Dismal Survival in COVID-19 Patients Requiring ECMO as Rescue Therapy after Corticosteroid Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Purposes
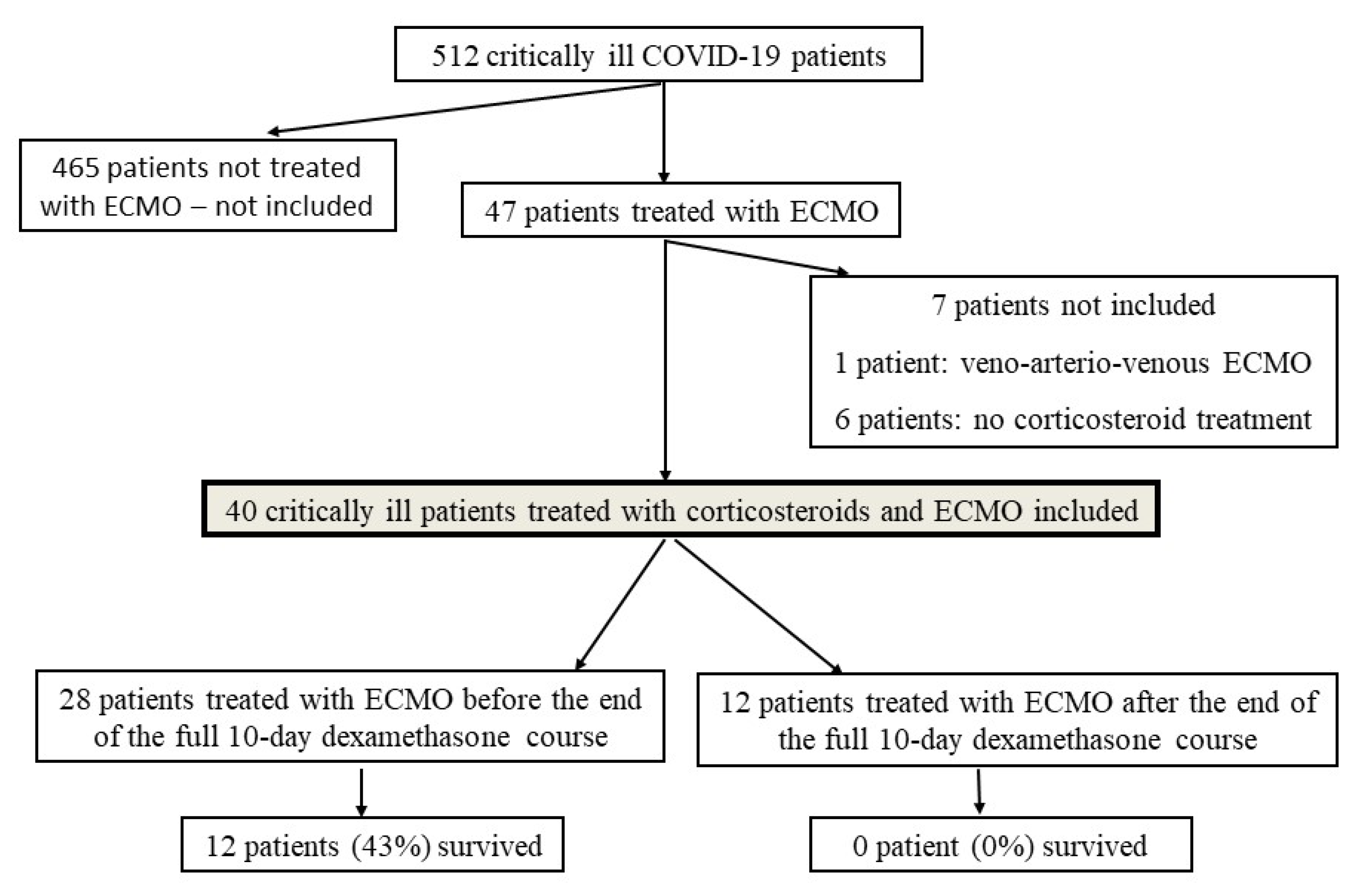
2.2. Study Population
2.3. Ventilation and ECMO Management in the ICU
2.4. Data Collection
2.5. Statistical Analysis
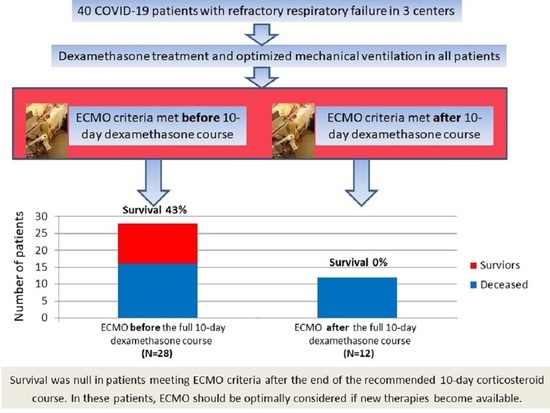
3. Results
3.1. Patient Characteristics
3.2. Dexamethasone and Other Immunomodulatory Treatments
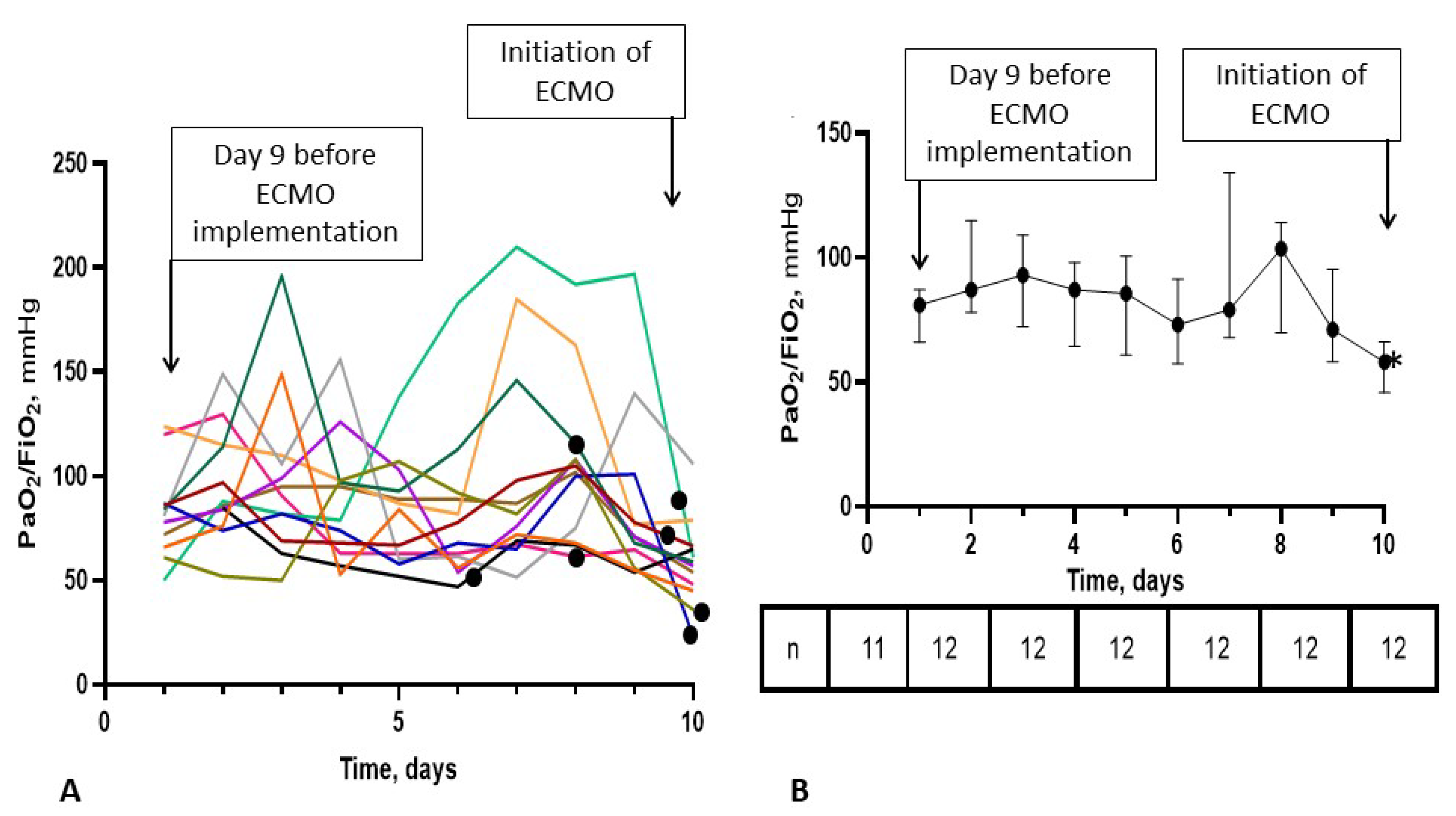
3.3. ECMO Treatment and Correlates of Survival
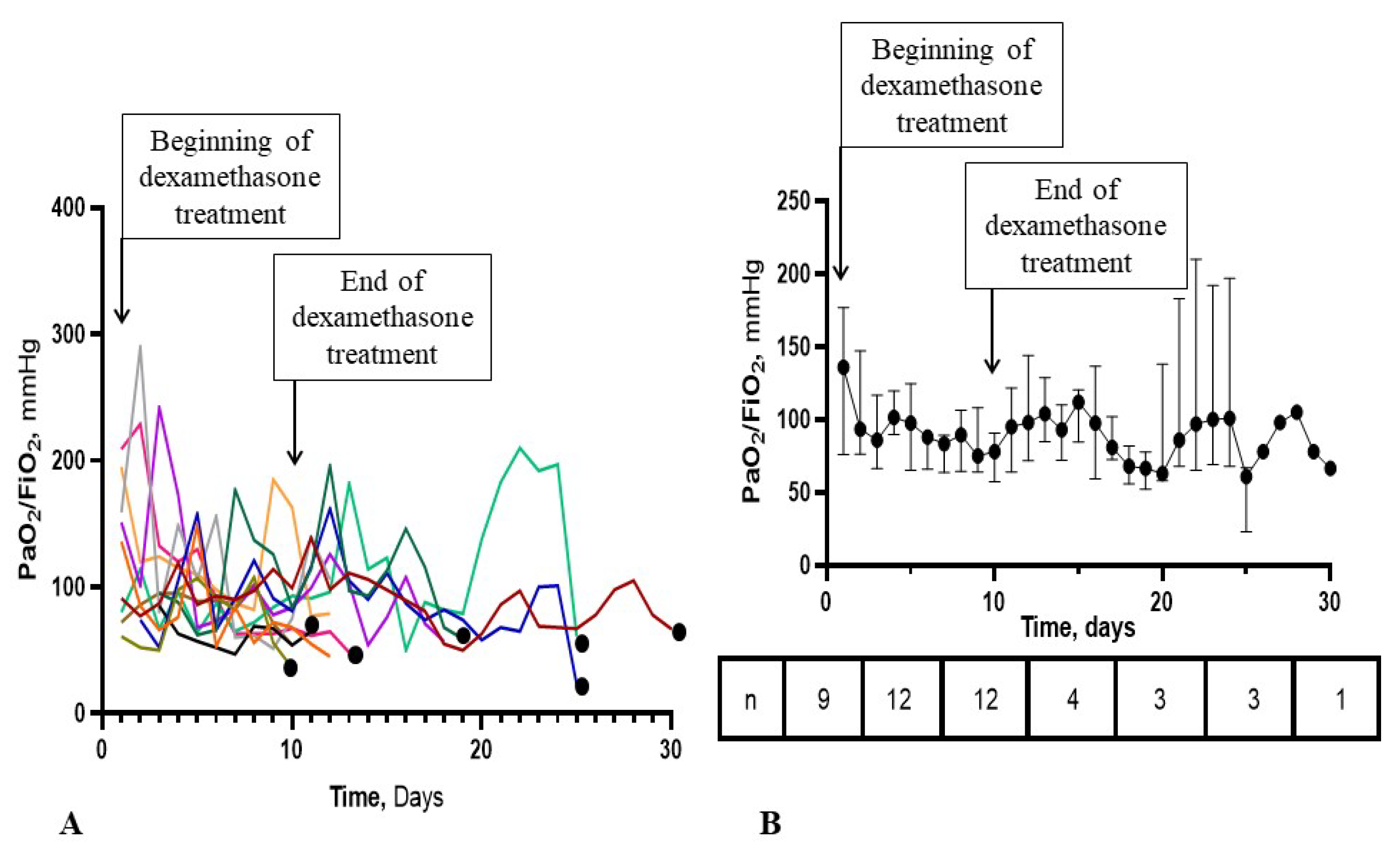
3.4. Time-Course of the PaO2/FiO2 Ratio in the ECMO-after Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [PubMed]
- REMAP-CAP Investigators; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [PubMed]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef]
- Lebreton, G.; Schmidt, M.; Ponnaiah, M.; Folliguet, T.; Para, M.; Guihaire, J.; Lansac, E.; Sage, E.; Cholley, B.; Mégarbane, B.; et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: A multicentre cohort study. Lancet Respir. Med. 2021, 9, 851–862. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Cano, E.J.; Fonseca Fuentes, X.; Corsini Campioli, C.; O’Horo, J.C.; Abu Saleh, O.; Odeyemi, Y.; Yadav, H.; Temesgen, Z. Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes: Systematic Review and Meta-analysis. Chest 2021, 159, 1019–1040. [Google Scholar] [CrossRef] [PubMed]
