Therapeutic Interaction of Apatinib and Chidamide in T-Cell Acute Lymphoblastic Leukemia through Interference with Mitochondria Associated Biogenesis and Intrinsic Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Flow Cytometry Analyses
2.5. Metabolic and Biochemical Assays
2.6. Oxygen Consumption Assay
2.7. Western Blot Analysis
2.8. RNA Isolation and Real-Time Quantitative PCR
2.9. In Vivo Tumor Growth Assay
2.10. Histological Analysis
2.11. Statistical Analysis
3. Results
3.1. The Additive Antileukemia Activity of Apatinib in Combination with Chidamide in T-ALL Cells
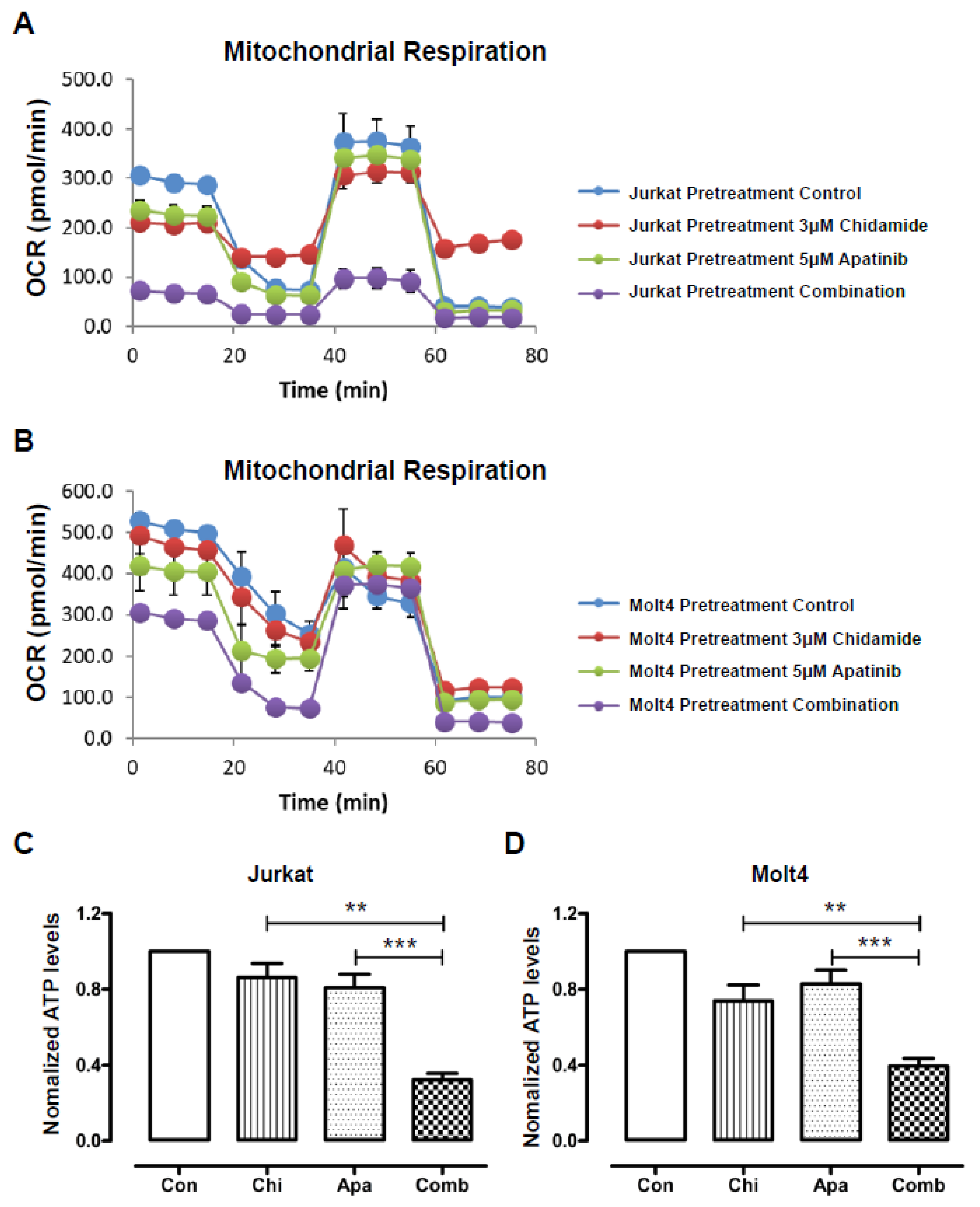
3.2. The Cytotoxic Effects of Apatinib and Chidamide on T-ALL Cells Are Associated with Blockade of Mitochondrial Respiration
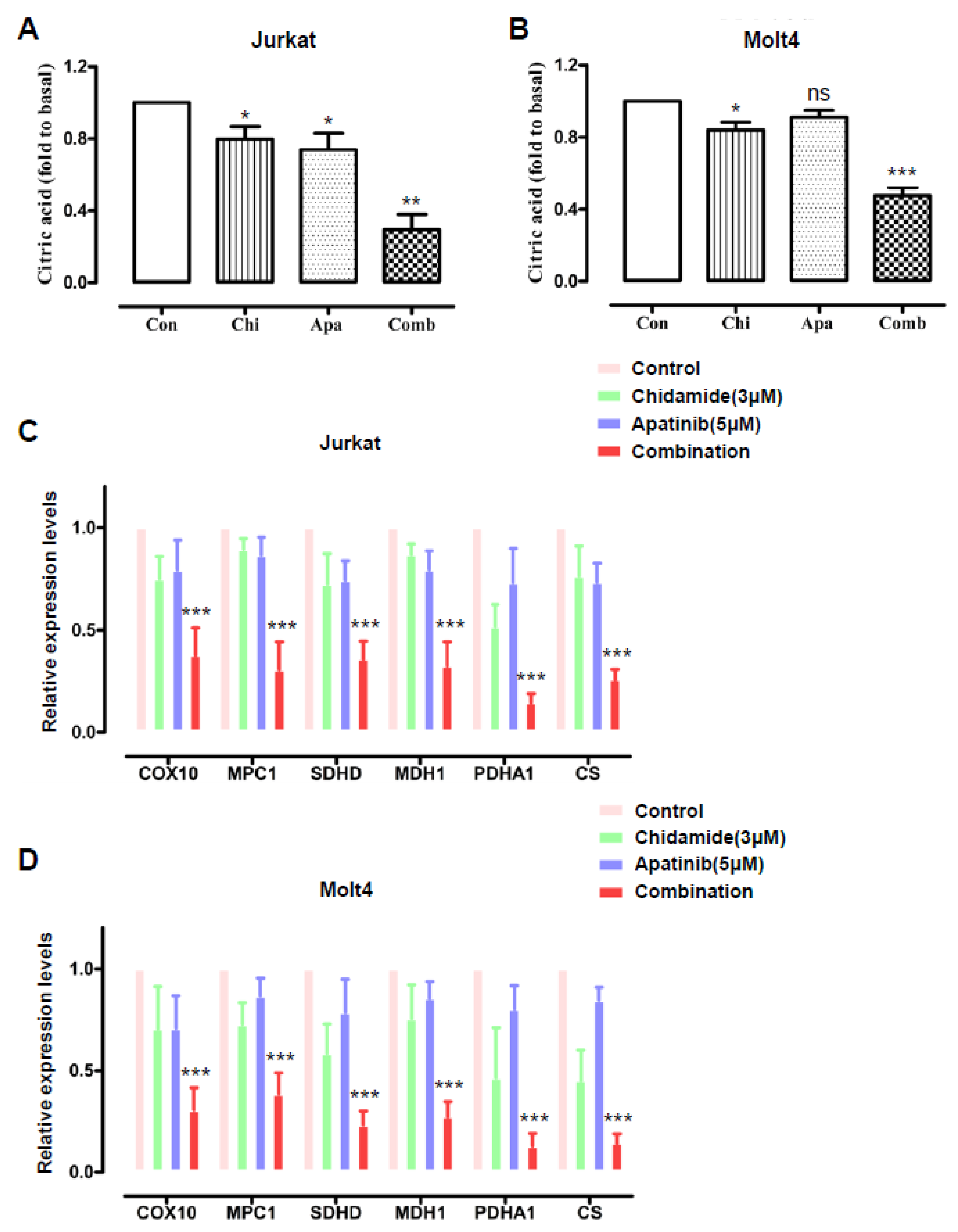
3.3. Cotreatment of Apatinib and Chidamide Interferes with the Citric Acid Cycle
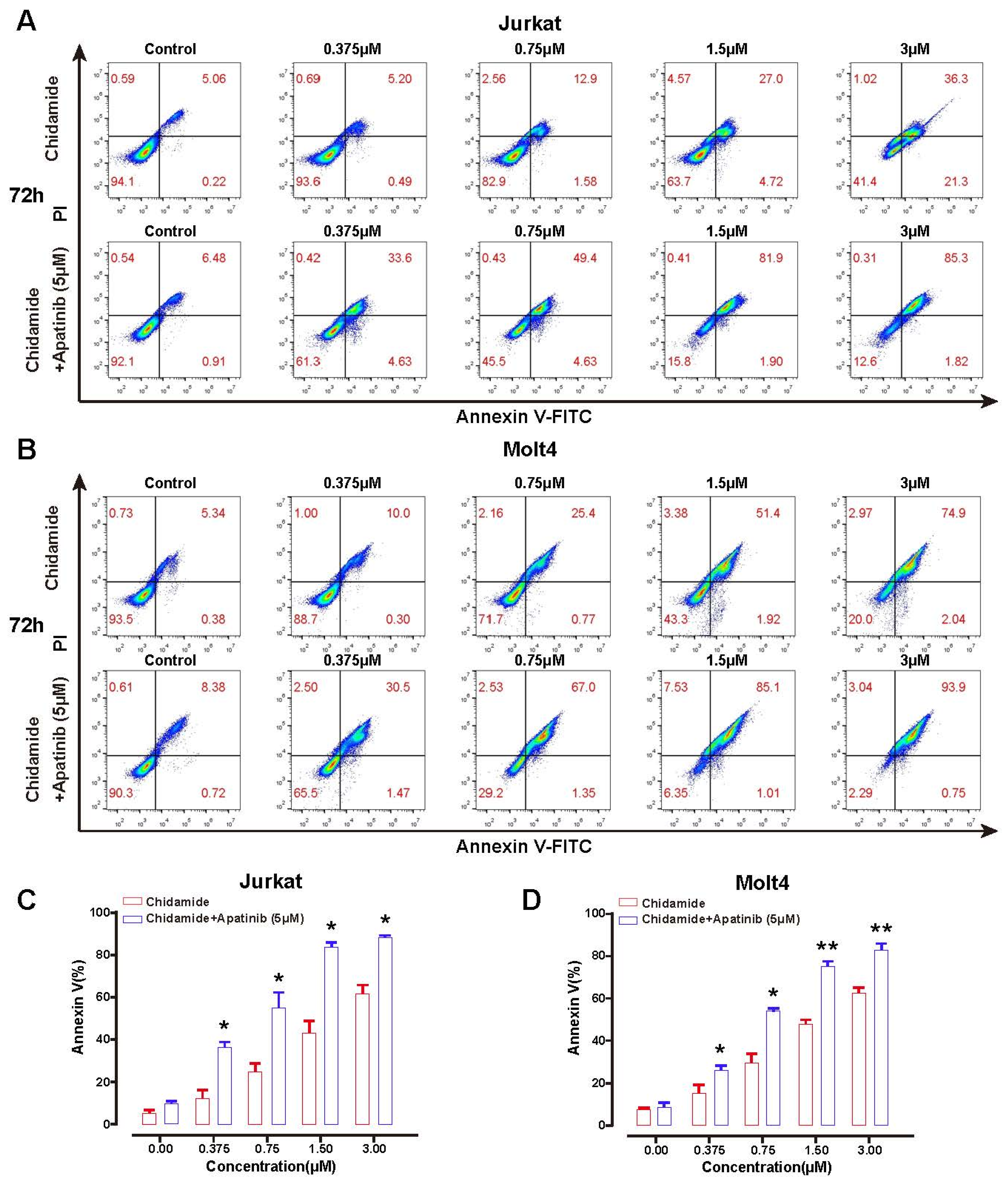
3.4. Apatinib Potentiates Chidamide Induced Apoptosis via Deregulation of Anti- and Pro-Apoptotic BCL-2 Family Components
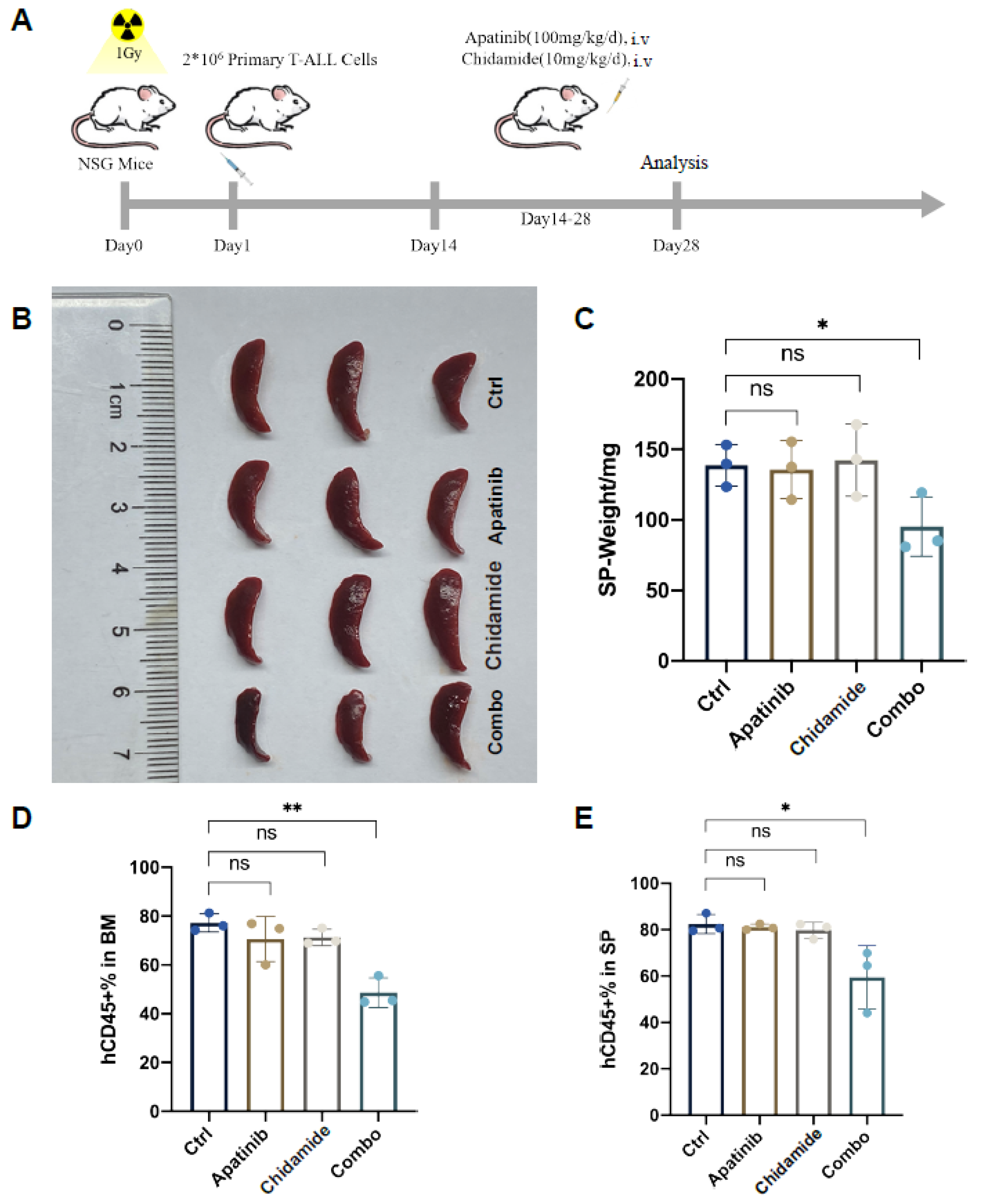
3.5. Apatinib and Chidamide Are Active to Abrogate Leukemic Burden in a T-ALL PDX Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T-ALL | T-cell acute lymphoblastic leukemia |
| B-ALL | B cell acute lymphoblastic leukemia |
| VEGF | Vascular endothelial cell growth factor |
| VEGFR | Vascular endothelial cell growth factor receptor |
| HDACs | Histone deacetylases |
| PTCL | Peripheral T-cell lymphoma |
| CR | Complete response |
| ORR | Overall response rate |
| PFS | Progress-free survival |
| DMSO | Dimethyl sulfoxide |
| OCR | Cellular Oxygen Consumption Rate |
| FCCP | Carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone |
| qRT-PCR | Real-time quantitative PCR |
| OXPHOS | Oxidative phosphorylation |
| CA | Citric acid |
| PDHA1 | Pyruvate dehydrogenase E1 alpha 1 subunit |
| MPC1 | Mitochondrial pyruvate carrier1 |
| CS | Citrate synthase |
