Soft-Tissue Simulation for Computational Planning of Orthognathic Surgery
Abstract
:1. Introduction
1.1. Contributions
1.2. Background
1.2.1. Classification of Previous Work
1.2.2. Simulation Meshes
1.2.3. Soft-Tissue Model
1.2.4. Performance and Validation
2. Materials and Methods
2.1. Mathematical Modeling of Soft Tissue
2.2. Mathematical Modeling of Bones
2.3. Mathematical Modeling of Boundary Conditions
2.3.1. Sliding Contact
2.3.2. Tissue Fixing and Coupling
2.3.3. Smooth Coupling at Bone Cuts
2.4. Constrained Optimization Problem
2.5. Preparation of Simulation Meshes and Couplings
2.5.1. Bones
2.5.2. Soft Tissue
2.6. Textured Output Visualization
3. Results
3.1. Validation Methodology
3.2. Test Cases
- In maxillary procedures, the maxilla is separated from the skull through a Lefort osteotomy, classified based on its anatomical level. In this cohort, the distribution of cases is: 8 Lefort I cases and 1 Lefort II case; one patient did not undergo maxillary surgery. Moreover, after a Lefort I osteotomy, the maxilla may be segmented (typically into three fragments) in order to expand the upper arch. Maxilla segmentation was applied to 6 patients in this cohort.
- In mandibular procedures, the mandible may be sagittally split on both rami (bilateral sagittal split osteotomy, BSSO) or only one ramus (unilateral sagittal split osteotomy, USSO). In this cohort, the distribution of cases is: 7 BSSO cases, 1 USSO case; two patients did not undergo mandibular surgery. Additionally, a chin osteotomy or genioplasty may be also performed. Genioplasty was applied to 1 patient in this cohort.
3.3. Simulation Error and Performance
4. Discussion
4.1. Analysis of Simulation Accuracy
- Chin. Overall, the amount of error at the chin area is very low. This could be explained by the fact that the skin at the chin is very thin, and the coupling to the mandible makes the simulation highly predictive.
- Lips. In other regions, such as the lips, skin slides strongly over the underlying bones and teeth, and the deformation result is more difficult to predict. Overall, we observe higher variability in the error at the lips, and also some patients with higher error.
- Nose. The quality of the prediction of the deformation of the nose varies strongly across patients. In this case, the variability may depend on the type of surgery performed on each patient’s anterior nasal spine. This type of surgery is not easy to identify in the post-operative CBCT image due to the presence of bone grafts or fixation plates.
- Neck. Finally, we observe large error in the neck area (e.g., patients M5 and M8), and specifically at the junction point between the submental area and the neck (“C point” or “cervical point” in cephalometric analysis). This error was accounted for in our quantitative analysis, which negatively biased the overall results. However, this area is not of special interest to orthognathic surgeons. The deformation is known to be produced by a retraction of skin after surgery, but surgeons do not account for this effect during pre-operative planning.
4.2. Analysis of Clinical Cases and Patients
- Ethnicity. The predicted deformation of the central area of the face is visually more accurate for the Latin American patients M3 and M8) than for Caucasian patients (rest of patients). As discussed with collaborating surgeons, this may be due to stiffer soft tissue in the case of patients of Latin American ethnicity, which deforms in a more predictable way when bones are displaced, compared to Caucasian patients. However, the group of Latin American patients in the study is very small, and such ethnicity differences could be analyzed in a more thorough study.
- Diagnosis. Patients with Class II diagnosis exhibit distinct results with respect to the rest. In these patients (M5 and M6), the simulation result shows error in the deformation of the lower lip. Initially everted lips, such as those of these patients, do not reach the full deformation visible in the post-operative scans, where they appear in front of the teeth, but instead remain slightly everted. This simulation error may be caused by a lip stretching effect that is not correctly captured by the simulation model, and remains as one of the items to be improved in the future. Patients with Class III, asymmetry and open bite diagnoses do not exhibit any common error pattern within their groups.