- López Zúñiga, M.Á.; Moreno-Moral, A.; Ocaña-Granados, A.; Padilla-Moreno, F.A.; Castillo-Fernández, A.M.; Guillamón-Fernández, D.; Ramírez-Sánchez, C.; Sanchez-Palop, M.; Martínez-Colmenero, J.; Pimentel-Villar, M.A.; et al. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS ONE 2021, 16, e0243964. [Google Scholar] [CrossRef]
- So, C.; Ro, S.; Murakami, M.; Imai, R.; Jinta, T. High-dose, short-term corticosteroids for ARDS caused by COVID-19: A case series. Respirol. Case Rep. 2020, 8, e00596. [Google Scholar] [CrossRef] [PubMed]
- Pinzón, M.A.; Ortiz, S.; Holguín, H.; Betancur, J.F.; Cardona Arango, D.; Laniado, H.; Arias Arias, C.; Muñoz, B.; Quiceno, J.; Jaramillo, D.; et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS ONE 2021, 16, e0252057. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E.A.; Sen, P.; Schoenfeld, S.R.; Neilan, T.G.; Frigault, M.J.; Stone, J.H.; Kim, A.Y.; Mansour, M.K. Immunomodulation as Treatment for Severe COVID-19: A systematic review of current modalities and future directions. Clin. Infect. Dis. 2020, 72, e1130–e1143. [Google Scholar] [CrossRef] [PubMed]
- COVID STEROID 2 Trial Group; Munch, M.W.; Myatra, S.N.; Vijayaraghavan, B.K.T.; Saseedharan, S.; Benfield, T.; Wahlin, R.R.; Rasmussen, B.S.; Andreasen, A.S.; Poulsen, L.M.; et al. Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA 2021, in press. [Google Scholar]
- Giraud, R.; Legouis, D.; Assouline, B.; De Charriere, A.; Decosterd, D.; Brunner, M.E.; Moret-Bochatay, M.; Fumeaux, T.; Bendjelid, K. Timing of VV-ECMO therapy implementation influences prognosis of COVID-19 patients. Physiol. Rep. 2021, 9, e14715. [Google Scholar] [CrossRef] [PubMed]