| SDHD | Succinate dehydrogenase complex subunit D |
| MDH1 | Malate dehydrogenase1 |
| COX10 | Cytochrome c oxidase 10 |
| XIAP | X-linked inhibitor of apoptosis protein |
| PDX | Patient-derived xenograft |
| VDAC | Voltage-dependent anion channels |
| OMM | Outer mitochondrial membrane |
References
- Hefazi, M.; Litzow, M.R. Recent Advances in the Biology and Treatment of T Cell Acute Lymphoblastic Leukemia. Curr. Hematol. Malig. Rep. 2018, 13, 265–274. [Google Scholar] [CrossRef]
- Dores, G.M.; Devesa, S.S.; Curtis, R.E.; Linet, M.S.; Morton, L.M. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 2012, 119, 34–43. [Google Scholar] [CrossRef]
- Karrman, K.; Johansson, B. Pediatric T-cell acute lymphoblastic leukemia. Genes Chromosom. Cancer 2017, 56, 89–116. [Google Scholar] [CrossRef] [Green Version]
- Fielding, A.K.; Richards, S.M.; Chopra, R.; Lazarus, H.M.; Litzow, M.R.; Buck, G.; Durrant, I.J.; Luger, S.M.; Marks, D.I.; Franklin, I.M.; et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007, 109, 944–950. [Google Scholar] [CrossRef] [Green Version]
- Aguayo, A.; Kantarjian, H.; Manshouri, T.; Gidel, C.; Estey, E.; Thomas, D.; Koller, C.; Estrov, Z.; O’Brien, S.; Keating, M.; et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 2000, 96, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Negaard, H.F.; Iversen, N.; Bowitz-Lothe, I.M.; Sandset, P.M.; Steinsvik, B.; Ostenstad, B.; Iversen, P.O. Increased bone marrow microvascular density in haematological malignancies is associated with differential regulation of angiogenic factors. Leukemia 2009, 23, 162–169. [Google Scholar] [CrossRef] [PubMed]
- De Raeve, H.; Van Marck, E.; Van Camp, B.; Vanderkerken, K. Angiogenesis and the role of bone marrow endothelial cells in haematological malignancies. Histol. Histopathol. 2004, 19, 935–950. [Google Scholar] [CrossRef]