- Lefort type. There appears to be a correlation between the type of Lefort osteotomy and the amount of error in the deformation of the nose. Specifically, the deformation of the nose is correctly predicted in the case of Lefort II osteotomy (patient M3), but it appears less predictable for patients with Lefort I osteotomy. This is probably due to the uncertainty of the intervention carried out on anterior nasal spine, as discussed earlier. Obviously, if a Lefort osteotomy is not performed (patient M9), there is no deformation and the prediction is correct.
- Segmentation of the maxilla and mandible. For all patients, the highest error (except for the neck, which is not clinically relevant as discussed above) appears near the cut areas, both of the maxilla (e.g., patients M5 and M7) and the mandible (e.g., patients M1 and M3). This is probably due to the presence of fixation plates and/or bone grafts in the real result (e.g., patient M10, whose maxilla was not segmented, but where the presence of bone graft has been confirmed by the surgeon who carried out the intervention). As a consequence, patients with a segmented maxilla and/or mandible show in general larger error than those without segmented bones. However, the smooth coupling method proposed in Section 2.3.3 reduces considerably the error in cut areas, as shown in Figure 2.
- Genioplasty. Error in the chin area appears low for patients who did not undergo genioplasty, but also for those who did (patient M4), as already mentioned. The analysis of genioplasty could be extended to a larger cohort.
4.3. Comparison of Fine and Coarse Meshes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Murphy, C.; Kearns, G.; Sleeman, D.; Cronin, M.; Allen, P.F. The clinical relevance of orthognathic surgery on quality of life. Int. J. Oral Maxillofac. Surg. 2011, 40, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Juggins, K.J.; Nixon, F.; Cunningham, S.J. Patient- and clinician-perceived need for orthognathic surgery. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Wolford, L.M.; Goncalves, J.R. Surgical Planning in Orthognathic Surgery and Outcome Stability, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1048–1126. [Google Scholar]
- Buchbinder, D. Esthetics and Oral and Maxillofacial Surgery, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 405–422. [Google Scholar]
- Swennen, G.R.; Mollemans, W.; Schutyser, F. Three-Dimensional Treatment Planning of Orthognathic Surgery in the Era of Virtual Imaging. J. Oral Maxillofac. Surg. 2009, 67, 2080–2092. [Google Scholar] [CrossRef]
- Mollemans, W.; Schutyser, F.; Nadjmi, N.; Maes, F.; Suetens, P. Predicting soft tissue deformations for a maxillofacial surgery planning system: From computational strategies to a complete clinical validation. Med. Image Anal. 2007, 11, 282–301. [Google Scholar] [CrossRef]
- Olivetti, E.C.; Nicotera, S.; Marcolin, F.; Vezzetti, E.; Jacqueline, J.P.; Zavattero, E.; Ramieri, G. 3D Soft-tissue prediction methodologies for orthognathic surgery-a literature review. Appl. Sci. 2019, 9, 4550. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Ho, D.C.Y.; Mai, H.; Zhang, X.; Shen, S.G.; Shen, S.; Yuan, P.; Liu, S.; Zhang, G.; Zhou, X.; et al. A clinically validated prediction method for facial soft-tissue changes following double-jaw surgery. Med. Phys. 2017, 44, 4252–4261. [Google Scholar] [CrossRef]
- Lutz, J.C.; Hostettler, A.; Agnus, V.; Nicolau, S.; George, D.; Soler, L.; Rémond, Y. A New Software Suite in Orthognathic Surgery: Patient Specific Modeling, Simulation and Navigation. Surg. Innov. 2019, 26, 5–20. [Google Scholar] [CrossRef]
- Marchetti, C.; Bianchi, A.; Bassi, M.; Gori, R.; Lamberti, C.; Sarti, A. Mathematical modeling and numerical simulation in maxillofacial virtual surgery. J. Craniofacial Surg. 2007, 18, 826–832. [Google Scholar] [CrossRef]
