Automated Assessment of the Substantia Nigra Pars Compacta in Parkinson’s Disease: A Diffusion Tensor Imaging Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Acquisition
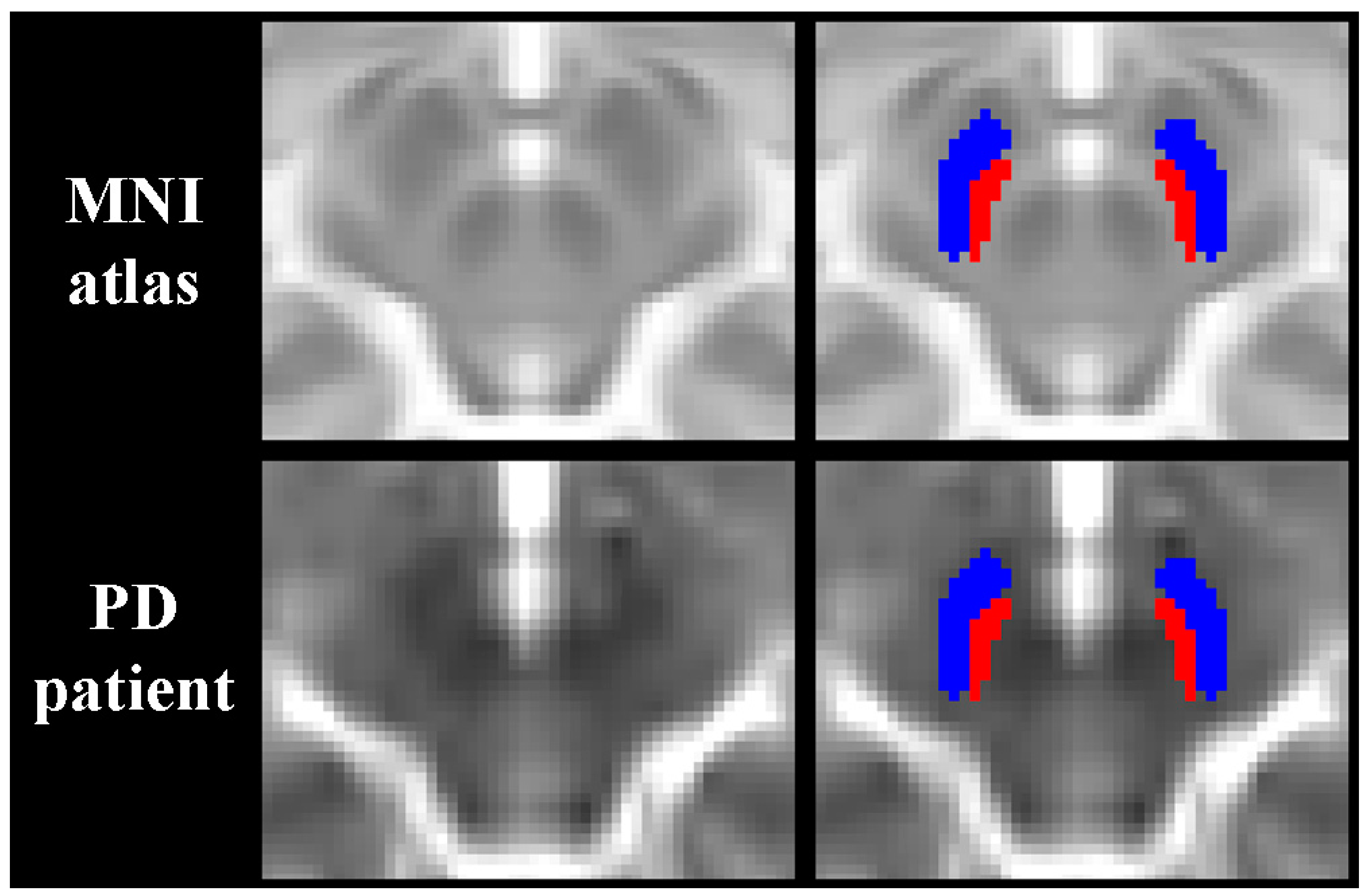
2.3. MRI Assessment
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Differences between Groups
3.3. Associations with Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeed, U.; Compagnone, J.; Aviv, R.I.; Strafella, A.P.; Black, S.E.; Lang, A.E.; Masellis, M. Imaging biomarkers in Parkinson’s disease and Parkinsonian syndromes: Current and emerging concepts. Transl. Neurodegener. 2017, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, J.; Huddleston, D.E.; Merritt, M.; Chen, X.; McMurray, R.; Silver, M.; Factor, S.A.; Hu, X. Diffusion tensor imaging of the substantia nigra in Parkinson’s disease revisited. Hum. Brain Mapp. 2016, 37, 2547–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, J.; Huddleston, D.E.; Sedlacik, J.; Boelmans, K.; Hu, X.P. Parkinson’s disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Mov. Disord. 2017, 32, 441–449. [Google Scholar] [CrossRef]
- Bergsland, N.; Zivadinov, R.; Schweser, F.; Hagemeier, J.; Lichter, D.; Guttuso, T., Jr. Ventral posterior substantia nigra iron increases over 3 years in Parkinson’s disease. Mov. Disord. 2019, 34, 1006–1013. [Google Scholar] [CrossRef]
- Guttuso, T., Jr.; Bergsland, N.; Hagemeier, J.; Lichter, D.G.; Pasternak, O.; Zivadinov, R. Substantia nigra free water increases longitudinally in Parkinson Disease. Am. J. Neuroradiol. 2018, 39, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Ofori, E.; Krismer, F.; Burciu, R.G.; Pasternak, O.; McCracken, J.L.; Lewis, M.M.; Du, G.; McFarland, N.R.; Okun, M.S.; Poewe, W.; et al. Free water improves detection of changes in the substantia nigra in parkinsonism: A multisite study. Mov. Disord. 2017, 32, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Ofori, E.; Pasternak, O.; Planetta, P.J.; Li, H.; Burciu, R.G.; Snyder, A.F.; Lai, S.; Okun, M.S.; Vaillancourt, D.E. Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain 2015, 138, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Spraker, M.B.; Prodoehl, J.; Abraham, I.; Corcos, D.M.; Zhou, X.J.; Comella, C.L.; Little, D.M. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 2009, 72, 1378–1384. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.Y.; Wang, L.; Yang, T.T.; Li, R.; Yu, G. A meta-analysis of diffusion tensor imaging of substantia nigra in patients with Parkinson’s disease. Sci. Rep. 2018, 8, 2941. [Google Scholar] [CrossRef]
