A Sneak-Peek into the Physician’s Brain: A Retrospective Machine Learning-Driven Investigation of Decision-Making in TAVR versus SAVR for Young High-Risk Patients with Severe Symptomatic Aortic Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. The TAVR Cohort
2.2. The iSAVR Cohort
2.3. Baseline Parameters
2.4. Statistical Analysis and the Machine Learning Model
3. Results
3.1. Baseline Characteristics
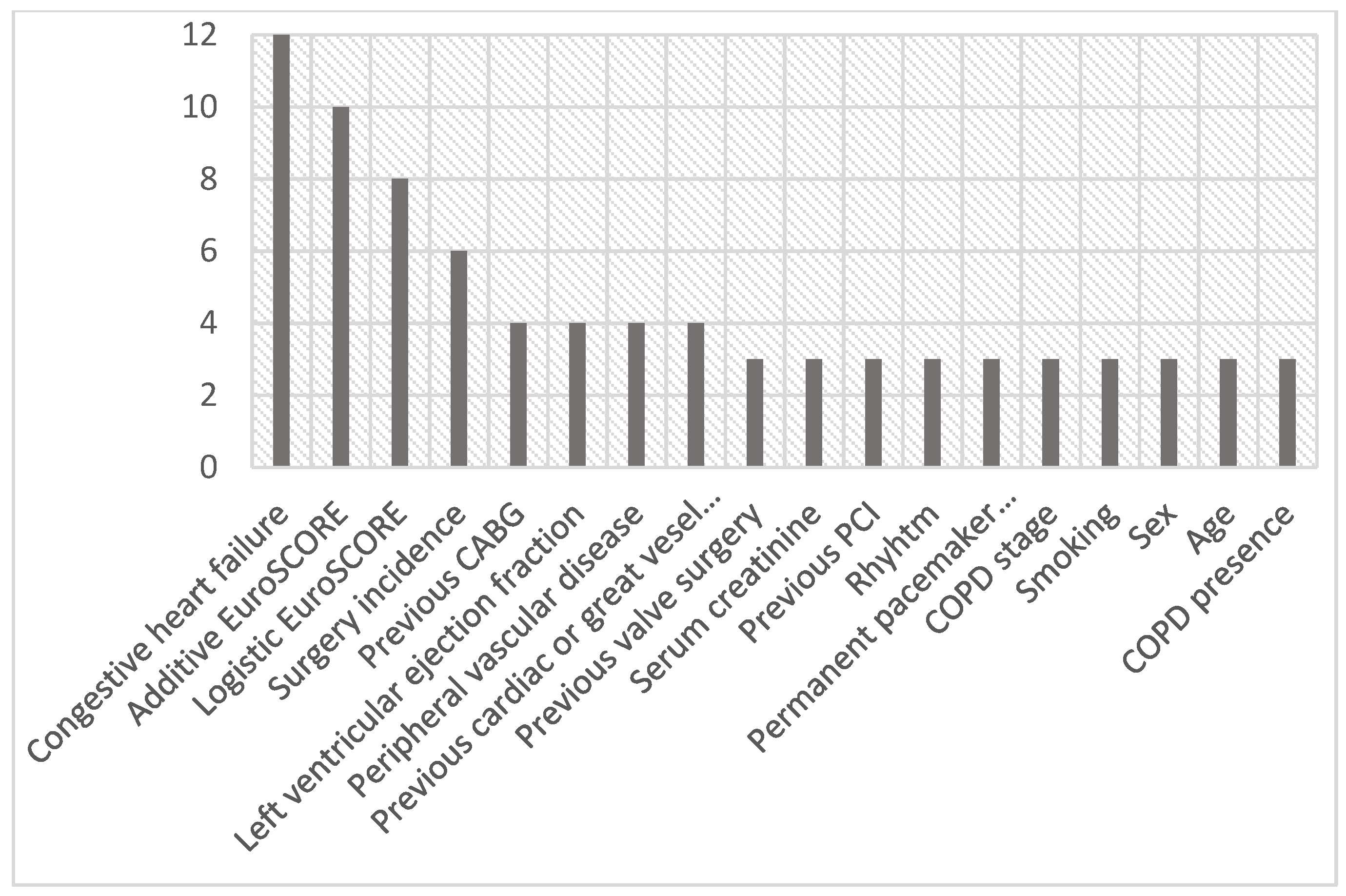
3.2. Heart Team Decision—Stated Reasons for TAVR
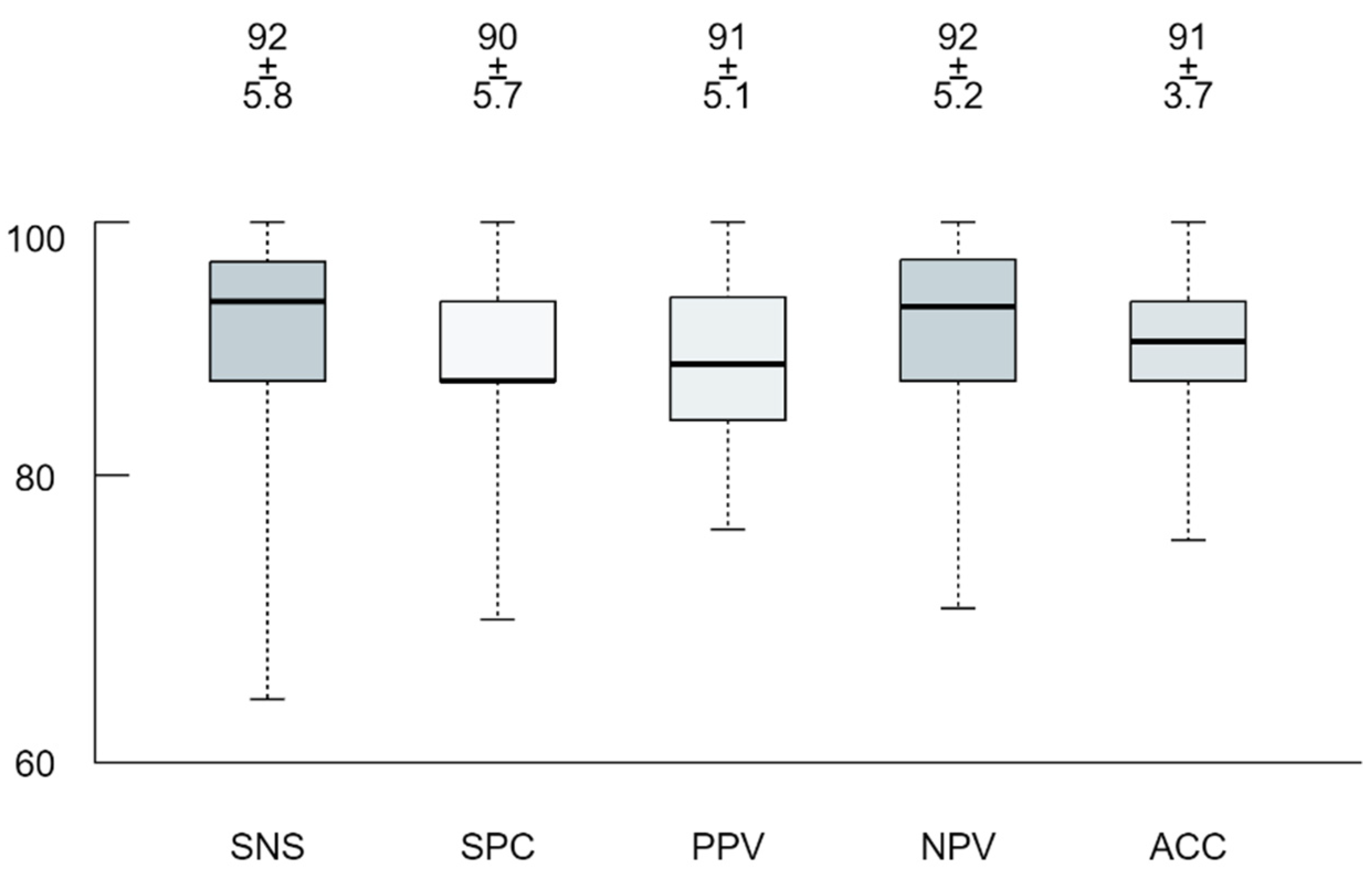
3.3. Machine Learning Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis: First Human Case Description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Mylotte, D.; Osnabrugge, R.L.J.; Windecker, S.; Lefèvre, T.; De Jaegere, P.; Jeger, R.; Wenaweser, P.; Maisano, F.; Moat, N.; Søndergaard, L.; et al. Transcatheter Aortic Valve Replacement in Europe: Adoption Trends and Factors Influencing Device Utilization. J. Am. Coll. Cardiol. 2013, 62, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durko, A.P.; Osnabrugge, R.L.; Van Mieghem, N.M.; Milojevic, M.; Mylotte, D.; Nkomo, V.T.; Pieter Kappetein, A. Annual Number of Candidates for Transcatheter Aortic Valve Implantation per Country: Current Estimates and Future Projections. Eur. Heart J. 2018, 39, 2635–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahanian, A.; Baumgartner, H.; Bax, J.; Butchart, E.; Dion, R.; Filippatos, G.; Flachskampf, F.; Hall, R.; Lung, B.; Kasprzak, J.; et al. Guidelines on the Management of Valvular Heart Disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur. Heart J. 2007, 28, 230–268. [Google Scholar] [PubMed] [Green Version]
- Vahanian, A.; Alfieri, O.; Al-Attar, N.; Antunes, M.; Bax, J.; Cormier, B.; Cribier, A.; De Jaegere, P.; Fournial, G.; Kappetein, A.P.; et al. Transcatheter Valve Implantation for Patients with Aortic Stenosis: A Position Statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in Collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2008, 29, 1463–1470. [Google Scholar]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Guidelines on the Management of Valvular Heart Disease (Version 2012). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2017, 38, 2739–2786. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar]
- De Backer, O.; Søndergaard, L. Challenges When Expanding Transcatheter Aortic Valve Implantation to Younger Patients. Front. Cardiovasc. Med. 2018, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Voigtländer, L.; Seiffert, M. Expanding TAVI to Low and Intermediate Risk Patients. Front. Cardiovasc. Med. 2018, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Kendall, F.; Khozin, S.; Goosen, R.; Hu, J.; Laramie, J.; Ringel, M.; Schork, N. Artificial Intelligence and Machine Learning in Clinical Development: A Translational Perspective. npj Digit. Med. 2019, 2, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, A.; Mago, V. Role of Machine Learning in Medical Research: A Survey. Comput. Sci. Rev. 2021, 40, 100370. [Google Scholar] [CrossRef]