- Kim, H.; Jürgens, P.; Weber, S.; Nolte, L.P.; Reyes, M. A new soft-tissue simulation strategy for cranio-maxillofacial surgery using facial muscle template model. Prog. Biophys. Mol. Biol. 2010, 103, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Chabanas, M.; Luboz, V.; Payan, Y. Patient specific Finite Element model of the face soft tissue for computer-assisted maxillofacial surgery. Med. Image Anal. 2003, 7, 131–151. [Google Scholar] [CrossRef]
- Zachow, S.; Hierl, T.; Erdmann, B. A Quantitative Evaluation of 3D Soft Tissue Prediction in Maxillofacial Surgery Planning. In Proceedings of the 3 Jahrestagung der Deautschen Gesellschaft fur Computer und Robot-Assistierte Chirurgie, Munchen, Germany, 8–9 October 2004; pp. 75–79. [Google Scholar]
- Knoops, P.G.; Borghi, A.; Ruggiero, F.; Badiali, G.; Bianchi, A.; Marchetti, C.; Rodriguez-Florez, N.; Breakey, R.W.; Jeelani, O.; Dunaway, D.J.; et al. A novel soft tissue prediction methodology for orthognathic surgery based on probabilistic finite element modelling. PLoS ONE 2018, 13, e0197209. [Google Scholar]
- Bobek, S.; Farrell, B.; Choi, C.; Farrell, B.; Weimer, K.; Tucker, M. Virtual surgical planning for orthognathic surgery using digital data transfer and an intraoral fiducial marker: The charlotte method. J. Oral Maxillofac. Surg. 2015, 73, 1143–1158. [Google Scholar] [CrossRef]
- Lee, Y.S.; Suh, H.Y.; Lee, S.J.; Donatelli, R.E. A more accurate soft-tissue prediction model for class III 2-jaw surgeries. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 724–733. [Google Scholar] [CrossRef]
- Xia, J.J.; Gateno, J.; Teichgraeber, J.F.; Yuan, P.; Li, J.; Zhang, X.; Alfi, D.M. HHS Public Access. Int. J. Oral Maxillofac. Surg. 2016, 44, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Zachow, S. Computational Planning in Facial Surgery. Facial Plast. Surg. 2015, 31, 446–462. [Google Scholar] [CrossRef] [Green Version]
- Koch, R.M.; Gross, M.H.; Carls, F.R.; von Büren, D.F.; Fankhauser, G.; Parish, Y.I.H. Simulating Facial Surgery Using Finite Element Models. In Proceedings of the 23rd Annual Conference on Computer Graphics and Interactive Techniques; Association for Computing Machinery: New York, NY, USA, 1996; pp. 421–428. [Google Scholar]
- Keeve, E.; Girod, S.; Pfeifle, P.; Girod, B. Anatomy-Based Facial Tissue Modeling Using the Finite Element Method. In Proceedings of the Seventh Annual IEEE Visualization ’96, San Francisco, CA, USA, 27 October–1 November 1996. [Google Scholar]
- Gladilin, E.; Zachow, S.; Deuflhard, P.; Hege, H.C. A biomechanical model for soft tissue simulation in craniofacial surgery. In Proceedings of the International Workshop on Medical Imaging and Augmented Reality, MIAR 2001, Washington, DC, USA, 10–12 June 2001; pp. 137–141. [Google Scholar]
- Bian, J.; Chen, J.; Sun, M. Simulation of soft tissue deformation in virtual surgery based on physics engine. In Proceedings of the 3rd International Conference on Multimedia Information Networking and Security, MINES 2011, Shanghai, China, 4–6 November 2011; pp. 60–64. [Google Scholar]
- Wang, S.; Yang, J. Efficient collision detection for soft tissue simulation in a surgical planning system. In Proceedings of the 2009 11th IEEE International Conference on Computer-Aided Design and Computer Graphics, CAD/Graphics 2009, Huangshan, China, 19–21 August 2009; pp. 49–53. [Google Scholar]
- Xia, J.; Qi, F.; Yuan, W.; Wang, D.; Qiu, W.; Sun, Y.; Huang, Y.; Shen, G.; Wu, H. Computer aided simulation system for orthognathic surgery. In Proceedings of the IEEE Symposium on Computer-Based Medical Systems, Lubbock, TX, USA, 9–10 June 1995; pp. 237–244. [Google Scholar]
- Ip, H.H.; Kot, C.S.; Xia, J. Simulated patient for orthognathic surgery. In Proceedings of the Computer Graphics International Conference, CGI, Geneva, Switzerland, 19–24 June 2000. pp. 239–245.