- Markovic, A.; MacKenzie, K.L.; Lock, R.B. Induction of vascular endothelial growth factor secretion by childhood acute lymphoblastic leukemia cells via the FLT-3 signaling pathway. Mol. Cancer Ther. 2012, 11, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munch, V.; Trentin, L.; Herzig, J.; Demir, S.; Seyfried, F.; Kraus, J.M.; Kestler, H.A.; Kohler, R.; Barth, T.F.E.; Te Kronnie, G.; et al. Central nervous system involvement in acute lymphoblastic leukemia is mediated by vascular endothelial growth factor. Blood 2017, 130, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Ravelli, A.; Polom, K.; Petrioli, R.; Marano, L.; Marrelli, D.; Roviello, F.; Generali, D. Apatinib: A novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett. 2016, 372, 187–191. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Xu, B.; Jiang, Z.; Ragaz, J.; Tong, Z.; Zhang, Q.; Wang, X.; Feng, J.; Pang, D.; et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int. J. Cancer 2014, 135, 1961–1969. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, J.; Guo, W.; Xiong, J.; Bai, Y.; Sun, G.; Yang, Y.; Wang, L.; Xu, N.; et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: Results from a randomized, placebo-controlled, parallel-arm, phase II trial. J. Clin. Oncol 2013, 31, 3219–3225. [Google Scholar] [CrossRef] [Green Version]
- Roviello, G.; Ravelli, A.; Fiaschi, A.I.; Cappelletti, M.R.; Gobbi, A.; Senti, C.; Zanotti, L.; Polom, K.; Reynolds, A.R.; Fox, S.B.; et al. Apatinib for the treatment of gastric cancer. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, Y.; Jia, R.; Yue, C.; Chang, L.; Liu, R.; Zhang, G.; Zhao, C.; Zhang, Y.; Chen, C.; et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin. Cancer Res. 2019, 25, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhu, T.; Cao, B.; Wang, J.; Liang, L. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur. J. Cancer 2017, 84, 184–192. [Google Scholar] [CrossRef]