- Biancari, F.; Mariscalco, G.; Dalén, M.; Settembre, N.; Welp, H.; Perrotti, A.; Wiebe, K.; Leo, E.; Loforte, A.; Chocron, S.; et al. Six-Month Survival After Extracorporeal Membrane Oxygenation for Severe COVID-19. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Combes, A.; Agerstrand, C.; Annich, G.; Diaz, R.; Fan, E.; Hryniewicz, K.; Lorusso, R.; et al. Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 2021, 398, 1230–1238. [Google Scholar] [CrossRef]
- Walter, K. Lung Transplants for COVID-19-The Option of Last Resort. JAMA 2021, in press. [Google Scholar] [CrossRef] [PubMed]
| Parameters * | All Patients (N = 40) | ECMO before/during the 10-Day Dexamethasone Course (N = 28) | ECMO after the 10-Day Dexamethasone Course (N = 12) | p-Value |
|---|---|---|---|---|
| Age (years) | 57 (51–62) | 57 (51–59) | 62 (57–67) | 0.053 |
| Male gender, N (%) | 30 (75) | 22 (79) | 8 (67) | 0.46 |
| Body mass index (kg/m2) | 31 (28–35) | 32 (28–35) | 30 (27–32) | 0.38 |
| History of hypertension, N (%) | 16 (40) | 9 (32) | 7 (58) | 0.17 |
| Diabetes mellitus, N (%) | 16 (40) | 9 (32) | 7 (58) | 0.17 |
| Ischemic heart disease, N (%) | 3 (8) | 2 (7) | 1 (8) | 1.0 |
| History of heart failure, N (%) | 1 (3) | 1 (4) | 0 (0) | 1.0 |
| Long-term anticoagulation, N (%) | 2 (5) | 1 (4) | 1 (8) | 0.52 |
| Long-term aspirin treatment, N (%) | 6 (15) | 3 (11) | 3 (25) | 0.34 |
| PaO2/FiO2 (mm Hg) | 84 (71–136) | 79 (61–111) | 93 (76–158) | 0.36 |
| SOFA score on admission | 8 (4–12) | 9 (6–13) | 5 (3–6) | 0.02 |