- Cevidanes, L.H.; Tucker, S.; Styner, M.; Kim, H.; Chapuis, J.; Reyes, M.; Proffit, W.; Turvey, T.; Jaskolka, M. Three-dimensional surgical simulation. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xiao, R.; He, Z. Real-time deformations simulation of soft tissue by combining mass-spring model with pressure based method. In Proceedings of the 2011 3rd International Conference on Advanced Computer Control, Harbin, China, 18–20 January 2011; pp. 506–510. [Google Scholar]
- Grauer, D.; Cevidanes, L.S.; Proffit, W.R. Working with DICOM craniofacial images. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 460–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchetti, C.; Bianchi, A.; Muyldermans, L.; Di Martino, M.; Lancellotti, L.; Sarti, A. Validation of new soft tissue software in orthognathic surgery planning. Int. J. Oral Maxillofac. Surg. 2011, 40, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Turner, P.J.; Khambay, B.S. Accuracy of three-dimensional soft tissue predictions in orthognathic surgery after le Fort i advancement osteotomies. Br. J. Oral Maxillofac. Surg. 2015, 53, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kuang, T.; Rodrigues, Y.L.; Gateno, J.; Shen, S.G.; Wang, X.; Deng, H.; Yuan, P.; Alfi, D.M.; Liebschner, M.A.; et al. A New Approach of Predicting Facial Changes Following Orthognathic Surgery Using Realistic Lip Sliding Effect. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: New York, NY, USA, 2019; pp. 336–344. [Google Scholar]
- El-Molla, M.M.; El-Beialy, A.R.; Kandil, A.H.; El-Bialy, A.M.; Mostafa, Y.A. Three Dimensional approach for realistic simulation of facial soft tissue response: A pilot study. Prog. Orthod. 2011, 12, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Si, H. TetGen, a delaunay-based quality tetrahedral mesh generator. ACM Trans. Math. Softw. 2015, 41, 1–36. [Google Scholar] [CrossRef]
- Shahim, K.; Jürgens, P.; Cattin, P.C.; Nolte, L.P.; Reyes, M. Prediction of cranio-maxillofacial surgical planning using an inverse soft tissue modelling approach. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: New York, NY, USA, 2013; pp. 18–25. [Google Scholar]
- Chabanas, M.; Payan, Y.; Marécaux, C.; Swider, P.; Boutault, F. Comparison of linear and non-linear soft tissue models with post-operative CT scan in maxillofacial surgery. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: New York, NY, USA, 2004; Volume 3078, pp. 19–27. [Google Scholar]
