An Artificial Intelligence-Based Alarm Strategy Facilitates Management of Acute Myocardial Infarction
Abstract
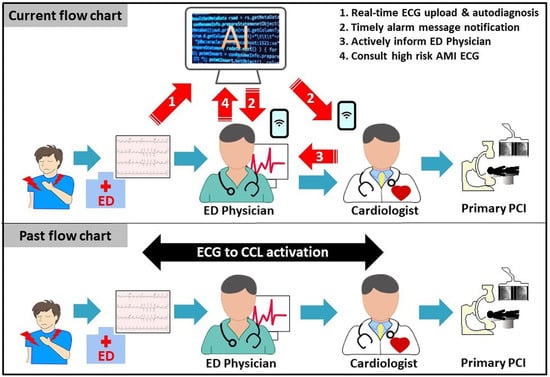
:1. Introduction
2. Method
2.1. Study Design and Setting
2.2. Study Population for AI-S Development
2.3. Definition of AMI, DtoB Time Metrics and STEMI Protocol
2.4. Data Collection
2.5. AI-S
2.5.1. First Part of AI-S: The Set-Up of AI-S
2.5.2. Second Part of AI-S: Automatic Active Alarm System with Notification by Short Message
2.6. Study Outcomes
2.7. Statistical Analysis
3. Results
3.1. The Development of AI-S
3.2. The Performance of AI-S for STEMI Detection
3.3. The Performance of AI-S for NSTEMI and Not-AMI Detection
3.4. Delayed/Misdiagnosed Cases by Front-Line Physicians
3.5. The DtoB Time Metrics before and after AI-S Implantation
3.6. The Performance of AI-S in Different Groups and Clinical Features
4. Discussion
5. Limitations and Strengths
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth universal definition of myocardial infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar]
- Li, Y.-H.; Lee, C.-H.; Huang, W.-C.; Wang, Y.-C.; Su, C.-H.; Sung, P.-H.; Chien, S.-C.; Hwang, J.-J. 2020 Focused Update of the 2012 Guidelines of the Taiwan Society of Cardiology for the Management of ST-Segment Elevation Myocardial Infarction. Acta Cardiol. Sin. 2020, 36, 285–307. [Google Scholar] [CrossRef]
- O’gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, 529–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, M.C.; Syndergaard, T.; Bowler, J.; Doxey, R. A systematic review of factors predicting door to balloon time in ST-segment elevation myocardial infarction treated with percutaneous intervention. Int. J. Cardiol. 2012, 157, 8–23. [Google Scholar] [CrossRef]
- Foo, C.Y.; Bonsu, K.O.; Nallamothu, B.K.; Reid, C.M.; Dhippayom, T.; Reidpath, D.D.; Chaiyakunapruk, N. Coronary intervention door-to-balloon time and outcomes in ST-elevation myocardial infarction: A meta-analysis. Heart 2018, 104, 1362–1369. [Google Scholar] [CrossRef]
- Terkelsen, C.J.; Jensen, L.O.; Tilsted, H.-H.; Trautner, S.; Johnsen, S.P.; Vach, W.; Bøtker, H.E.; Thuesen, L.; Lassen, J.F. Health Care System Delay and Heart Failure in Patients with ST-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention: Follow-up of Population-Based Medical Registry Data. Ann. Intern. Med. 2011, 155, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.-M.; Lai, C.-L.; Li, A.-H.; Chung, K.-P.; Yang, M.-C. Shorter Door-to-Balloon Time in ST-Elevation Myocardial Infarction Saves Insurance Payments: A Single Hospital Experience in Taiwan. Acta Cardiol. Sin. 2015, 31, 127–135. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wu, H.-P.; Lo, P.-H.; Liang, H.-Y.; Chang, K.-C. Impact of Prolonged Door-to-Balloon Times on the Diastolic Function in Acute ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention. Acta Cardiol. Sin. 2015, 31, 281–291. [Google Scholar] [CrossRef]
- Chen, F.-C.; Lin, Y.-R.; Kung, C.-T.; Cheng, C.-I.; Li, C.-J. The Association between Door-to-Balloon Time of Less than 60 Minutes and Prognosis of Patients Developing ST Segment Elevation Myocardial Infarction and Undergoing Primary Percutaneous Coronary Intervention. BioMed Res. Int. 2017, 2017, 1910934. [Google Scholar] [CrossRef] [Green Version]
- Antman, E.M. Time Is Muscle: Translation into Practice. J. Am. Coll. Cardiol. 2008, 52, 1216–1221. [Google Scholar] [CrossRef] [Green Version]