- Sidey-Gibbons, J.A.M.; Sidey-Gibbons, C.J. Machine Learning in Medicine: A Practical Introduction. BMC Med. Res. Methodol. 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of Machine Learning in Drug Discovery and Development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A Guide to Deep Learning in Healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Peiffer-Smadja, N.; Rawson, T.M.; Ahmad, R.; Buchard, A.; Pantelis, G.; Lescure, F.X.; Birgand, G.; Holmes, A.H. Machine Learning for Clinical Decision Support in Infectious Diseases: A Narrative Review of Current Applications. Clin. Microbiol. Infect. 2020, 26, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Wardrope, A.; Jamnadas-Khoda, J.; Broadhurst, M.; Grünewald, R.A.; Heaton, T.J.; Howell, S.J.; Koepp, M.; Parry, S.W.; Sisodiya, S.; Walker, M.C.; et al. Machine Learning as a Diagnostic Decision Aid for Patients with Transient Loss of Consciousness. Neurol. Clin. Pract. 2020, 10, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Senders, J.T.; Staples, P.C.; Karhade, A.V.; Zaki, M.M.; Gormley, W.B.; Broekman, M.L.D.; Smith, T.R.; Arnaout, O. Machine Learning and Neurosurgical Outcome Prediction: A Systematic Review. World Neurosurg. 2018, 109, 476–486.e1. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Barnaby, D.P.; Coppa, K.; Makhnevich, A.; Kim, E.J.; Chatterjee, S.; Tóth, V.; Levy, T.J.; Paradis, M.d.; Cohen, S.L.; et al. Machine Learning to Assist Clinical Decision-Making during the COVID-19 Pandemic. Bioelectron. Med. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.; Spielvogel, C.P.; Grubmüller, B.; Grahovac, M.; Krajnc, D.; Ecsedi, B.; Sareshgi, R.A.M.; Mohamad, D.; Hamboeck, M.; Rausch, I.; et al. Supervised Machine Learning Enables Non-Invasive Lesion Characterization in Primary Prostate Cancer with [68Ga]Ga-PSMA-11 PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1795–1805. [Google Scholar] [CrossRef]
- Amin, A.; Anwar, S.; Adnan, A.; Nawaz, M.; Howard, N.; Qadir, J.; Hawalah, A.; Hussain, A. Comparing Oversampling Techniques to Handle the Class Imbalance Problem: A Customer Churn Prediction Case Study. IEEE Access 2016, 4, 7940–7957. [Google Scholar] [CrossRef]
- Krajnc, D.; Papp, L.; Nakuz, T.S.; Magometschnigg, H.F.; Grahovac, M.; Spielvogel, C.P.; Ecsedi, B.; Bago-Horvath, Z.; Haug, A.; Karanikas, G.; et al. Breast Tumor Characterization Using [18F]FDG-PET/CT Imaging Combined with Data Preprocessing and Radiomics. Cancers 2021, 13, 1249. [Google Scholar] [CrossRef] [PubMed]