| Parameters * | All Patients (N = 40) | ECMO before/during the 10-Day Dexamethasone Course (N = 28) | ECMO after the 10-Day Dexamethasone Course (N = 12) | p-Value |
|---|---|---|---|---|
| Serum creatinine (µmol/L) | 116 (64–265) | 152 (71–283) | 92 (61–139) | 0.14 |
| Serum alanine aminotransferase (IU/L) | 38 (24–80) | 38 (26–66) | 44 (22–92) | 0.95 |
| Blood lactate (mmol/L) | 1.5 (1.2–2.8) | 1.5 (1.3–2.9) | 1.6 (1.2–2.2) | 0.81 |
| C-reactive protein (mg/L) | 155 (94–264) | 155 (97–222) | 177 (56–306) | 0.83 |
| Procalcitonin (µg/L) | 1.40 (0.2–3.14) | 1.25 (0.62–2.85) | 2.19 (0.19–5.49) | 0.95 |
| Fibrinogen (g/L) | 6.0 (4.8–7.6) | 5.8 (4.4–7.1) | 6.3 (5.4–8.9) | 0.18 |
| D-dimer (ng/mL) | 3195 (1858–6208) | 2940 (1735–3550) | 5750 (2975–16,310) | 0.09 |
| PaO2/FiO2 (mm Hg) | 56 (48–66) | 55 (48–66) | 56 (53–67) | 0.58 |
| PaCO2 (mmHg) | 58 (47–67) | 56 (45–65) | 59 (48–69) | 0.55 |
| Arterial pH | 7.31 (7.23–7.37) | 7.32 (7.25–7.38) | 7.27 (7.20–7.34) | 0.23 |
| Shock, N (%) | 36 (90) | 25 (89) | 11 (92) | 1.0 |
| Renal replacement therapy, N (%) | 20 (50) | 14 (50) | 6 (50) | 1.0 |
| Time from first symptoms to ECMO (days) | 13 (10–17) | 12 (10–14) | 19 (16–28) | 0.0002 |
| Length of ICU stay (days) | 31 (19–46) | 30 (17–46) | 37 (22–45) | 0.42 |
| Length of hospital stay (days) | 39 (22–59) | 37 (21–62) | 39 (25–47) | 0.64 |
| Parameters * | All Patients (N = 40) | ECMO before/during the 10-Day Dexamethasone Course (N = 28) | ECMO after the 10-Day Dexamethasone Course (N = 12) | p-Value |
