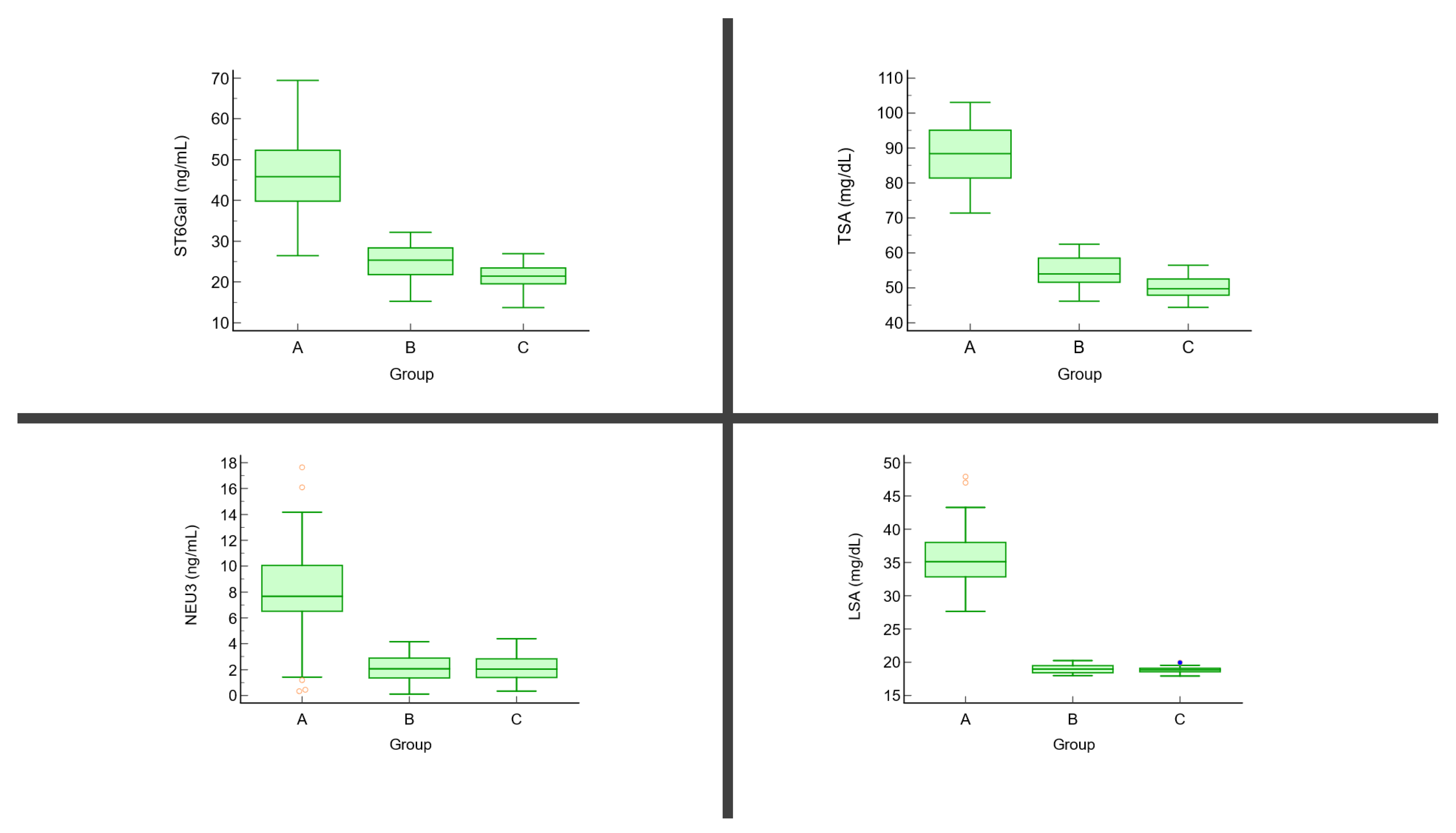
| TSA (mg/dL) | 86.26 ± 8.58 | 56.41 ± 4.27 | 49.71 ± 3.56 | p < 0.01 | A vs. B: <0.001 A vs. C: <0.001 B vs. C: <0.001 |
| LSA (mg/dL) | 36.59 ± 7.14 | 18.92 ± 0.56 | 18.77 ± 0.49 | p < 0.01 | A vs. B: <0.001 A vs. C: <0.001 B vs. C: 0.79 |
| ST6GalI (ng/mL) | 49.06 ± 10.02 | 24.62 ± 3.71 | 22.31 ± 2.90 | p < 0.01 | A vs. B: <0.001 A vs. C: <0.001 B vs. C: 0.052 |
| NEU3 (ng/mL) | 7.64 ± 4.22 | 2.32 ± 1.16 | 2.27 ± 1.01 | p < 0.01 | A vs. B: <0.001 A vs. C: <0.001 B vs. C: 0.89 |
| Parameter | TSA | LSA | ST6GalI | NEU3 | ||||
|---|---|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | rho | p | |
| Diameter | 0.63 | <0.001 * | 0.58 | <0.010 * | 0.78 | 0.01 * | 0.58 | 0.07 |
| Depth of invasion | 0.39 | 0.02 * | 0.46 | 0.01 * | 0.29 | 0.13 | 0.46 | 0.11 |
| Ki67 | 0.34 | 0.04 * | 0.66 | 0.01 * | 0.25 | 0.82 | 0.26 | 0.102 |
| Ulceration | 0.12 | 0.29 | −0.02 | 0.78 | 0.33 | 0.42 | 0.47 | 0.03 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tampa, M.; Nicolae, I.; Mitran, C.I.; Mitran, M.I.; Ene, C.; Matei, C.; Georgescu, S.R.; Ene, C.D. Serum Sialylation Changes in Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Patients. J. Pers. Med. 2021, 11, 1027. https://doi.org/10.3390/jpm11101027
Tampa M, Nicolae I, Mitran CI, Mitran MI, Ene C, Matei C, Georgescu SR, Ene CD. Serum Sialylation Changes in Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Patients. Journal of Personalized Medicine. 2021; 11(10):1027. https://doi.org/10.3390/jpm11101027
Chicago/Turabian StyleTampa, Mircea, Ilinca Nicolae, Cristina Iulia Mitran, Madalina Irina Mitran, Cosmin Ene, Clara Matei, Simona Roxana Georgescu, and Corina Daniela Ene. 2021. "Serum Sialylation Changes in Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Patients" Journal of Personalized Medicine 11, no. 10: 1027. https://doi.org/10.3390/jpm11101027
APA StyleTampa, M., Nicolae, I., Mitran, C. I., Mitran, M. I., Ene, C., Matei, C., Georgescu, S. R., & Ene, C. D. (2021). Serum Sialylation Changes in Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Patients. Journal of Personalized Medicine, 11(10), 1027. https://doi.org/10.3390/jpm11101027








