The Role of IL-6 and ET-1 in the Diagnosis of Coronary MicroVascular Disease in Women
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ong, P.; Athanasiadis, A.; Borgulya, G.; Vokshi, I.; Bastiaenen, R.; Kubik, S.; Hill, S.; Schäufele, T.; Mahrholdt, H.; Kaski, J.C.; et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation 2014, 129, 1723–1730. [Google Scholar] [CrossRef] [Green Version]
- Lanza, G.A.; Crea, F. Primary coronary microvascular dysfunction: Clinical presentation, pathophysiology, and management. Circulation 2010, 121, 2317–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocco, G.; Jerie, P. Angina pectoris in patients without flow-limiting coronary artery disease (cardiac syndrome X). A forest of a variety of trees. Cardiol. J. 2015, 22, 605–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Rocca, D.G.; Pepine, C.J. Some thoughts on the continuing dilemma of angina pectoris. Eur. Heart J. 2014, 35, 1361–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, T.J.; Ong, P.; Sechtem, U.; Beltrame, J.; Camici, P.G.; Crea, F.; Kaski, J.-C.; Merz, C.N.B.; Pepine, C.J.; Shimokawa, H.; et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc. Interv. 2020, 13, 1847–1864. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Sutton, G.; Pugh, D.; Dhaun, N. Developments in the Role of Endothelin-1 in Atherosclerosis: A Potential Therapeutic Target? Am. J. Hypertens. 2019, 32, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Dhaun, N.; Webb, D.J. Endothelins in cardiovascular biology and therapeutics. Nat. Rev. Cardiol. 2019, 16, 491–502. [Google Scholar] [CrossRef]
- Guddeti, R.R.; Prasad, A.; Matsuzawa, Y.; Aoki, T.; Rihal, C.; Holmes, D.; Best, P.J.; Lennon, R.; Lerman, L.O.; Lerman, A. Role of endothelin in microvascular dysfunction following percutaneous coronary intervention for non-ST elevation acute coronary syndromes: A single-centre randomised controlled trial. Open Heart 2016, 3, e000428. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.; Choy, E. Clinical experience of IL-6 blockade in rheumatic diseases—Implications on IL-6 biology and disease pathogenesis. Semin. Immunol. 2014, 26, 97–104. [Google Scholar] [CrossRef]
- Bacchiega, B.C.; Bacchiega, A.B.; Usnayo, M.J.G.; Bedirian, R.; Singh, G.; Pinheiro, G. Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study. J. Am. Heart Assoc. 2017, 6, e005038. [Google Scholar] [CrossRef]