- Holzinger, D.; Juergens, P.; Shahim, K.; Reyes, M.; Schicho, K.; Millesi, G.; Perisanidis, C.; Zeilhofer, H.F.; Seemann, R. Accuracy of soft tissue prediction in surgery-first treatment concept in orthognathic surgery: A prospective study. J. Cranio-Maxillofac. Surg. 2018, 46, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Bonet, J.; Wood, R.D. Nonlinear Continuum Mechanics for Finite Element Analysis, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Hughes, T.J. The Finite Element Method: Linear Static and Dynamic Finite Element Analysis; Dover Publications: Mineola, NY, USA, 2000. [Google Scholar]
- Sifakis, E.; Barbic, J. FEM Simulation of 3D Deformable Solids: A Practitioner’s Guide to Theory, Discretization and Model Reduction. In ACM SIGGRAPH 2012 Courses; Association for Computing Machinery: New York, NY, USA, 2012; pp. 20:1–20:50. [Google Scholar]
- Taylor, C.J.; Kriegman, D.J. Minimization on the Lie Group SO(3) and Related Manifolds; Technical Report; Yale University: New Haven, CT, USA, 1994. [Google Scholar]
- Magnenat-Thalmann, N.; Laperrière, R.; Thalmann, D. Joint-Dependent Local Deformations for Hand Animation and Object Grasping. In Proceedings of the Graphics Interface ’88; Canadian Information Processing Society: Calgary, AB, Canada, 1988; pp. 26–33. [Google Scholar]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrens, J.; Geveci, B.; Law, C. 36 ParaView: An End-User Tool for Large-Data Visualization; Elsevier Inc.: Amsterdam, The Netherlands, 2005; pp. 717–731. [Google Scholar]
- Olivetti, E.C.; Marcolin, F.; Moos, S.; Ferrando, A.; Vezzetti, E.; Autorino, U.; Borbon, C.; Zavattero, E.; Gerbino, G.; Ramieri, G. Three-Dimensional Evaluation of Soft Tissue Malar Modifications after Zygomatic Valgization Osteotomy via Geometrical Descriptors. J. Pers. Med. 2021, 11, 205. [Google Scholar] [CrossRef]
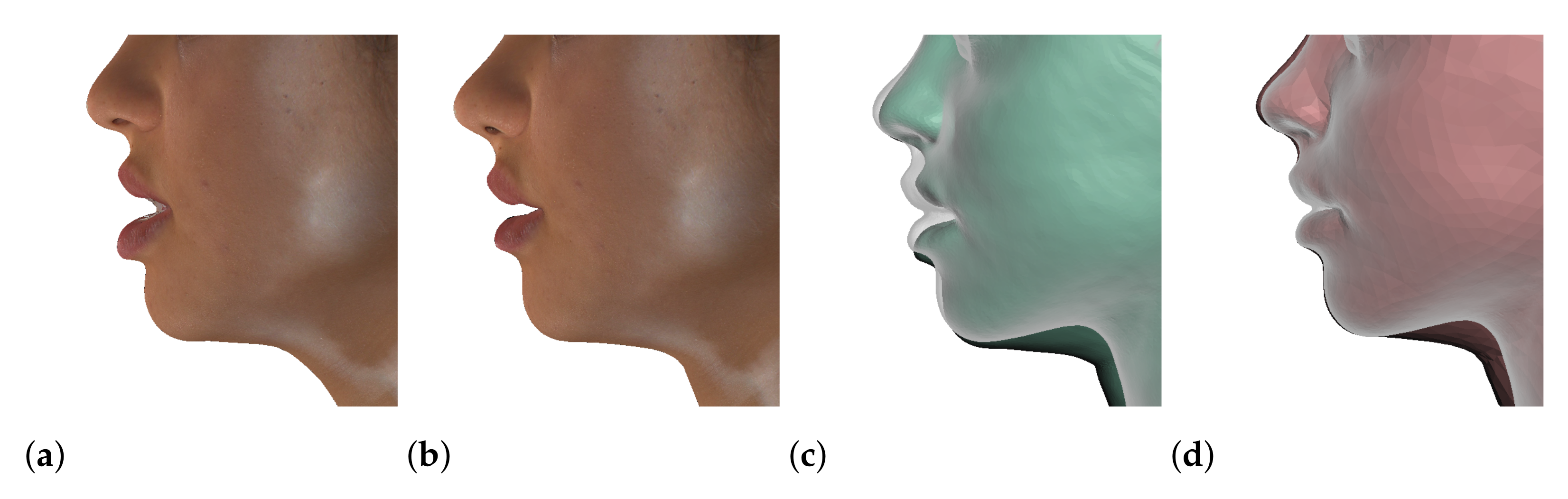










| ID | Gender | Age | Ethnic Group | Diagnosis | Maxilla Surgery | Mandible Surgery | ||
|---|---|---|---|---|---|---|---|---|
| Lefort Type | Segmented | Sagittal Split | Genioplasty | |||||
| M1 | M | 41 | Caucasian | Class III | I | Yes | BSSO | No |
| M2 | F | 31 | Caucasian | Open bite | I | Yes | BSSO | No |
| M3 | F | 36 | Latin American | Class III | II | No | BSSO | No |
| M4 | F | 28 | Caucasian | Asymmetry | I | No | USSO | Yes |
| M5 | F | 25 | Caucasian | Class II | I | Yes | BSSO | No |
| M6 | M | 51 | Caucasian | Class II | I | Yes | BSSO | No |