- Schwarz, S.T.; Abaei, M.; Gontu, V.; Morgan, P.S.; Bajaj, N.; Auer, D.P. Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: A region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. Neuroimage Clin. 2013, 3, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wu, I.W.; Tosun, D.; Foster, E.; Schuff, N. Parkinson’s progression markers I. Progression of regional microstructural degeneration in Parkinson’s Disease: A multicenter diffusion tensor imaging study. PLoS ONE 2016, 11, e0165540. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.J.; Lin, W.Y.; Lu, C.S.; Weng, Y.H.; Ng, S.H.; Wang, C.H.; Liu, H.-L.; Hsieh, R.-H.; Wan, Y.-L.; Wai, Y.-Y. Parkinson disease: Diagnostic utility of diffusion kurtosis imaging. Radiology 2011, 261, 210–217. [Google Scholar] [CrossRef]
- Pelizzari, L.; Lagana, M.M.; Di Tella, S.; Rossetto, F.; Bergsland, N.; Nemni, R.; Clerici, M.; Baglio, F. Combined assessment of diffusion parameters and cerebral blood flow within basal ganglia in early Parkinson’s Disease. Front. Aging Neurosci. 2019, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Hardman, C.D.; McRitchie, D.A.; Halliday, G.M.; Cartwright, H.R.; Morris, J.G. Substantia nigra pars reticulata neurons in Parkinson’s disease. Neurodegener. 1996, 5, 49–55. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.; Skare, S.; Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. NeuroImage 2003, 20, 870–888. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2016, 125, 1063–1078. [Google Scholar] [CrossRef] [Green Version]
- Andersson, J.L.R.; Graham, M.S.; Zsoldos, E.; Sotiropoulos, S.N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage 2016, 141, 556–572. [Google Scholar] [CrossRef] [Green Version]
- Avants, B.B.; Epstein, C.L.; Grossman, M.; Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.; Andersson, J.; Ardekani, B.A.; Ashburner, J.; Avants, B.; Chiang, M.C.; Christensen, G.E.; Collins, D.L.; Gee, J.; Hellier, P.; et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage 2009, 46, 786–802. [Google Scholar] [CrossRef] [Green Version]