- Deng, M.; Zha, J.; Jiang, Z.; Jia, X.; Shi, Y.; Li, P.; Chen, X.L.; Fang, Z.; Du, Z.; Xu, B. Apatinib exhibits anti-leukemia activity in preclinical models of acute lymphoblastic leukemia. J. Transl. Med. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- San Jose-Eneriz, E.; Agirre, X.; Rodriguez-Otero, P.; Prosper, F. Epigenetic regulation of cell signaling pathways in acute lymphoblastic leukemia. Epigenomics 2013, 5, 525–538. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Downing, J.R. Genome-wide profiling of genetic alterations in acute lymphoblastic leukemia: Recent insights and future directions. Leukemia 2009, 23, 1209–1218. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.J.; Seto, E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhong, J.F.; Stucky, A.; Chen, X.L.; Press, M.F.; Zhang, X. Histone acetylation: Novel target for the treatment of acute lymphoblastic leukemia. Clin. Epigenet. 2015, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkenberg, K.J.; Johnstone, R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014, 13, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Glozak, M.A.; Seto, E. Histone deacetylases and cancer. Oncogene 2007, 26, 5420–5432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, B.; Jin, J.; Xiang, R.; Liu, M.; Yang, L.; Tong, Y.; Xiao, X.; Lei, H.; Liu, W.; Xu, H.; et al. Vorinostat and quinacrine have synergistic effects in T-cell acute lymphoblastic leukemia through reactive oxygen species increase and mitophagy inhibition. Cell Death Dis. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Z.; Liu, J. Role of HDACs in normal and malignant hematopoiesis. Mol. Cancer 2020, 19, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Zhang, L.; Chen, K.; Jiang, Z.; Deng, M.; Zha, J.; Guo, X.; Li, P.; Xu, B. Low-dose decitabine enhances chidamide-induced apoptosis in adult acute lymphoblast leukemia, especially for p16-deleted patients through DNA damage. Pharmacogenomics 2017, 18, 1259–1270. [Google Scholar] [CrossRef]