- Bradley, E.H.; Herrin, J.; Wang, Y.; Barton, B.; Webster, T.R.; Mattera, J.A.; Roumanis, S.A.; Curtis, J.P.; Nallamothu, B.K.; Magid, D.J.; et al. Strategies for Reducing the Door-to-Balloon Time in Acute Myocardial Infarction. N. Engl. J. Med. 2006, 355, 2308–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, S.-K.; Cheng, J.-J.; Shyu, K.-G.; Kuo, J.-Y.; Ko, Y.-L.; Wang, C.-C.; Chang, K.-C.; Ku, P.-M.; Lee, S.-H. Improvement in Door-to-Balloon (D2B) Time in Acute ST-Elevation Myocardial Infarction through the D2B Alliance–Experience of 15 Primary Percutaneous Coronary Intervention Centers in Taiwan. Circ. J. 2013, 77, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-K.; Juang, J.-M.J.; Chiang, J.-Y.; Li, Y.-H.; Tsai, C.-T.; Chiang, F.-T. The Taiwan Heart Registries: Its influence on cardiovascular patient care. J. Am. Coll. Cardiol. 2018, 71, 1273–1283. [Google Scholar] [CrossRef]
- McLaren, J.T.; Kapoor, M.; Soojin, L.Y.; Chartier, L.B. Using ECG-To-Activation Time to Assess Emergency Physicians’ Diagnostic Time for Acute Coronary Occlusion. J. Emerg. Med. 2021, 60, 25–34. [Google Scholar] [CrossRef]
- Calder, L.A.; Forster, A.J.; Stiell, I.; Carr, L.K.; Perry, J.; Vaillancourt, C.; Brehaut, J. Mapping Out the Emergency Department Disposition Decision for High-Acuity Patients. Ann. Emerg. Med. 2012, 60, 567–576.e4. [Google Scholar] [CrossRef]
- Moy, E.; Barrett, M.; Coffey, R.M.; Hines, A.L.; Newman-Toker, D.E. Missed diagnoses of acute myocardial infarction in the emergency department: Variation by patient and facility characteristics. Diagnosis 2015, 2, 29–40. [Google Scholar] [CrossRef]
- Morley, C.; Unwin, M.; Peterson, G.M.; Stankovich, J.; Kinsman, L. Emergency department crowding: A systematic review of causes, consequences and solutions. PLoS ONE 2018, 13, e0203316. [Google Scholar] [CrossRef]
- Hussain, F.; Cooper, A.; Carson-Stevens, A.; Donaldson, L.; Hibbert, P.; Hughes, T.; Edwards, A. Diagnostic error in the emergency department: Learning from national patient safety incident report analysis. BMC Emerg. Med. 2019, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Masoudi, F.A.; Magid, D.J.; Vinson, D.R.; Tricomi, A.J.; Lyons, E.E.; Crounse, L.; Ho, P.M.; Peterson, P.N.; Rumsfeld, J.S. Implications of the Failure to Identify High-Risk Electrocardiogram Findings for the Quality of Care of Patients with Acute Myocardial Infarction. Circulation 2006, 114, 1565–1571. [Google Scholar] [CrossRef]
- Wu, J.; Gale, C.P.; Hall, M.; Dondo, T.; Metcalfe, E.; Oliver, G.; Batin, P.D.; Hemingway, H.; Timmis, A.; West, R.M. Editor’s Choice—Impact of initial hospital diagnosis on mortality for acute myocardial infarction: A national cohort study. Eur. Heart J. Acute Cardiovasc. Care 2016, 7, 139–148. [Google Scholar] [CrossRef]
- Cho, Y.; Kwon, J.-M.; Kim, K.-H.; Medina-Inojosa, J.R.; Jeon, K.-H.; Cho, S.; Lee, S.Y.; Park, J.; Oh, B.-H. Artificial intelligence algorithm for detecting myocardial infarction using six-lead electrocardiography. Sci. Rep. 2020, 10, 20495. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, J.; Hou, Y.; Zhu, M.; Lu, Y.; Xu, Y.; Teliewubai, J.; Liu, W.; Xu, X.; Li, X.; et al. Early detection of ST-segment elevated myocardial infarction by artificial intelligence with 12-lead electrocardiogram. Int. J. Cardiol. 2020, 317, 223–230. [Google Scholar] [CrossRef]
- Liu, W.-C.; Lin, C.-S.; Tsai, C.-S.; Tsao, T.-P.; Cheng, C.-C.; Liou, J.-T.; Lin, W.-S.; Cheng, S.-M.; Lou, Y.-S.; Lee, C.-C. A deep learning algorithm for detecting acute myocardial infarction. EuroIntervention 2021, 17, 765–773. [Google Scholar] [CrossRef]
- McCabe, J.M.; Armstrong, E.J.; Hoffmayer, K.S.; Bhave, P.D.; MacGregor, J.S.; Hsue, P.; Stein, J.C.; Kinlay, S.; Ganz, P. Impact of door-to-activation time on door-to-balloon time in primary percutaneous coronary intervention for ST-segment elevation myocardial infarctions: A report from the Activate-SF registry. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.-K. Minimizing false activation of cath lab for STEMI—A realistic goal? Int. J. Cardiol. 2014, 172, e91–e93. [Google Scholar] [CrossRef]
