COVID-19 Pandemic: Public Health Risk Assessment and Risk Mitigation Strategies
Abstract
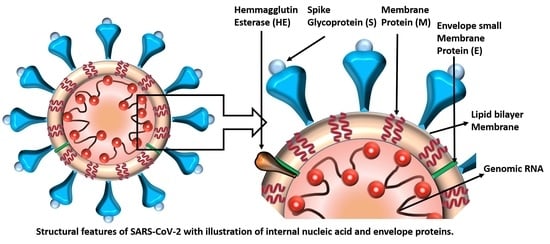
:1. Introduction
2. Risk Assessment Perspective
2.1. Risk Group
2.2. Host Range
2.3. Possible Transmission Routes
2.4. Fomite-Mediated Transmission
2.5. Surface Survival
2.6. Wastewater-Based Epidemiology
2.7. Reproduction Number
2.8. Viral Dose
2.9. Case Fatality Ratio (CFR)
3. Risk Mitigation Strategies
3.1. Administrative Control Measures
3.2. Engineering Control Measures
3.3. Personal Protective Equipment (PPE)
3.4. Herd Immunity via Vaccination
4. Experimental Section
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fidler, D.P. Brief History of the Global SARS Outbreak of 2002–03. In SARS, Governance and the Globalization of Disease; Fidler, D.P., Ed.; Palgrave Macmillan: London, UK, 2004; pp. 71–105. [Google Scholar] [CrossRef]
- Ahmad, A.; Krumkamp, R.; Reintjes, R. Controlling SARS: A review on China’s response compared with other SARS-affected countries. Trop. Med. Int. Health 2009, 14, 36–45. [Google Scholar] [CrossRef]
- Hajjar, S.A.; Memish, Z.A.; McIntosh, K. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): A perpetual challenge. Ann. Saudi Med. 2013, 33, 427–436. [Google Scholar] [CrossRef]
- Farooq, H.Z.; Davies, E.; Ahmad, S.; Machin, N.; Hesketh, L.; Guiver, M.; Turner, A.J. Middle East respiratory syndrome coronavirus (MERS-CoV)—Surveillance and testing in North England from 2012 to 2019. Int. J. Infect. Dis. 2020, 93, 237–244. [Google Scholar] [CrossRef]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Chapter Eight—Hosts and Sources of Endemic Human Coronaviruses. In Advances in Virus Research; Kielian, M., Mettenleiter, T.C., Roossinck, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 100, pp. 163–188. [Google Scholar]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China. Life Sci. 2020, 63, 457–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, E.; Tan, M.M.J.; Turk, E.; Sridhar, D.; Leung, G.M.; Shibuya, K.; Asgari, N.; Oh, J.; García-Basteiro, A.L.; Hanefeld, J.; et al. Lessons learnt from easing COVID-19 restrictions: An analysis of countries and regions in Asia Pacific and Europe. Lancet 2020, 396, 1525–1534. [Google Scholar] [CrossRef]
- Abbasi, A.Z.; Kiyani, D.A.; Hamid, S.M.; Saalim, M.; Fahim, A.; Jalal, N. Spiking dependence of SARS-CoV-2 pathogenicity on TMPRSS2. J. Med Virol. 2021, 93, 4205–4218. [Google Scholar] [CrossRef]
- Tan, J.; Liu, S.; Zhuang, L.; Chen, L.; Dong, M.; Zhang, J.; Xin, Y. Transmission and clinical characteristics of asymptomatic patients with SARS-CoV-2 infection. Future Virol. 2020, 15, 373–380. [Google Scholar] [CrossRef]
- Sagnelli, C.; Celia, B.; Monari, C.; Cirillo, S.; De Angelis, G.; Bianco, A.; Coppola, N. Management of SARS-CoV-2 pneumonia. J. Med. Virol. 2021, 93, 1276–1287. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Syed, A.; Elgorban, A.M.; Marraiki, N.; Kim, D.-Y. Biological characteristics and biomarkers of novel SARS-CoV-2 facilitated rapid development and implementation of diagnostic tools and surveillance measures. Biosens. Bioelectron. 2021, 177, 112969. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; Chu, D.K.; Akl, E.A.; El-harakeh, A.; Bognanni, A.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Bush, J.W.M. A guideline to limit indoor airborne transmission of COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2018995118. [Google Scholar] [CrossRef]
- Sah, P.; Fitzpatrick, M.C.; Zimmer, C.F.; Abdollahi, E.; Juden-Kelly, L.; Moghadas, S.M.; Singer, B.H.; Galvani, A.P. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2109229118. [Google Scholar] [CrossRef]
- Artika, I.M.; Ma’roef, C.N. Laboratory biosafety for handling emerging viruses. Asian Pac. J. Trop. Biomed. 2017, 7, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Wang, R.; Zhou, Y.; Hu, D.; Li, J.; Zhou, Y. Enhancing the capabilities of biosafety laboratories through the established accreditation system: Development of the biosafety laboratory accreditation system in China. J. Biosaf. Biosecurity 2019, 1, 86–89. [Google Scholar] [CrossRef]