- Ning, Z.Q.; Li, Z.B.; Newman, M.J.; Shan, S.; Wang, X.H.; Pan, D.S.; Zhang, J.; Dong, M.; Du, X.; Lu, X.P. Chidamide (CS055/HBI-8000): A new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother. Pharmacol. 2012, 69, 901–909. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Zang, J.; Xu, W.; Zhang, Y. Preclinical and Clinical Studies of Chidamide (CS055/HBI-8000), An Orally Available Subtype-selective HDAC Inhibitor for Cancer Therapy. Anticancer Agents Med. Chem. 2017, 17, 802–812. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, M.; Hong, X.; Zhang, W.; Feng, J.; Zhu, J.; Yu, L.; Ke, X.; Huang, H.; Shen, Z.; et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann. Oncol. 2015, 26, 1766–1771. [Google Scholar] [CrossRef]
- Chan, T.S.; Tse, E.; Kwong, Y.L. Chidamide in the treatment of peripheral T-cell lymphoma. Onco Targets Ther. 2017, 10, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.; Jing, Y.; Dou, L.; Wang, M.; Xiao, Y.; Yu, L. Chidamide in combination with chemotherapy in refractory and relapsed T lymphoblastic lymphoma/leukemia. Leuk. Lymphoma 2020, 61, 855–861. [Google Scholar] [CrossRef]
- Chi, Z.; Gao, H.; Liu, H.; Wu, B.; Zhang, B.; Gu, M.; Yang, W. Chidamide induces necroptosis via regulation of cFLIPL expression in Jurkat and HUT78 cells. Mol. Med. Rep. 2020, 21, 936–944. [Google Scholar] [CrossRef]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Yi, Q.; Xu, B.; Li, S.; Wang, T.; Liu, F.; Zhu, B.; Hoffmann, P.R.; Ji, G.; Lei, P.; et al. ORP4L is essential for T-cell acute lymphoblastic leukemia cell survival. Nat. Commun. 2016, 7, 12702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes-Nesi, A.; Araujo, W.L.; Obata, T.; Fernie, A.R. Regulation of the mitochondrial tricarboxylic acid cycle. Curr. Opin. Plant. Biol. 2013, 16, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastre, J.; Pallardo, F.V.; Vina, J. Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life 2000, 49, 427–435. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Lopez, J.; Tait, S.W. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Li, J.; Xie, Q.; Diao, L.; Gai, L.; Yang, W. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: An observational study. Cancer Biol. Ther. 2018, 19, 198–204. [Google Scholar] [CrossRef]
- Kuwana, T.; Mackey, M.R.; Perkins, G.; Ellisman, M.H.; Latterich, M.; Schneiter, R.; Green, D.R.; Newmeyer, D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 2002, 111, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tozzi, F.; Chen, J.; Fan, F.; Xia, L.; Wang, J.; Gao, G.; Zhang, A.; Xia, X.; Brasher, H.; et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012, 72, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Nobrega-Pereira, S.; Caiado, F.; Carvalho, T.; Matias, I.; Graca, G.; Goncalves, L.G.; Silva-Santos, B.; Norell, H.; Dias, S. VEGFR2-Mediated Reprogramming of Mitochondrial Metabolism Regulates the Sensitivity of Acute Myeloid Leukemia to Chemotherapy. Cancer Res. 2018, 78, 731–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Qiao, Z.; Wang, Y.; Kuai, Q.; Li, C.; Wang, Y.; Jiang, X.; Wang, X.; Li, W.; He, M.; et al. Chidamide Inhibits Aerobic Metabolism to Induce Pancreatic Cancer Cell Growth Arrest by Promoting Mcl-1 Degradation. PLoS ONE 2016, 11, e0166896. [Google Scholar] [CrossRef]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Li, J.; Guo, Z.; Sun, L.; Juan, C.; Zhou, Y.; Gu, H.; Yu, Y.; Hu, Q.; Kan, Q.; et al. Overexpression of Pyruvate Dehydrogenase E1alpha Subunit Inhibits Warburg Effect and Induces Cell Apoptosis Through Mitochondria-Mediated Pathway in Hepatocellular Carcinoma. Oncol. Res. 2019, 27, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Schormann, N.; Hayden, K.L.; Lee, P.; Banerjee, S.; Chattopadhyay, D. An overview of structure, function, and regulation of pyruvate kinases. Protein Sci. 2019, 28, 1771–1784. [Google Scholar] [CrossRef]