- Rahhab, Z.; El Faquir, N.; Tchetche, D.; Delgado, V.; Kodali, S.; Mara Vollema, E.; Bax, J.; Leon, M.B.; Van Mieghem, N.M. Expanding the Indications for Transcatheter Aortic Valve Implantation. Nat. Rev. Cardiol. 2020, 17, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Briasoulis, A.; Holmes, A.A.; Afonso, L.; Schreiber, T.; Kondur, A. Transcatheter Aortic Valve Replacement versus Surgical Aortic Valve Replacement in Patients with Previous Coronary Artery Bypass Surgery: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2016, 215, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Regev, E.; Finkelstein, A.; Assali, A.; Barbash, I.; Fefer, P.; Ben-Shoshan, J.; Orvin, K.; Konigstein, M.; Guetta, V.; Kornowski, R.; et al. Comparison of Outcome of Transcatheter Aortic Valve Implantation for Severe Aortic Stenosis in 3 Age Groups (≤70; 71 to 80, and ≥81 Years). Am. J. Cardiol. 2017, 120, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Ler, A.; Ler, A.; Ying, Y.J.; Ying, Y.J.; Sazzad, F.; Sazzad, F.; Sazzad, F.; Choong, A.M.T.L.; Kofidis, T.; Kofidis, T.; et al. Structural Durability of Early-Generation Transcatheter Aortic Valve Replacement Valves Compared with Surgical Aortic Valve Replacement Valves in Heart Valve Surgery: A Systematic Review and Meta-Analysis. J. Cardiothorac. Surg. 2020, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Belluschi, I.; Buzzatti, N.; Castiglioni, A.; de Bonis, M.; Montorfano, M.; Alfieri, O. Severe Aortic Stenosis in the Young, with or without Bicuspid Valve: Is Transcatheter Aortic Valve Implantation the First Choice? Eur. Heart J. Suppl. 2020, 22, L1–L5. [Google Scholar] [CrossRef]
- Inamdar, A.; Inamdar, A. Heart Failure: Diagnosis, Management and Utilization. J. Clin. Med. 2016, 5, 62. [Google Scholar] [CrossRef]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef] [Green Version]
- Rosenhek, R.; Iung, B.; Tornos, P.; Antunes, M.J.; Prendergast, B.D.; Otto, C.M.; Kappetein, A.P.; Stepinska, J.; Kaden, J.J.; Naber, C.K.; et al. ESC Working Group on Valvular Heart Disease Position Paper: Assessing the Risk of Interventions in Patients with Valvular Heart Disease. Eur. Heart J. 2012, 33, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Van Rosendael, P.J.; Delgado, V.; Bax, J.J. Pacemaker Implantation Rate after Transcatheter Aortic Valve Implantation with Early and New-Generation Devices: A Systematic Review. Eur. Heart J. 2018, 39, 2003–2013. [Google Scholar] [CrossRef]
- Kamperidis, V.; Delgado, V.; Van Mieghem, N.M.; Kappetein, A.P.; Leon, M.B.; Bax, J.J. Diagnosis and Management of Aortic Valve Stenosis in Patients with Heart Failure. Eur. J. Heart Fail. 2016, 18, 469–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassileva, C.M.; Telila, T.; Markwell, S.; Hazelrigg, S. Magnitude of Negative Impact of Preoperative Heart Failure on Mortality during Aortic Valve Replacement in the Medicare Population. Ann. Thorac. Surg. 2015, 99, 1503–1510. [Google Scholar] [CrossRef]