| M7 | F | 22 | Caucasian | Class III | I | Yes | No | No |
| M8 | F | 22 | Latin American | Class III | I | Yes | No | No |
| M9 | F | 36 | Caucasian | Asymmetry | No | No | BSSO | No |
| M10 | F | 29 | Caucasian | Asymmetry | I | No | BSSO | No |
| ID | Skin Pre | Skin Post | Bones | Fine Mesh Error | Coarse Mesh Error | |
|---|---|---|---|---|---|---|
| M1 |  |  |  |  |  |  |
| M2 |  |  |  |  |  | |
| M3 |  |  |  |  |  | |
| M4 |  |  |  |  |  | |
| M5 |  |  |  |  |  |
| ID | Skin Pre | Skin Post | Bones | Fine Mesh Error | Coarse Mesh Error | |
|---|---|---|---|---|---|---|
| M6 |  |  |  |  |  |  |
| M7 |  |  |  |  |  | |
| M8 |  |  |  |  |  | |
| M9 |  |  |  |  |  | |
| M10 |  |  |  |  |  |
| Patient ID | Number of Triangles | Simulation Time (s) | Surface with Error <= 3 mm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fine | Coarse | Reduction | Fine | Coarse | Reduction | Fine | Coarse | Reduction | |
| M1 | 22,970 | 2600 | 89% | 90.7 | 5.7 | 93% | 98% | 92% | 6% |
| M2 | 18,000 | 3400 | 81% | 111.6 | 11.8 | 90% | 98% | 96% | 2% |
| M3 | 18,600 | 3800 | 80% | 68.5 | 12.6 | 82% | 95% | 90% | 5% |
| M4 | 22,750 | 4250 | 81% | 107.3 | 14.9 | 86% | 95% | 93% | 2% |
| M5 | 19,500 | 3848 | 80% | 202.7 | 11.8 | 94% | 89% | 85% | 4% |
| M6 | 22,560 | 2720 | 88% | 102.4 | 10.7 | 92% | 94% | 91% | 3% |
| M7 | 23,576 | 2632 | 89% | 111.9 | 4.8 | 96% | 91% | 91% | 0% |
| M8 | 22,888 | 2354 | 90% | 77.5 | 3.8 | 95% | 93% | 86% | 7% |
| M9 | 18,738 | 2646 | 86% | 38.6 | 3.3 | 92% | 100% | 100% | 0% |
| M10 | 20,640 | 3390 | 84% | 65.9 | 9.3 | 87% | 96% | 94% | 2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcañiz, P.; Pérez, J.; Gutiérrez, A.; Barreiro, H.; Villalobos, Á.; Miraut, D.; Illana, C.; Guiñales, J.; Otaduy, M.A. Soft-Tissue Simulation for Computational Planning of Orthognathic Surgery. J. Pers. Med. 2021, 11, 982. https://doi.org/10.3390/jpm11100982
Alcañiz P, Pérez J, Gutiérrez A, Barreiro H, Villalobos Á, Miraut D, Illana C, Guiñales J, Otaduy MA. Soft-Tissue Simulation for Computational Planning of Orthognathic Surgery. Journal of Personalized Medicine. 2021; 11(10):982. https://doi.org/10.3390/jpm11100982
Chicago/Turabian StyleAlcañiz, Patricia, Jesús Pérez, Alessandro Gutiérrez, Héctor Barreiro, Ángel Villalobos, David Miraut, Carlos Illana, Jorge Guiñales, and Miguel A. Otaduy. 2021. "Soft-Tissue Simulation for Computational Planning of Orthognathic Surgery" Journal of Personalized Medicine 11, no. 10: 982. https://doi.org/10.3390/jpm11100982