- Su, D.; Li, Z.; Li, X.; Chen, Y.; Zhang, Y.; Ding, D.; Xia, M.; Qiu, J.; Ling, W. Association between serum interleukin-6 concentration and mortality in patients with coronary artery disease. Med. Inflamm. 2013, 2013, 726178. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Laghi-Pasini, F.; Acampa, M.; Srivastava, U.; Bertolozzi, I.; Giabbani, B.; Finizola, F.; Vanni, F.; Dokollari, A.; Natale, M.; et al. Systemic Inflammation Rapidly Induces Reversible Atrial Electrical Remodeling: The Role of Interleukin-6-Mediated Changes in Connexin Expression. J. Am. Heart Assoc. 2019, 8, e011006. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. ESC Scientific Document Group, 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Chen, A.Y.; Peterson, E.D.; Newby, L.K.; Pollack Jr, V.C.; Brindis, R.G.; Gibson, C.M.; Kleiman, N.S.; Saucedo, J.F.; Bhatt, D.L.; et al. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: Results from the Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am. Heart J. 2006, 152, 641–647. [Google Scholar] [PubMed]
- Lanza, G.A.; Crea, F. Acute coronary syndromes without obstructive coronary atherosclerosis: The tiles of a complex puzzle. Circ. Cardiovasc. Interv. 2014, 7, 278–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recio-Mayoral, A.; Rimoldi, O.E.; Camici, P.G.; Kaski, J.C. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc. Imaging 2013, 6, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Kei, A.; Koutsouka, F.; Makri, A.; Elisaf, M. Uric acid and cardiovascular risk: What genes can say. Int. J. Clin. Pract. 2018, 72, e13048. [Google Scholar] [CrossRef]
- Wainstein, M.V.; Mossmann, M.; Araujo, G.N.; Gonçalves, S.C.; Gravina, G.L.; Sangalli, M.; Veadrigo, F.; Matte, R.; Reich, R.; Costa, F.G.; et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate risk overweight patients referred for coronary angiography. Diabetol. Metab. Syndr. 2017, 9, 67. [Google Scholar] [CrossRef]
- Schroder, J.; Mygind, N.D.; Frestad, D.; Michelsen, M.; Suhrs, H.E.; Bove, K.B.; Gustafsson, I.; Kastrup, J.; Prescott, E. Pro-inflammatory biomarkers in women with non-obstructive angina pectoris and coronary microvascular dysfunction. Int. J. Cardiol. Heart Vasc. 2019, 24, 100370. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K. C-reactive protein, inflammation and coronary heart disease. Egypt. Heart J. 2015, 67, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Tong, D.C.; Whitbourn, R.; MacIsaac, A.; Wilson, A.; Burns, A.; Palmer, S.; Layland, J. High-Sensitivity C-Reactive Protein Is a Predictor of Coronary Microvascular Dysfunction in Patients with Ischemic Heart Disease. Front. Cardiovasc. Med. 2018, 4, 81. [Google Scholar] [CrossRef] [Green Version]
- Tomai, F. C reactive protein and microvascular function. Heart 2004, 90, 727–728. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, J.; Jiang, L.; Xu, L.; Liu, J.; Zhao, X.; Feng, X.; Wang, D.; Zhang, Y.; Sun, K.; et al. Prognostic Value of Plasma Big Endothelin-1 Level among Patients with Three-Vessel Disease: A Cohort Study. J. Atheroscler. Thromb. 2019, 26, 959–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, A.J.; Gano, L.B.; Eskurza, I.; Silver, A.E.; Gates, P.E.; Jablonski, K.; Seals, D.R. Vascular endothelial dysfunction with aging: Endothelin-1 and endothelial nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H425–H432. [Google Scholar] [CrossRef] [Green Version]