- Gotzmann, M.; Rahlmann, P.; Hehnen, T.; Müller, P.; Lindstaedt, M.; Mügge, A.; Ewers, A. Heart Failure in Severe Aortic Valve Stenosis: Prognostic Impact of Left Ventricular Ejection Fraction and Mean Gradient on Outcome after Transcatheter Aortic Valve Implantation. Eur. J. Heart Fail. 2012, 14, 1155–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer-Rasokat, U.; Renker, M.; Liebetrau, C.; Weferling, M.; Rolf, A.; Doss, M.; Möllmann, H.; Walther, T.; Hamm, C.W.; Kim, W.K. Outcome of Patients with Heart Failure after Transcatheter Aortic Valve Implantation. PLoS ONE 2019, 14, e0225473. [Google Scholar] [CrossRef]
- Krittanawong, C.; Kumar, A.; Wang, Z.; Johnson, K.W.; Rastogi, U.; Narasimhan, B.; Kaplin, S.; Virk, H.U.H.; Baber, U.; Tang, W.; et al. Predictors of In-Hospital Mortality after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 125, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.B.; Tsigkas, G.; Kalogeropoulos, A.P. Transcatheter Aortic Valve Replacement after Heart Failure Hospitalization: Too Little, Too Late? Eur. J. Heart Fail. 2020, 22, 1875–1877. [Google Scholar] [CrossRef] [PubMed]
- Al-khadra, Y.; Sattar, Y.; Ullah, W.; Moussa Pacha, H.; Baibars, M.; Darmoch, F.; Abu-Mahfouz, M.; Afonso, L.; Devireddy, C.; Anwaruddin, S.; et al. Temporal Trends and Outcomes in Utilisation of Transcatheter and Surgical Aortic Valve Therapies in Aortic Valve Stenosis Patients with Heart Failure. Int. J. Clin. Pract. 2021, 75, 13711. [Google Scholar] [CrossRef]
- Skipper, N.C.; Matingal, J.; Zamvar, V. Assessment of EuroSCORE in Patients Undergoing Aortic Valve Replacement. J. Card. Surg. 2011, 26, 124–129. [Google Scholar] [CrossRef]
- Ben-Dor, I.; Gaglia, M.A.; Barbash, I.M.; Maluenda, G.; Hauville, C.; Gonzalez, M.A.; Sardi, G.; Laynez-Carnicero, A.; Torguson, R.; Okubagzi, P.; et al. Comparison between Society of Thoracic Surgeons Score and Logistic EuroSCORE for Predicting Mortality in Patients Referred for Transcatheter Aortic Valve Implantation. Cardiovasc. Revasc. Med. 2011, 12, 345–349. [Google Scholar] [CrossRef]
- Thalji, N.; Suri, R.; Greason, K.; Schaff, H.V. Risk Assessment Methods for Cardiac Surgery and Intervention. Nat. Rev. Cardiol 2014, 11, 704–714. [Google Scholar] [CrossRef]
- Azadani, A.N.; Tseng, E.E. Transcatheter Heart Valves for Failing Bioprostheses: State-of-the-Art Review of Valve-in-Valve Implantation. Circ. Cardiovasc. Interv. 2011, 4, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Tam, D.Y.; Vo, T.X.; Wijeysundera, H.C.; Dvir, D.; Friedrich, J.O.; Fremes, S.E. Transcatheter Valve-in-Valve versus Redo Surgical Aortic Valve Replacement for the Treatment of Degenerated Bioprosthetic Aortic Valve: A Systematic Review and Meta-Analysis. Catheter. Cardiovasc. Interv. 2018, 92, 1404–1411. [Google Scholar] [CrossRef]