- Pauli, W.M.; Nili, A.N.; Tyszka, J.M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data 2018, 5, 180063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Du, G.; Lewis, M.M.; Sica, C.; He, L.; Connor, J.R.; Kong, L.; Mailman, R.B.; Huang, X. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson’s patients. Mov. Disord. 2018, 33, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.; He, N.; Huddleston, D.E.; Chen, S.; Yan, F.; Crosson, B.; Factor, S.; Hu, X. Reproducible detection of nigral iron deposition in 2 Parkinson’s disease cohorts. Mov. Disord. 2018, 34, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, M.G.; Silva, D.; Bergsland, N.; Horakova, D.; Ramasamy, D.; Durfee, J.; Vaneckova, M.; Havrdova, E.; Zivadinov, R. Neurological software tool for reliable atrophy measurement (NeuroSTREAM) of the lateral ventricles on clinical-quality T2-FLAIR MRI scans in multiple sclerosis. Neuroimage Clin. 2017, 15, 769–779. [Google Scholar] [CrossRef]
- Visser, E.; Keuken, M.C.; Douaud, G.; Gaura, V.; Bachoud-Levi, A.C.; Remy, P.; Forstmann, B.U.; Jenkinson, M. Automatic segmentation of the striatum and globus pallidus using MIST: Multimodal Image Segmentation Tool. NeuroImage 2016, 125, 479–497. [Google Scholar] [CrossRef] [Green Version]
- Visser, E.; Keuken, M.C.; Forstmann, B.U.; Jenkinson, M. Automated segmentation of the substantia nigra, subthalamic nucleus and red nucleus in 7T data at young and old age. NeuroImage 2016, 139, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Burciu, R.G.; Ofori, E.; Archer, D.B.; Wu, S.S.; Pasternak, O.; McFarland, N.R.; Okun, M.S.; Vaillancourt, D.E. Progression marker of Parkinson’s disease: A 4-year multi-site imaging study. Brain 2017, 140, 2183–2192. [Google Scholar] [CrossRef] [Green Version]

| PD (n = 29) | HC (n = 28) | p | |
|---|---|---|---|
| Age in years, mean (SD) | 66.1 (8.1) | 65.4 (8.4) | 0.742 ^ |
| Males, n (%) | 23 (79.3) | 19 (67.9) | 0.379 % |
| Disease duration in years, median (IQR) | 2.8 (2.1–5.3) | - | - |
| UPDRS-III, median (IQR) | 21 (8–34) | - | - |
| H&Y stage, median (IQR) | 1.5 (1.25–2) | - | - |
| LEDD, mean (IQR) | 230 (125–325) | - | - |
| PD (n = 29) | HC (n = 28) | Partial η2 | FDR-Corrected p | |
|---|---|---|---|---|
| Subtantia nigra pars compacta | ||||
| FA | 0.442 (0.040) | 0.432 (0.037) | 0.014 | 0.454 |
| MD | 0.803 (0.054) | 0.772 (0.038) | 0.098 | 0.08 |
| AD | 1.207 (0.068) | 1.156 (0.045) | 0.152 | 0.024 |
| RD | 0.601 (0.053) | 0.579 (0.045) | 0.042 | 0.454 |