- Yang, H.; Sürer, Ö.; Duque, D.; Morton, D.P.; Singh, B.; Fox, S.J.; Pasco, R.; Pierce, K.; Rathouz, P.; Valencia, V.; et al. Design of COVID-19 staged alert systems to ensure healthcare capacity with minimal closures. Nat. Commun. 2021, 12, 3767. [Google Scholar] [CrossRef]
- Rabi, F.A.; Al Zoubi, M.S.; Kasasbeh, G.A.; Salameh, D.M.; Al-Nasser, A.D. SARS-CoV-2 and Coronavirus Disease 2019: What We Know So Far. Pathogens 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Iwen, P.C.; Stiles, K.L.; Pentella, M.A. Safety Considerations in the Laboratory Testing of Specimens Suspected or Known to Contain the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Lab. Med. 2020, 51, 239–242. [Google Scholar] [CrossRef]
- Wu, D.; Wu, T.; Liu, Q.; Yang, Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Dis. 2020, 94, 44–48. [Google Scholar] [CrossRef]
- Chang, L.; Yan, Y.; Wang, L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus. Med. Rev. 2020, 34, 75–80. [Google Scholar] [CrossRef]
- Schröder, I. COVID-19: A Risk Assessment Perspective. ACS Chem. Health Saf. 2020, 27, 160–169. [Google Scholar] [CrossRef]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.Q.; Hoang, N.-A.; Quach, H.-L.; Nguyen, K.C.; Colquhoun, S.; Lambert, S.; Duong, L.H.; Tran, Q.D.; Ha, D.A.; Phung, D.C.; et al. Timeliness of contact tracing among flight passengers during the COVID-19 epidemic in Vietnam. BMC Infect. Dis. 2021, 21, 393. [Google Scholar] [CrossRef]
- Chinazzi, M.; Davis, J.T.; Ajelli, M.; Gioannini, C.; Litvinova, M.; Merler, S.; Pastore y Piontti, A.; Mu, K.; Rossi, L.; Sun, K.; et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science 2020, 368, 395–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, I.; Vetrivel, U.; Harish, D.R.; Chattophadhyay, D. Coding-Complete Genome Sequences of NITMA1086 and NITMA1139, Two SARS-CoV-2 Isolates from Belagavi District, Karnataka State, India, Harboring the D614G Mutation. Microbiol. Resour. Announc. 2021, 10, e00016-21. [Google Scholar] [CrossRef]
- Drake, J.W.; Holland, J.J. Mutation rates among RNA viruses. Proc. Natl. Acad. Sci. USA 1999, 96, 13910–13913. [Google Scholar] [CrossRef] [Green Version]
- Koonin, E.V.; Gorbalenya, A.E. Evolution of RNA genomes: Does the high mutation rate necessitate high rate of evolution of viral proteins? J. Mol. Evol. 1989, 28, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018, 16, e3000003. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, T.; Gandon, S.; Lion, S.; Otto, S.P. On the evolutionary epidemiology of SARS-CoV-2. Curr. Biol. 2020, 30, R849–R857. [Google Scholar] [CrossRef]
- Choi, Y.K. Emerging and re-emerging fatal viral diseases. Exp. Mol. Med. 2021, 53, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.L.; Suresh, M.; Huisingh, D. What can we learn from previous pandemics to reduce the frequency of emerging infectious diseases like COVID-19? Glob. Transit. 2020, 2, 202–220. [Google Scholar] [CrossRef]
- Graham, R.L.; Baric, R.S. Recombination, Reservoirs, and the Modular Spike: Mechanisms of Coronavirus Cross-Species Transmission. J. Virol. 2010, 84, 3134–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Kulsum, U.; Rufai, S.B.; Mudliar, S.R.; Singh, S. Mutations in SARS-CoV-2 Leading to Antigenic Variations in Spike Protein: A Challenge in Vaccine Development. J. Lab. Physicians 2020, 12, 154–160. [Google Scholar] [CrossRef]
- Kumar, S.; Maurya, V.K.; Prasad, A.K.; Bhatt, M.L.B.; Saxena, S.K. Structural, glycosylation and antigenic variation between 2019 novel coronavirus (2019-nCoV) and SARS coronavirus (SARS-CoV). Virus Dis. 2020, 31, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Abdelghany, T.M.; Ganash, M.; Bakri, M.M.; Qanash, H.; Al-Rajhi, A.M.H.; Elhussieny, N.I. SARS-CoV-2, the other face to SARS-CoV and MERS-CoV: Future predictions. Biomed. J. 2021, 44, 86–93. [Google Scholar] [CrossRef]
- Khailany, R.A.; Safdar, M.; Ozaslan, M. Genomic characterization of a novel SARS-CoV-2. Gene. Rep. 2020, 19, 100682. [Google Scholar] [CrossRef]