- Takagi, H.; Mitta, S.; Ando, T. Meta-Analysis of Valve-in-Valve Transcatheter versus Redo Surgical Aortic Valve Replacement. Thorac. Cardiovasc. Surg. 2019, 67, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Silaschi, M.; Wendler, O.; Seiffert, M.; Castro, L.; Lubos, E.; Schirmer, J.; Blankenberg, S.; Reichenspurner, H.; Schäfer, U.; Treede, H.; et al. Transcatheter Valve-in-Valve Implantation versus Redo Surgical Aortic Valve Replacement in Patients with Failed Aortic Bioprostheses. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 63–70. [Google Scholar] [CrossRef]
- Malik, A.H.; Yandrapalli, S.; Zaid, S.; Shetty, S.S.; Aronow, W.S.; Ahmad, H.; Tang, G.H.L. Valve-in-Valve Transcatheter Implantation Versus Redo Surgical Aortic Valve Replacement. Am. J. Cardiol. 2020, 125, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.P.B.O.; Van den Eynde, J.; Simonato, M.; Cavalcanti, L.R.P.; Doulamis, I.P.; Weixler, V.; Kampaktsis, P.N.; Gallo, M.; Laforgia, P.L.; Zhigalov, K.; et al. Valve-in-Valve Transcatheter Aortic Valve Replacement Versus Redo Surgical Aortic Valve Replacement: An Updated Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Wilbring, M.; Tugtekin, S.M.; Alexiou, K.; Simonis, G.; Matschke, K.; Kappert, U. Transapical Transcatheter Aortic Valve Implantation vs. Conventional Aortic Valve Replacement in High-Risk Patients with Previous Cardiac Surgery: A Propensity-Score Analysis. Eur. J. Cardio-Thorac. Surg. 2013, 44, 42–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehada, S.E.; Elhmidi, Y.; Öztürk, Ö.; Kasel, M.; Frangieh, A.H.; Mourad, F.; Benedik, J.; El Bahi, J.; El Gabry, M.; Thielmann, M.; et al. Transcatheter versus Surgical Aortic Valve Replacement after Previous Cardiac Surgery: A Systematic Review and Meta-Analysis. Cardiol. Res. Pract. 2018, 2018, 4615043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, T.; Khera, S.; Kolte, D.; Goel, K.; Kalra, A.; Villablanca, P.A.; Aronow, H.D.; Abbott, J.D.; Fonarow, G.C.; Taub, C.C.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Prior Coronary Artery Bypass Grafting: Trends in Utilization and Propensity-Matched Analysis of In-Hospital Outcomes. Circ. Cardiovasc. Interv. 2018, 11, e006179. [Google Scholar] [CrossRef] [PubMed]
- Bagur, R.; Rodés-Cabau, J.; Gurvitch, R.; Dumont, É.; Velianou, J.L.; Manazzoni, J.; Toggweiler, S.; Cheung, A.; Ye, J.; Natarajan, M.K.; et al. Need for Permanent Pacemaker as a Complication of Transcatheter Aortic Valve Implantation and Surgical Aortic Valve Replacement in Elderly Patients with Severe Aortic Stenosis and Similar Baseline Electrocardiographic Findings. JACC Cardiovasc. Interv. 2012, 5, 540–551. [Google Scholar] [CrossRef] [Green Version]