- Cai, Z.; Li, C.F.; Han, F.; Liu, C.; Zhang, A.; Hsu, C.C.; Peng, D.; Zhang, X.; Jin, G.; Rezaeian, A.H.; et al. Phosphorylation of PDHA by AMPK Drives TCA Cycle to Promote Cancer Metastasis. Mol. Cell 2020, 80, 263–278.e7. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Ban, H.S.; Jang, K.; Kim, I.; Xu, X.; Harmalkar, D.; Shin, S.A.; Kim, M.; Kim, B.K.; Park, J.; et al. Methyl 3-(3-(4-(2,4,4-Trimethylpentan-2-yl)phenoxy)-propanamido)benzoate as a Novel and Dual Malate Dehydrogenase (MDH) 1/2 Inhibitor Targeting Cancer Metabolism. J. Med. Chem. 2017, 60, 8631–8646. [Google Scholar] [CrossRef]
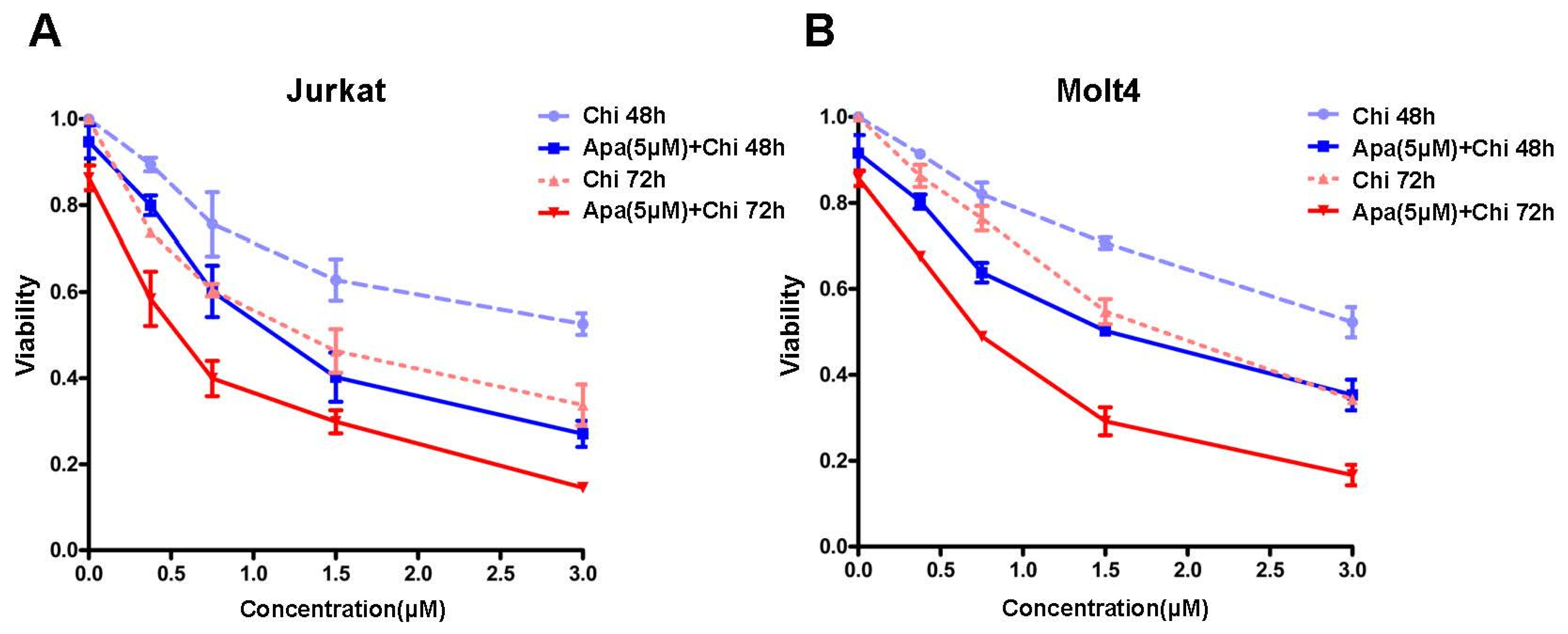



| Cell Lines | IC50 (μM) of 48 h | Fold | p Value | IC50 (μM) of 72 h | Fold | p Value | ||
|---|---|---|---|---|---|---|---|---|
| Single | Combination | Single | Combination | |||||
| Jurkat | 3.22 ± 1.12 | 1.17 ± 0.28 | 2.75 | 0.037 | 1.34 ± 0.36 | 0.53 ± 0.22 | 2.52 | 0.031 |
| Molt4 | 3.92 ± 1.28 | 1.52 ± 0.26 | 2.57 | 0.034 | 1.72 ± 0.08 | 0.73 ± 0.03 | 2.35 | 0.001 |
| Treatment Timepoints | Chidamide (μM) | Apoptosis Rate (%) | p Value | |
|---|---|---|---|---|
| Chidamide | Apatinib (5 μM) + Chidamide | |||
| 48 h | 0 | 4.63 ± 1.22 | 10.16 ± 4.17 | 0.1396 |
| 0.375 | 9.34 ± 3.17 | 25.66 ± 8.97 | 0.0743 | |
| 0.75 | 16.03 ± 7.02 | 33.99 ± 9.23 | 0.0592 | |
| 1.5 | 21.00 ± 7.13 | 46.68 ± 7.91 | 0.0142 | |
| 3 | 30.50 ± 4.79 | 64.53 ± 5.47 | 0.0013 | |
| 72 h | 0 | 5.28 ± 1.93 | 9.75 ± 1.92 | 0.0566 |
| 0.375 | 12.60 ± 6.88 | 36.66 ± 4.50 | 0.0104 | |
| 0.75 | 24.78 ± 7.27 | 55.52 ± 12.14 | 0.0282 | |
| 1.5 | 43.22 ± 10.52 | 84.34 ± 3.73 | 0.0132 | |
| 3 | 62.00 ± 6.80 | 88.24 ± 1.70 | 0.0169 | |
| Treatment Timepoints | Chidamide (μM) | Apoptosis Rate (%) | p Value | |
|---|---|---|---|---|
| Chidamide | Apatinib (5 μM) + Chidamide | |||
| 48 h | 0 | 4.69 ± 0.84 | 7.33 ± 0.61 | 0.0544 |
| 0.375 | 8.40 ± 2.05 | 17.92 ± 2.01 | 0.0345 | |