|---|---|---|---|---|
| Survival, N (%) | 12 (30) | 12 (43) | 0 (0) | 0.007 |
| Dexamethasone treatment, N (%) | 40 (100) | 28 (100) | 12 (100) | 1.0 |
| Dexamethasone 6 mg/day regimen, N (%) | 21 (53) | 9 (32) | 12 (100) | <0.0001 |
| Dexamethasone 20/1 mg/day regimen, N (%) | 19 (47) | 19 (78) | 0 (0) | <0.0001 |
| Time from first symptoms to dexamethasone (days) | 9 (7–13) | 12 (8–15) | 7 (6–7) | 0.003 |
| Tocilizumab treatment, N (%) | 8 (20) | 4 (14) | 4 (33) | 0.21 |
| Number of prone sessions before ECMO | 3 (2–4) | 3 (2–4) | 3 (2–5) | 0.75 |
| Inspired tidal volume (mL) | 352 (335–406) | 360 (343–400) | 350 (300–391) | 0.49 |
| Plateau pressure (cm H2O) | 30 (28–32) | 29 (25–30) | 32 (29–34) | 0.013 |
| Positive end-expiratory pressure (cm H2O) | 12 (10–14) | 12 (10–14) | 12 (10–14) | 0.65 |
| Static compliance (mL/cm H2O) | 22 (16–29) | 25 (21–30) | 16 (13–19) | 0.02 |
| Respiratory rate (cycle/min) | 30 (28–34) | 29 (27–32) | 33 (32–35) | 0.03 |
| Total duration of mechanical ventilation (days) | 28 (16–35) | 26 (16–36) | 30 (19–36) | 0.82 |
| Time from ICU admission to ECMO (days) | 7 (4–9) | 5 (2–7) | 12 (10–19) | <0.0001 |
| Time from intubation to ECMO (days) | 4 (1–6) | 3 (1–5) | 7 (2–11) | 0.04 |
| ECMO duration (days) | 14 (7–26) | 13 (6–21) | 26 (11–31) | 0.20 |
| Parameters * | Survivors (N = 12) | Non-Survivors (N = 28) | p-Value |
|---|---|---|---|
| Age (years) | 51 (43–56) | 60 (57–64) | 0.0006 |
| SOFA score on admission | 9 (4–11) | 7 (5–12) | 0.84 |