- Fujita, B.; Schmidt, T.; Bleiziffer, S.; Bauer, T.; Beckmann, A.; Bekeredjian, R.; Möllmann, H.; Walther, T.; Landwehr, S.; Hamm, C.; et al. Impact of New Pacemaker Implantation Following Surgical and Transcatheter Aortic Valve Replacement on 1-Year Outcome. Eur. J. Cardio-Thorac. Surg. 2020, 57, 151–159. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Praz, F.; Pilgrim, T.; Mavridis, D.; Verma, S.; Salanti, G.; Søndergaard, L.; Jüni, P.; Windecker, S. Transcatheter Aortic Valve Implantation vs. Surgical Aortic Valve Replacement for Treatment of Severe Aortic Stenosis: A Meta-Analysis of Randomized Trials. Eur. Heart J. 2016, 37, 3503–3512. [Google Scholar] [CrossRef] [Green Version]
- Rosato, S.; Santini, F.; Barbanti, M.; Biancari, F.; D’Errigo, P.; Onorati, F.; Tamburino, C.; Ranucci, M.; Covello, R.D.; Santoro, G.; et al. Transcatheter Aortic Valve Implantation Compared with Surgical Aortic Valve Replacement in Low-Risk Patients. Circ. Cardiovasc. Interv. 2016, 9, e003326. [Google Scholar] [CrossRef] [PubMed]
- Rajula, H.S.R.; Verlato, G.; Manchia, M.; Antonucci, N.; Fanos, V. Comparison of Conventional Statistical Methods with Machine Learning in Medicine: Diagnosis, Drug Development, and Treatment. Medicina 2020, 56, 455. [Google Scholar] [CrossRef] [PubMed]
| Screening Parameter | Parameter Description |
|---|---|
| Age | Age in years |
| Sex | Male/female |
| Weight | Weight in kilograms |
| Height | Height in metres |
| Body Mass Index | |
| Body Surface Area | |
| Additive EuroSCORE | |
| Logistic EuroSCORE | |
| STS Score | Society of Thoracic Surgeons Score |
| HAS-BLED-Score | Hypertension, Abnormal Kidney and Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs or Alcohol Concomitantly Score |
| CHADS-VASC-Score | Congestive Heart Failure, Hypertension, Age, Diabetes mellitus, Stroke, Vascular disease, Age, Sex Category Score |
| Arterial hypertension | |
| Smoking | Any period of smoking in lifetime |
| Active smoker | At the time of intervention |
| COPD | Presence of COPD according to the STS classification |
| COPD severity | No symptoms, mild, moderate, severe symptoms |
| Dialysis | Active dialysis |
| Creatinine in serum | Creatinine in serum in mg/dL |
| Diabetes mellitus | No diabetes mellitus Insulin dependent diabetes mellitus Diabetes mellitus treated with dietary measures Diabetes mellitus treated with oral medication Diabetes mellitus diagnosed but not currently treated |
| Immunosuppression | Active immunosuppression |
| Peripheral vessel disease | |
| Cerebrovascular disease | |
| Previous cerebrovascular event | |
| Surgery incidence | First valve intervention or redo |
| Previous CABG | |
| Previous valve surgery | Previous surgery on any cardiac valve |
| Previous cardiac surgery—other | |