- Barton, M. Aging and endothelin: Determinants of disease. Life Sci. 2014, 118, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, G.M.; Whooley, M.A.; Glidden, D.V.; Pawlikowska, L.; Zaroff, J.G.; Olgin, J.E. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: Data from the Heart and Soul Study. Am. Heart J. 2008, 155, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Mayyas, F.; Niebauer, M.; Zurick, A.; Barnard, J.; Gillinov, A.M.; Chung, M.K.; Wagoner, D.R.V. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ. Arrhythm. Electrophysiol. 2010, 3, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, S.; Thomas, R.; Shihab, P.; Sriraman, D.; Behbehani, K.; Ahmad, R. Obesity Is a Positive Modulator of IL-6R and IL-6 Expression in the Subcutaneous Adipose Tissue: Significance for Metabolic Inflammation. PLoS ONE 2015, 10, e0133494. [Google Scholar] [CrossRef] [Green Version]
- El-Mikkawy, D.M.E.; EL-Sadek, M.A.; EL-Badawy, M.A.; Samaha, D. Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egypt. Rheumatol. Rehabil. 2020, 47, 7. [Google Scholar] [CrossRef]
- Solarz, D.E.; Mullington, J.M.; Meier-Ewert, H.K. Sleep, Inflammation and cardiovascular disease. Front. Biosci. 2012, 4, 2490–2501. [Google Scholar] [CrossRef]
- Kosacka, M.; Brzecka, A. Endothelin-1 and LOX-1 as Markers of Endothelial Dysfunction in Obstructive Sleep Apnea Patients. Int. J. Environ. Res. Public Health 2021, 18, 1319. [Google Scholar] [CrossRef]
- Shaafi, S.; Sharifipour, E.; Rahmanifar, R.; Hejazi, S.; Andalib, S.; Nikanfar, M.; Baradarn, B.; Mehdizadeh, R. Interleukin-6, a reliable prognostic factor for ischemic stroke. Iran. J. Neurol. 2014, 13, 70–76. [Google Scholar] [PubMed]
- Gunnoo, T.; Hasan, N.; Khan, M.S.; Slark, J.; Bentley, P.; Sharma, P. Quantifying the risk of heart disease following acute ischaemic stroke: A meta-analysis of over 50,000 participants. BMJ Open 2016, 6, e009535. [Google Scholar] [CrossRef] [Green Version]
- Putaala, J.; Nieminen, T. Stroke Risk Period After Acute Myocardial Infarction Revised. J. Am. Heart Assoc. 2018, 7, e011200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koton, S.; Schneider, A.L.C.; Windham, B.G.; Mosley, T.H.; Gottesman, R.F.; Coresh, J. Microvascular Brain Disease Progression and Risk of Stroke: The ARIC Study. Stroke 2020, 51, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.T.; Yan, R.T.; Cushman, M.; Redheuil, A.; Tracy, R.P.; Arnett, D.K.; Rosen, B.D.; McClelland, R.L.; Bluemke, D.A.; Lima, J.A.C. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: Insights from the Multi-Ethnic Study of Atherosclerosis. Eur. Heart J. 2010, 31, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory cytokines in heart failure: Mediators and markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef]