| Time from first symptom to ECMO (days) | 12 (12–14) | 14 (10–17) | 0.31 |
| Time from ICU admission to ECMO (days) | 4 (2–6) | 7 (5–11) | 0.017 |
| Time from intubation to ECMO (days) | 3 (0–4) | 5 (2–7) | 0.03 |
| Time from first symptom to dexamethasone (days) | 13 (7–15) | 9 (6–10) | 0.07 |
| Serum creatinine (µmol/L) | 112 (54–196) | 116 (78–272) | 0.53 |
| Renal replacement therapy, N (%) | 3 (25) | 17 (61) | 0.08 |
| Blood lactate (mmol/L) | 1.4 (1.2–2.0) | 1.6 (1.2–2.8) | 0.56 |
| PaO2/FiO2 at cannulation (mm Hg) | 55 (45–61) | 56 (48–68) | 0.43 |
| Presence of shock, N (%) | 10 (83) | 26 (93) | 0.57 |
| Arterial pH at cannulation | 7.37 (7.34–7.45) | 7.27 (7.20–7.33) | 0.002 |
| Plateau pressure (cmH2O) | 26 (21–30) | 30 (29–33) | 0.015 |
| Static compliance (ml/cmH2O) | 27 (20–30) | 21 (15–25) | 0.11 |
| Positive end-expiratory pressure (cmH2O) | 12 (10–14) | 12 (10–14) | 0.95 |
| Dexamethasone 6mg/day regimen, N (%) | 3 (25) | 18 (64) | 0.038 |
| ECMO after at least 10-day dexamethasone, N (%) | 0 (0) | 12 (43) | 0.007 |
| Parameters | Odds Ratio (CI) * | Area under the Curve of the Model (CI) | p-Value |
|---|---|---|---|
| Age | 0.855 (0.764–0.957) | 0.844 (0.694–0.939) | 0.0005 |
| pH at cannulation | 1.97 × 106 (44.600–87.3 × 109) | 0.854 (0.696–0.949) | 0.0008 |
| Time from ICU admission to ECMO | 0.796 (0.642–0.986) | 0.740 (0.577–0.865) | 0.007 |
| Plateau pressure | 0.762 (0.601–0.951) | 0.786 (0.606–0.911) | 0.005 |
| Time from intubation to ECMO | 0.759 (0.575–1.002) | 0.717 (0.553–0.848) | 0.019 |