| Previous PCI | Previous PCI with or without stent implantation |
| Myocardial infarction | Previous myocardial infarction |
| Congestive heart failure | |
| NYHA class | |
| LVEF | LVEF in percent |
| Rhythm | Physiological sinus rhythm Atrial fibrillation Atrial flutter Active pacing |
| Permanent pacemaker | Previous permanent pacemaker implantation |
| Mean pressure gradient | In mmHg |
| Overall n = 692 | iSAVR < 75 Yrs n = 604 | TAVR < 75 Yrs n = 88 | p Value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (±SD) | 64.1 (9.5) | 63.4 (9.8) | 68.9 (5.2) | 0.385 | |
| Female, n (%) | 287 (41.5) | 237 (39.2) | 50 (56.8) | 0.370 | |
| Body mass index kg/m2, median (IQR) | 28.7 (5.5) | 28.6 (5.4) | 29.2 (6.5) | 0.369 | |
| Risk profile | |||||
| EuroSCORE, median (IQR) | 2.7 (3.7) | 1.7 (2.2) | 5.9 (5.3) | 0.103 | |
| Logistic EuroSCORE, median (IQR) | 17.8 (20.4) | 21.6 (28.9) | 17.4 (19.6) | 0.032 | |
| STS score, median (IQR) | 4.5 (3.3) | 5.8 (4.7) | 4.6 (3.2) | 0.288 | |
| Incremental risk score, median (IQR) | 3 (8) | 3 (9) | 5 (11.5) | 0.889 | |
| HAS-BLED score, median (IQR) | 1 (1) | 1 (1) | 1 (1) | 0.085 | |
| CHADS-VASC Score, mean (±SD) | 5.3 (1.4) | 5.8 (1.4) | 5.2 (1.4) | 0.014 | |
| Chronic health conditions and risk factors | |||||
| Hypertension, n (%) | 538 (77.7) | 464 (76.8) | 74 (84.1) | 0.160 | |
| Dyslipidemia, n (%) | 433 (62.6) | 380 (62.9) | 53 (60.2) | 0.026 | |
| Diabetes mellitus, n (%) | 200 (28.9) | 164 (27.2) | 36 (40.9) | 0.012 | |
| Active smoker, n (%) | 126 (18.2) | 106 (17.5) | 20 (22.7) | 0.209 | |
| Serum creatinine mg/dL, mean (±SD) | 1.1 (0.6) | 1.0 (0.4) | 1.5 (1.2) | 0.433 | |
| Preoperative dialysis, n (%) | 6 (0.9) | 2 (0.3) | 4 (4.5) | 0.478 | |
| COPD, n (%) | 217 (31.4) | 168 (27.8) | 49 (7.1) | 0.908 | |
| Peripheral vascular disease, n (%) | 67 (9.7) | 43 (7.1) | 24 (27.3) | 0.891 | |
| Cerebrovascular disease, n (%) | 111 (16.0) | 87 (14.4) | 24 (3.5) | 0.478 | |
| Previous cerebrovascular event, n (%) | 17 (2.5) | 7 (1.2) | 10 (11.4) | 0.143 | |
| Atrial fibrillation, n (%) | 119 (17.2) | 101 (16.7) | 18 (20.5) | 0.841 | |
| Previous myocardial infarction, n (%) | 54 (7.8) | 37 (6.1) | 17 (19.3) | 0.298 | |
| NYHA class III/IV, n (%) | 367 (53.1) | 288 (47.7) | 79 (90) | 0.820 | |
| Previous PCI, n (%) | 43 (6.2) | 26 (4.3) | 17 (19.3) | 0.233 | |
| Previous pacemaker implantation, n (%) | 32 (4.6) | 17 (2.8) | 15 (17) | 0.558 | |
| Previous cardiac surgery, n (%) | 64 (9.2) | 26 (4.3) | 38 (43.2) | 0.256 | |
| Previous CABG, n (%) | 34 (4.9) | 11 (1.8) | 23 (26.1) | 0.569 | |
| Previous valve surgery, n (%) | 34 (4.9) | 16 (2.6) | 18 (20.5) | ||
| Previous other cardiac surgery, n (%) | 17 (2.5) | 2 (0.3) | 15 (17) | 0.161 | |
| Preoperative echocardiographic data | |||||
| Mean pressure gradient, mean (±SD) | 48 (17.3) | 48.6 (17.6) | 46.3 (18.3) | 0.565 | |