| Parameters | Details | Global | Group 1 Microvascular Disease (Non-Obstructive Coronary Artery Disease) | Group 2 Macrovascular Disease (Obstructive Coronary Artery Disease) | p Value |
|---|---|---|---|---|---|
| Number of patients | 45 (50%) | 45 (50%) | |||
| Age (Mean ± SD) | 69.02 ± 9.34 | 67.37 ± 9.03 | 70.66 ± 9.46 | p = ns | |
| Smoking (%) | Yes | 30 (33.3) | 13 (28.88) | 17 (37.77) | p = ns |
| No | 60 (67.6) | 32 (71.11) | 28 (62.22) | ||
| Symptoms (%-yes) | Typical angina | 54 (60) | 23 (51.11) | 31 (68.88) | p = ns |
| Atypical pain | 19 (21.1%) | 13 (28.88) | 6 (13.33) | p = ns | |
| Palpitations | 37 (41.1%) | 25 (55.55) | 12 (26.66) | p = 0.0101 | |
| Dyspnea | 67 (74.4%) | 33 (73.33) | 34 (75.55) | p = ns | |
| Decreased exercise tolerance | 74 (82.2%) | 35 (77.77) | 39 (86.66) | p = ns | |
| No of macrovascular coronary lesions (%) | One-vessel | 15 (33.3) | - | 15 (33.3) | p = ns |
| Two-vessel | 15 (33.3) | - | 15 (33.3) | ||
| Three-vessel | 15 (33.3) | - | 15 (33.3) | ||
| Total cholesterol (mg/dL) Mean ± SD | 171.9 ±46.55 | 174.24 ±50.18 | 169.55 ± 43.05 | p = ns | |
| LDL cholesterol (mg/dL) Mean ± SD | 101.18 ±42.15 | 105.80 ±43.15 | 96.57 ±41.09 | p = ns | |
| HDL cholesterol (mg/dL) Mean ± SD | 43.46 ±10.48 | 43.60 ± 11.85 | 43.33 ± 9.04 | p = ns | |
| Triglycerides (mg/dL) Mean ± SD | 140.14 ±69.56 (119.5) | 125.55 ± 50.88 (117) | 154.73 ±82.23 (137) | p = ns | |
| Uric acid (mg/dL) Mean ± SD | 6.76 ±2.08 | 6.61 ±1.96 | 6.91 ± 2.2 | p = ns | |
| CRP (mg/dL) Mean ± SD | 1.13 ± 1.26 | 1.24 ± 1.67 | 1.01 ± 0.65 | p = ns | |
| IL-6 * (pg/mL) Mean ± SD | 19.66 ± 55.09 (7.7) | 12.36 ± 16.36 (7.5) | 26.95± 75.9 (8) | p = ns | |
| Log IL-6 Mean ± SD | 0.8528 ± 0.5809 | 0.7760 ± 0.5664 | 0.9297 ± 0.5913 | p = ns | |
| ET-1 * (pmol/L) Mean ± SD | 1.67 ± 0.5 (1.7) | 1.63 ± 0.42 (1.7) | 1.7 ± 0.57 (1.7) | p = ns | |
| Log ET-1 Mean ± SD | 0.20 ± 0.12 () | 0.19 ± 0.11 (0.23) | 0.21 ± 0.13 (0.23) | p = ns | |
| Diabetes mellitus (%) | Yes | 41 (45.6%) | 18 (40) | 23 (51.11) | p = ns |
| No | 49 (54.4%) | 27 (60) | 22 (48.88) | ||
| Obesity (%) | Yes | 64 (71.1%) | 32 (71.11) | 32 (71.11) | p = ns |
| No | 26 (28.9%) | 13 (28.88) | 13 (28.88) | ||
| PPH of ACS (%) | Yes | 33 (36.7%) | 5 (11.11) | 28 (62.22) | p < 0.0001 |
| No | 57 (63.3%) | 40 (88.88) | 17 (37.77) | ||
| STEMI | 15 (45.5%) | 2 (4.44) | 13(28.88) | p < 0.0001 | |
| NSTEMI | 14 (42.4%) | 2(4.44) | 12 (26.66) | ||