| Dexamethasone 6mg/day regimen | 0.1852 (0.041–0.845) | 0.696 (0.531–0.832) | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voicu, S.; Goury, A.; Lacoste-Palasset, T.; Malissin, I.; Fanet, L.; Souissi, S.; Busto, J.; Legros, V.; Sutterlin, L.; Naim, G.; et al. Dismal Survival in COVID-19 Patients Requiring ECMO as Rescue Therapy after Corticosteroid Failure. J. Pers. Med. 2021, 11, 1238. https://doi.org/10.3390/jpm11111238
Voicu S, Goury A, Lacoste-Palasset T, Malissin I, Fanet L, Souissi S, Busto J, Legros V, Sutterlin L, Naim G, et al. Dismal Survival in COVID-19 Patients Requiring ECMO as Rescue Therapy after Corticosteroid Failure. Journal of Personalized Medicine. 2021; 11(11):1238. https://doi.org/10.3390/jpm11111238
Chicago/Turabian StyleVoicu, Sebastian, Antoine Goury, Thomas Lacoste-Palasset, Isabelle Malissin, Lucie Fanet, Samar Souissi, Julia Busto, Vincent Legros, Laetitia Sutterlin, Giulia Naim, and et al. 2021. "Dismal Survival in COVID-19 Patients Requiring ECMO as Rescue Therapy after Corticosteroid Failure" Journal of Personalized Medicine 11, no. 11: 1238. https://doi.org/10.3390/jpm11111238
APA StyleVoicu, S., Goury, A., Lacoste-Palasset, T., Malissin, I., Fanet, L., Souissi, S., Busto, J., Legros, V., Sutterlin, L., Naim, G., M’Rad, A., Pepin-Lehaleur, A., Deye, N., Mourvillier, B., & Mégarbane, B. (2021). Dismal Survival in COVID-19 Patients Requiring ECMO as Rescue Therapy after Corticosteroid Failure. Journal of Personalized Medicine, 11(11), 1238. https://doi.org/10.3390/jpm11111238