- Yayehd, K.; Ricard, C.; Ageron, F.-X.; Buscaglia, L.; Savary, D.; Audema, B.; Lacroix, D.; Barthes, M.; Joubert, P.; Gheno, G.; et al. Role of primary care physicians in treating patients with ST-segment elevation myocardial infarction located in remote areas (from the REseau Nord-Alpin des Urgences [RENAU], Network). Eur. Heart J. Acute Cardiovasc. Care 2014, 4, 41–50. [Google Scholar] [CrossRef]
- Williams, T.; Savage, L.; Whitehead, N.; Orvad, H.; Cummins, C.; Faddy, S.; Fletcher, P.; Boyle, A.J.; Inder, K.J. Missed Acute Myocardial Infarction (MAMI) in a rural and regional setting. Int. J. Cardiol. Heart Vasc. 2019, 22, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-C.; Chen, Y.-C.; Shih, C.-M.; Hou, S.-K.; Seethala, R.R.; Aisiku, I.P.; Huang, C.-C.; Hou, P.C.; Kao, W.-F. Smartphone transmission of electrocardiography images to reduce time of cardiac catheterization laboratory activation. J. Chin. Med. Assoc. 2018, 81, 505–510. [Google Scholar] [CrossRef]
- Chen, K.-C.; Yen, D.H.-T.; Chen, C.-D.; Young, M.S.; Yin, W.-H. Effect of Emergency Department in-Hospital Tele-Electrocardiographic Triage and Interventional Cardiologist Activation of the Infarct Team on Door-to-Balloon Times in ST-Segment-Elevation Acute Myocardial Infarction. Am. J. Cardiol. 2011, 107, 1430–1435. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Leu, F.-Y.; Yang, C.-W.; Lai, L.-S. Healthcare-based on Cloud Electrocardiogram System: A Medical Center Experience in Middle Taiwan. J. Med. Syst. 2018, 42, 39. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.R. Was there a Hawthorne effect? Am. J. Sociol. 1992, 98, 451–468. [Google Scholar]





| Prospective Validation Cohort | ||||
|---|---|---|---|---|
| STEMI | NSTEMI | not-AMI | Excluded | |
| All dataset | ||||
| Alarmed | 59 (100.0%) | 41 (62.1%) | 10 (0.1%) | 13 (100.0%) |
| Strategy-1 | 41 | 0 | 1 | 2 |
| Strategy-2 | 14 | 1 | 3 | 11 |
| Strategy-3 | 4 | 40 | 6 | 0 |
| Unalarmed | 0 (0.0%) | 25 (37.9%) | 14,161 (99.9%) | 0 (0.0%) |
| Subset-1 | ||||
| Alarmed | 4 (100.0%) | 6 (50.0%) | 1 (<0.1%) | 3 (100.0%) |
| Strategy-1 | 3 | 0 | 0 | 1 |
| Strategy-2 | 1 | 1 | 0 | 2 |
| Strategy-3 | 0 | 5 | 1 | 0 |
| Unalarmed | 0 (0.0%) | 6 (50.0%) | 3559 (>99.9%) | 0 (0.0%) |
| Subset-2 | ||||
| Alarmed | 55 (100.0%) | 35 (64.8%) | 9 (0.1%) | 10 (100.0%) |
| Strategy-1 | 38 | 0 | 1 | 1 |
| Strategy-2 | 13 | 0 | 3 | 9 |
| Strategy-3 | 4 | 35 | 5 | 0 |
| Unalarmed | 0 (0.0%) | 19 (35.2%) | 10,602 (99.9%) | 0 (0.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-C.; Lin, C.; Lin, C.-S.; Tsai, M.-C.; Chen, S.-J.; Tsai, S.-H.; Lin, W.-S.; Lee, C.-C.; Tsao, T.-P.; Cheng, C.-C. An Artificial Intelligence-Based Alarm Strategy Facilitates Management of Acute Myocardial Infarction. J. Pers. Med. 2021, 11, 1149. https://doi.org/10.3390/jpm11111149
Liu W-C, Lin C, Lin C-S, Tsai M-C, Chen S-J, Tsai S-H, Lin W-S, Lee C-C, Tsao T-P, Cheng C-C. An Artificial Intelligence-Based Alarm Strategy Facilitates Management of Acute Myocardial Infarction. Journal of Personalized Medicine. 2021; 11(11):1149. https://doi.org/10.3390/jpm11111149
Chicago/Turabian StyleLiu, Wen-Cheng, Chin Lin, Chin-Sheng Lin, Min-Chien Tsai, Sy-Jou Chen, Shih-Hung Tsai, Wei-Shiang Lin, Chia-Cheng Lee, Tien-Ping Tsao, and Cheng-Chung Cheng. 2021. "An Artificial Intelligence-Based Alarm Strategy Facilitates Management of Acute Myocardial Infarction" Journal of Personalized Medicine 11, no. 11: 1149. https://doi.org/10.3390/jpm11111149