| Subtantia nigra pars reticulata | ||||
| FA | 0.565 (0.051) | 0.569 (0.046) | 0.006 | 0.582 |
| MD | 0.766 (0.033) | 0.748 (0.032) | 0.070 | 0.136 |
| AD | 1.307 (0.062) | 1.290 (0.049) | 0.014 | 0.454 |
| RD | 0.495 (0.049) | 0.477 (.046) | 0.043 | 0.256 |
| AUC | SE | 95% CI | pFDR-Value | Youden Index J | Optimal Cut-Off | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| SNpc | ||||||||
| FA | 0.591 | 0.077 | 0.428 – 0.730 | 0.307 | 0.293 | >0.426 | 79.3% | 50.0% |
| MD | 0.664 | 0.074 | 0.498 – 0.787 | 0.102 | 0.335 | >0.782 | 62.1% | 71.4% |
| AD | 0.736 | 0.067 | 0.608 – 0.870 | 0.002 | 0.440 | >1.182 | 69.0% | 75.0% |
| RD | 0.619 | 0.076 | 0.510 – 0.808 | 0.082 | 0.297 | >0.580 | 69.0% | 60.7% |
| SNpr | ||||||||
| FA | 0.461 | 0.076 | 0.389 – 0.765 | 0.172 | 0.164 | ≤0.555 | 48.3% | 67.9% |
| MD | 0.642 | 0.072 | 0.517 – 0.799 | 0.082 | 0.256 | >0.741 | 82.8% | 42.9% |
| AD | 0.567 | 0.076 | 0.450 – 0.747 | 0.231 | 0.235 | >1.325 | 44.8% | 78.6% |
| RD | 0.642 | 0.074 | 0.519 – 0.808 | 0.082 | 0.335 | >0.495 | 62.1% | 71.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergsland, N.; Pelizzari, L.; Laganá, M.M.; Di Tella, S.; Rossetto, F.; Nemni, R.; Clerici, M.; Baglio, F. Automated Assessment of the Substantia Nigra Pars Compacta in Parkinson’s Disease: A Diffusion Tensor Imaging Study. J. Pers. Med. 2021, 11, 1235. https://doi.org/10.3390/jpm11111235
Bergsland N, Pelizzari L, Laganá MM, Di Tella S, Rossetto F, Nemni R, Clerici M, Baglio F. Automated Assessment of the Substantia Nigra Pars Compacta in Parkinson’s Disease: A Diffusion Tensor Imaging Study. Journal of Personalized Medicine. 2021; 11(11):1235. https://doi.org/10.3390/jpm11111235
Chicago/Turabian StyleBergsland, Niels, Laura Pelizzari, Maria Marcella Laganá, Sonia Di Tella, Federica Rossetto, Raffaello Nemni, Mario Clerici, and Francesca Baglio. 2021. "Automated Assessment of the Substantia Nigra Pars Compacta in Parkinson’s Disease: A Diffusion Tensor Imaging Study" Journal of Personalized Medicine 11, no. 11: 1235. https://doi.org/10.3390/jpm11111235
APA StyleBergsland, N., Pelizzari, L., Laganá, M. M., Di Tella, S., Rossetto, F., Nemni, R., Clerici, M., & Baglio, F. (2021). Automated Assessment of the Substantia Nigra Pars Compacta in Parkinson’s Disease: A Diffusion Tensor Imaging Study. Journal of Personalized Medicine, 11(11), 1235. https://doi.org/10.3390/jpm11111235