| LVEF in %, mean (±IQR) | 52.7 (9.9) | 53.4 (9.2) | 46.5 (11.9) | 0.368 | |
| TAVR < 75 Years (n = 88) | |
|---|---|
| High-risk reoperation, n (%) | 42 (47.7) |
| Significant respiratory impairment, n (%) | 41 (46.6) |
| Severely reduced LVEF, n (%) | 34 (38.6) |
| Severe renal insufficiency, n (%) | 32 (36.4) |
| Substance abuse, n (%) | 23 (26.1) |
| Adipositas per magna, n (%) | 16 (18.2) |
| Valve-in-Valve procedure, n (%) | 13 (12.5) |
| Neurological impairment, n (%) | 12 (14.8) |
| Hepatopathy, n (%) | 10 (11.4) |
| History of radiation to the chest, n (%) | 9 (10.2) |
| Severe mental disorder, n (%) | 9 (10.2) |
| Pulmonary hypertension, n (%) | 7 (8.0) |
| Frailty, n (%) | 3 (3.4) |
| Severe rhythm disorder, n (%) | 2 (2.3) |
| History of severe bleeding, n (%) | 1 (1.1) |
| Other, n (%) | 17 (19.3) |
| Patients with 2 or more reasons listed above | 74 (84.1) |
| Patients with 3 or more reasons listed above | 35 (39.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasimbegovic, E.; Papp, L.; Grahovac, M.; Krajnc, D.; Poschner, T.; Hasan, W.; Andreas, M.; Gross, C.; Strouhal, A.; Delle-Karth, G.; et al. A Sneak-Peek into the Physician’s Brain: A Retrospective Machine Learning-Driven Investigation of Decision-Making in TAVR versus SAVR for Young High-Risk Patients with Severe Symptomatic Aortic Stenosis. J. Pers. Med. 2021, 11, 1062. https://doi.org/10.3390/jpm11111062
Hasimbegovic E, Papp L, Grahovac M, Krajnc D, Poschner T, Hasan W, Andreas M, Gross C, Strouhal A, Delle-Karth G, et al. A Sneak-Peek into the Physician’s Brain: A Retrospective Machine Learning-Driven Investigation of Decision-Making in TAVR versus SAVR for Young High-Risk Patients with Severe Symptomatic Aortic Stenosis. Journal of Personalized Medicine. 2021; 11(11):1062. https://doi.org/10.3390/jpm11111062
Chicago/Turabian StyleHasimbegovic, Ena, Laszlo Papp, Marko Grahovac, Denis Krajnc, Thomas Poschner, Waseem Hasan, Martin Andreas, Christoph Gross, Andreas Strouhal, Georg Delle-Karth, and et al. 2021. "A Sneak-Peek into the Physician’s Brain: A Retrospective Machine Learning-Driven Investigation of Decision-Making in TAVR versus SAVR for Young High-Risk Patients with Severe Symptomatic Aortic Stenosis" Journal of Personalized Medicine 11, no. 11: 1062. https://doi.org/10.3390/jpm11111062
APA StyleHasimbegovic, E., Papp, L., Grahovac, M., Krajnc, D., Poschner, T., Hasan, W., Andreas, M., Gross, C., Strouhal, A., Delle-Karth, G., Grabenwöger, M., Adlbrecht, C., & Mach, M. (2021). A Sneak-Peek into the Physician’s Brain: A Retrospective Machine Learning-Driven Investigation of Decision-Making in TAVR versus SAVR for Young High-Risk Patients with Severe Symptomatic Aortic Stenosis. Journal of Personalized Medicine, 11(11), 1062. https://doi.org/10.3390/jpm11111062