| 0.75 | 14.12 ± 2.33 | 28.50 ± 6.93 | 0.0372 | |
| 1.5 | 24.18 ± 3.33 | 45.02 ± 10.20 | 0.0397 | |
| 3 | 38.81 ± 2.60 | 59.34 ± 7.86 | 0.031 | |
| 72 h | 0 | 7.74 ± 0.96 | 8.85 ± 2.21 | 0.4913 |
| 0.375 | 15.78 ± 6.09 | 25.86 ± 3.59 | 0.0435 | |
| 0.75 | 29.34 ± 5.89 | 53.95 ± 1.75 | 0.0129 | |
| 1.5 | 48.05 ± 2.93 | 74.82 ± 4.51 | 0.0019 | |
| 3 | 62.09 ± 3.82 | 82.33 ± 4.58 | 0.0046 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, M.; Lin, F.; Jiang, Y.; Pan, G.; Tan, J.; Zhou, H.; Lai, Q.; Chen, Q.; Deng, M.; Zha, J.; et al. Therapeutic Interaction of Apatinib and Chidamide in T-Cell Acute Lymphoblastic Leukemia through Interference with Mitochondria Associated Biogenesis and Intrinsic Apoptosis. J. Pers. Med. 2021, 11, 977. https://doi.org/10.3390/jpm11100977
Zhong M, Lin F, Jiang Y, Pan G, Tan J, Zhou H, Lai Q, Chen Q, Deng M, Zha J, et al. Therapeutic Interaction of Apatinib and Chidamide in T-Cell Acute Lymphoblastic Leukemia through Interference with Mitochondria Associated Biogenesis and Intrinsic Apoptosis. Journal of Personalized Medicine. 2021; 11(10):977. https://doi.org/10.3390/jpm11100977
Chicago/Turabian StyleZhong, Mengya, Fusheng Lin, Yuelong Jiang, Guangchao Pan, Jinshui Tan, Hui Zhou, Qian Lai, Qinwei Chen, Manman Deng, Jie Zha, and et al. 2021. "Therapeutic Interaction of Apatinib and Chidamide in T-Cell Acute Lymphoblastic Leukemia through Interference with Mitochondria Associated Biogenesis and Intrinsic Apoptosis" Journal of Personalized Medicine 11, no. 10: 977. https://doi.org/10.3390/jpm11100977
APA StyleZhong, M., Lin, F., Jiang, Y., Pan, G., Tan, J., Zhou, H., Lai, Q., Chen, Q., Deng, M., Zha, J., & Xu, B. (2021). Therapeutic Interaction of Apatinib and Chidamide in T-Cell Acute Lymphoblastic Leukemia through Interference with Mitochondria Associated Biogenesis and Intrinsic Apoptosis. Journal of Personalized Medicine, 11(10), 977. https://doi.org/10.3390/jpm11100977