| UA | 4 (12.1%) | 1 (2.22) | 3 (6.66) | ||
| Without | 57 (63.3%) | 40 (88.88) | 17 (37.77) | ||
| PAD (%) | Yes | 14 (15.6%) | 5 (11.11) | 9 (20) | p = ns |
| No | 76 (84.4%) | 40 (88.88) | 36 (80) | ||
| CHF/LVF (%) | Yes | 68 (75.6%) | 28 (62.22) | 40(88.88) | p = 0.0070 |
| No | 22 (24.4%) | 17 (37.77) | 5(11.11) | ||
| AFi (%) | Yes | 48 (53.3%) | 30 (66.66) | 18 (40) | p = 0.0201 |
| No | 42 (46.7%) | 15 (33.33) | 27 (60) | ||
| Permanent | 14 (15.6%) | 9 (20) | 5 (11.11) | p = ns | |
| Persistent | 10 (11.1%) | 6 (13.33) | 4 (8.88) | ||
| Paroxysmal | 24 (26.7%) | 15 (33.33) | 9 (20) | ||
| Without | 42 (46.7%) | 15(33.33) | 27(60) | ||
| Ischemic stroke (%) | Yes | 26 (28.9%) | 11 (24.44) | 15 (33.33) | p = ns |
| No | 64 (71.1%) | 34 (75.55) | 30(66.66) | ||
| CKD (%) | Yes | 22 (24.4%) | 9 (25) | 13 (28.88) | p = ns |
| No | 68 (75.6%) | 36 (75) | 32 (71.11) | ||
| Anxiety-depressive disorder (%) | Yes | 34 (37.8%) | 18 (40) | 16 (35.55) | p = ns |
| No | 56 (62.2%) | 27(60) | 29 (64.44) |
| Parameter | Number of Coronary Lesions | p Value | |||
|---|---|---|---|---|---|
| without | One-Vessel | Two-Vessel | Three-Vessel | ||
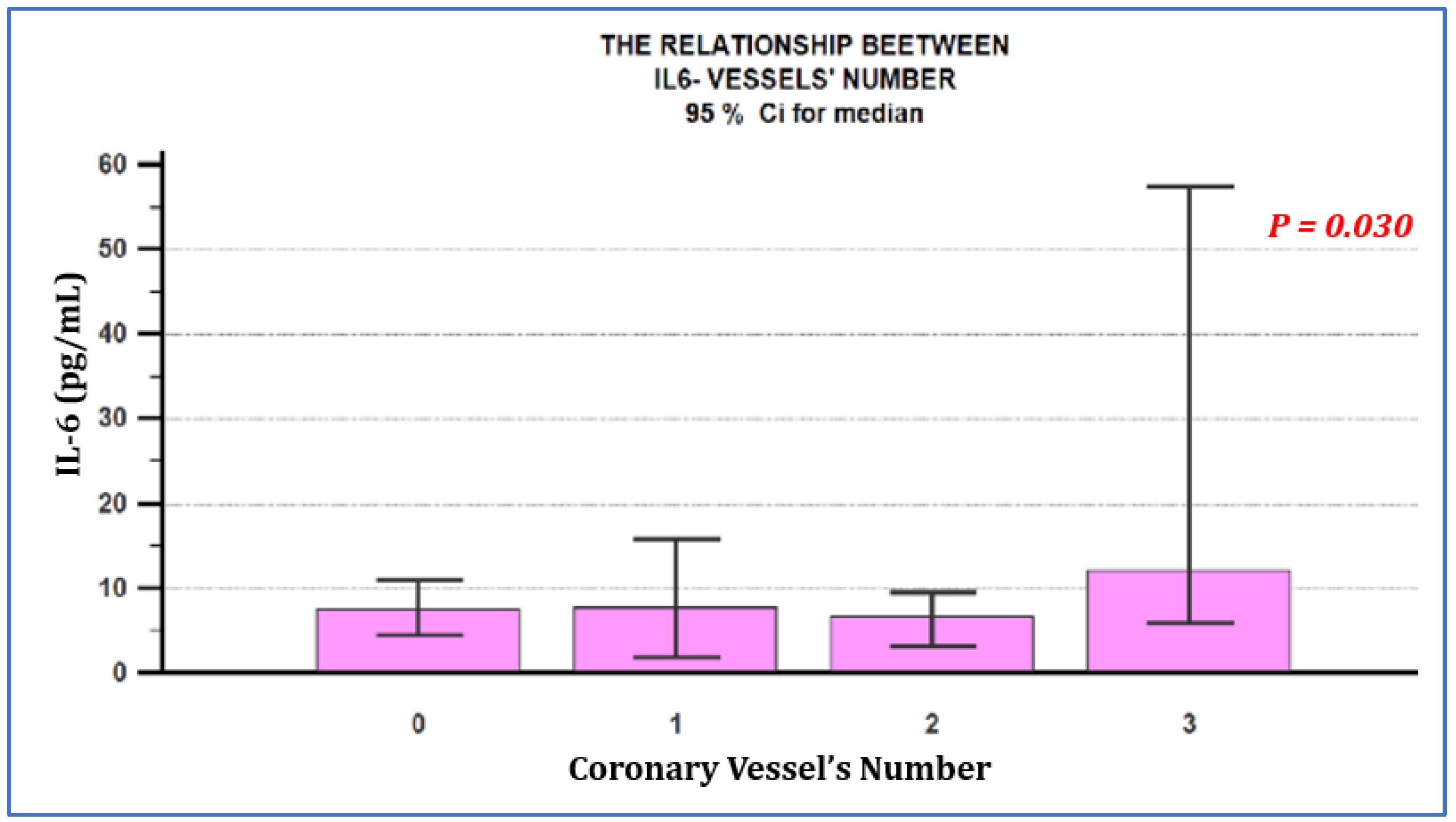
| IL-6 (pg/mL) Mean ± SD | 12.36 ± 16.36 (7.5) | 10.06 ± 10.41 (7.7) | 12.71 ± 19.17 (6.6) | 58.08 ± 126.76 (12.10) | p = 0.030 |
| Log IL-6 Mean ± SD | 0.7760 ± 0.5664 | 0.7752 ± 0.4852 | 0.8335 ±0.4597 | 1.1803 ± 0.7412 | p = 0.018 |
| SD-standard deviation, p value obtained in the Kruskal–Wallis test and Mann–Whitney tests. | |||||
| ET-1 (pmol/L) Mean ± SD | 1.63 ± 0.42 (1.7) | 1.78 ± 0.7 (1.7) | 1.57 ± 0.41 (1.6) | 1.76 ± 0.58 (1.7) | p = 0.7316 |
| Log ET-1 Mean ± SD | 0.19 ± 0.11 (0.23) | 0.22 ± 0.14 (0.23) | 0.18 ± 0.12 (0.20) | 0.22 ± 0.14 (0.23) | p = 0.7316 |
| Global | Group 1—Microvascular Disease | Group 2—Macrovascular Disease | |
|---|---|---|---|
| IL-6–age | Rho 0.283 p = 0.0075 | Rho 0.301 p = 0.0459 | Rho 0.230 p = 0.1277 |
| Mean Age | 67.37 ± 9.03 | 70.66 ± 9.46 | |
| ET-1–age | Rho 0.0963 p = 0.3635 | Rho 0.0827 p = 0.5835 | Rho 0.0944 p = 0.5313 |
| Global | Group 1—Microvascular Disease | Group 2—Macrovascular Disease | |
|---|---|---|---|
| IL-6-LVEF% | Rho −0.186 p = 0.0789 | Rho −0.263 p = 0.0813 | Rho −0.121 p = 0.4225 |
| ET-1-LVEF% | Rho −0.203 p = 0.0561 | Rho −0.440 p = 0.0035 | Rho 0.0261 p = 0.8625 |
| IL-6–diastolic dysfunction | Rho 0.128 p = 0.2255 | Rho 0.0637 p = 0.6728 | Rho 0.197 p = 0.1905 |
| ET-1–diastolic dysfunction | Rho 0.0915 p = 0.3881 | Rho 0.265 p = 0.0783 | Rho −0.123 p = 0.4159 |
| Group 1-Microvascular Disease | Group 2-Macrovascular Disease | |||
|---|---|---|---|---|
| with AFi | without AFi | with AFi | without AFi | |
| IL-6-ET-1 | 30 patients- rho 0.193 p = 0.2980 | 15 patients- rho 0.418 p = 0.1181 | 18 patients- rho 0.161 p = 0.505 | 27 patients- rho 0.147 p = 0.4542 |
| Spearman correlation coefficient (R), and p > 0.05 was considered statistically significant. | ||||
| IL-6 | Log IL-6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Median | Mean | Standard Deviation | Median | ||||
| Diabetes mellitus | yes | 14.61 | 22.94 | 8.00 | p = 0.8174 | 0.8216 | 0.5667 | 0.9031 | p = 0.64 |
| no | 23.88 | 71.77 | 7.50 | 0.8790 | 0.5970 | 0.8751 | |||
| Obesity | yes | 24.51 | 64.66 | 8.10 | p = 0.0353 | 0.9377 | 0.5886 | 0.9085 | p = 0.0288 |
| no | 7.72 | 7.90 | 6.35 | 0.6439 | 0.5140 | 0.8024 | |||
| PAD | yes | 20.82 | 34.63 | 7.55 | p = 0.6047 | 0.7749 | 0.7610 | 0.8779 | p = 0.58 |
| no | 19.45 | 58.26 | 7.75 | 0.8672 | 0.5465 | 0.8893 | |||
| CKD | yes | 14.30 | 22.53 | 7.35 | p = 0.3312 | 0.7832 | 0.5703 | 0.8662 | p = 0.52 |
| no | 21.39 | 62.14 | 8.10 | 0.8754 | 0.5866 | 0.9085 | |||
| Ischemic stroke | yes | 39.69 | 97.12 | 9.55 | p = 0.0497 | 1.0791 | 0.6541 | 0.9793 | p = 0.0176 |
| no | 11.52 | 17.68 | 7.55 | ||||||
| ET-1 | Log ET-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Median | Mean | Standard Deviation | Median | ||||
| Diabetes mellitus | yes | 1.61 | 0.29 | 1.60 | p = 0.3190 | 0.20 | 0.08 | 0.20 | p = 0.31 |
| no | 1.72 | 0.63 | 1.70 | 0.20 | 0.15 | 0.23 | |||
| Obesity | yes | 1.71 | 0.54 | 1.70 | p = 0.5658 | 0.21 | 0.12 | 0.23 | p = 0.56 |
| no | 1.59 | 0.41 | 1.67 | 0.18 | 0.11 | 0.22 | |||
| PAD | yes | 1.85 | 0.53 | 1.75 | p = 0.0994 | 0.25 | 0.11 | 0.24 | p = 0.09 |
| no | 1.64 | 0.50 | 1.60 | 0.19 | 0.12 | 0.20 | |||
| CKD | yes | 1.76 | 0.62 | 1.70 | p = 0.4442 | 0.22 | 0.13 | 0.23 | p = 0.44 |
| no | 1.64 | 0.46 | 1.67 | 0.19 | 0.12 | 0.22 | |||
| Ischemic stroke | yes | 1.71 | 0.55 | 1.70 | p = 0.3319 | 0.21 | 0.14 | 0.23 | p = 0.33 |
| no | 1.66 | 0.49 | 1.60 | 0.20 | 0.11 | 0.20 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurzău, D.; Sitar-Tăut, A.; Caloian, B.; Guşetu, G.; Comşa, H.; Frîngu, F.; Zdrenghea, D.; Pop, D. The Role of IL-6 and ET-1 in the Diagnosis of Coronary MicroVascular Disease in Women. J. Pers. Med. 2021, 11, 965. https://doi.org/10.3390/jpm11100965
Gurzău D, Sitar-Tăut A, Caloian B, Guşetu G, Comşa H, Frîngu F, Zdrenghea D, Pop D. The Role of IL-6 and ET-1 in the Diagnosis of Coronary MicroVascular Disease in Women. Journal of Personalized Medicine. 2021; 11(10):965. https://doi.org/10.3390/jpm11100965
Chicago/Turabian StyleGurzău, Diana, Adela Sitar-Tăut, Bogdan Caloian, Gabriel Guşetu, Horaţiu Comşa, Florina Frîngu, Dumitru Zdrenghea, and Dana Pop. 2021. "The Role of IL-6 and ET-1 in the Diagnosis of Coronary MicroVascular Disease in Women" Journal of Personalized Medicine 11, no. 10: 965. https://doi.org/10.3390/jpm11100965