- Jia, Y.; Shen, G.; Zhang, Y.; Huang, K.-S.; Ho, H.-Y.; Hor, W.-S.; Yang, C.-H.; Li, C.; Wang, W.-L. Analysis of the mutation dynamics of SARS-CoV-2 reveals the spread history and emergence of RBD mutant with lower ACE2 binding affinity. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Amanat, F.; Krammer, F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Chan, J.F.-W.; Yuen, T.T.-T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.-Y.; Tsang, J.O.-L.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe. 2020, 1, e14–e23. [Google Scholar] [CrossRef]
- Luan, J.; Lu, Y.; Jin, X.; Zhang, L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem. Biophys. Res. Commun. 2020, 526, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.R.; Holmes, E.C.; Morens, D.M.; Park, E.-C.; Burke, D.S.; Calisher, C.H.; Laughlin, C.A.; Saif, L.J.; Daszak, P. Cross-Species Virus Transmission and the Emergence of New Epidemic Diseases. Microbiol. Mol. Biol. Rev. 2008, 72, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Reusken, C.B.E.M.; Haagmans, B.L.; Müller, M.A.; Gutierrez, C.; Godeke, G.-J.; Meyer, B.; Muth, D.; Raj, V.S.; Vries, L.S.-D.; Corman, V.M.; et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect. Dis. 2013, 13, 859–866. [Google Scholar] [CrossRef] [Green Version]
- Haagmans, B.L.; Al Dhahiry, S.H.S.; Reusken, C.B.E.M.; Raj, V.S.; Galiano, M.; Myers, R.; Godeke, G.-J.; Jonges, M.; Farag, E.; Diab, A.; et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect. Dis. 2014, 14, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Hu, Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008, 133, 74–87. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Pachetti, M.; Marini, B.; Benedetti, F.; Giudici, F.; Mauro, E.; Storici, P.; Masciovecchio, C.; Angeletti, S.; Ciccozzi, M.; Gallo, R.C.; et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J. Transl. Med. 2020, 18, 179. [Google Scholar] [CrossRef] [Green Version]
- Hemida, M.G.; Ba Abduallah, M.M. The SARS-CoV-2 outbreak from a one health perspective. One Health 2020, 10, 100127. [Google Scholar] [CrossRef]
- Danchin, A.; Timmis, K. SARS-CoV-2 variants: Relevance for symptom granularity, epidemiology, immunity (herd, vaccines), virus origin and containment? Environ. Microbiol. 2020, 22, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Smith, D.W. COVID-19: A novel zoonotic disease caused by a coronavirus from China: What we know and what we don’t. Microbiol. Aust. 2020, 41, 45–50. [Google Scholar] [CrossRef]
- Leroy, E.; Ar Gouilh, M.; Brugère-Picoux, J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health 2020, 10, 100133. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-W.; Yuan, S.; Yuen, K.-S.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020, 16, 1686–1697. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; Zhao, Y.; et al. Susceptibility of ferrets, cats, dogs, and different domestic animals to SARS-coronavirus-2. BioRxiv 2020. [Google Scholar] [CrossRef]
- Lam, T.T.-Y.; Jia, N.; Zhang, Y.-W.; Shum, M.H.-H.; Jiang, J.-F.; Zhu, H.-C.; Tong, Y.-G.; Shi, Y.-X.; Ni, X.-B.; Liao, Y.-S.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Newman, C.; Buesching, C.D.; Macdonald, D.W.; Zhou, Z.-M. Animal sales from Wuhan wet markets immediately prior to the COVID-19 pandemic. Sci. Rep. 2021, 11, 11898. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Dietrich, M.L.; Senior, R.A.; Wilcove, D.S. A better classification of wet markets is key to safeguarding human health and biodiversity. Lancet Planet. Health 2021, 5, e386–e394. [Google Scholar] [CrossRef]
- Morawska, L.; Tang, J.W.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.; Floto, A.; Franchimon, F.; et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020, 142, 105832. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef]
- Caputo, V.; Termine, A.; Fabrizio, C.; Calvino, G.; Luzzi, L.; Fusco, C.; Ingrascì, A.; Peconi, C.; Alessio, R.; Mihali, S.; et al. Age and Sex Modulate SARS-CoV-2 Viral Load Kinetics: A Longitudinal Analysis of 1735 Subjects. J. Pers. Med. 2021, 11, 882. [Google Scholar] [CrossRef]
- Meiksin, A. Dynamics of COVID-19 transmission including indirect transmission mechanisms: A mathematical analysis. Epidemiol. Infect. 2020, 148, e257. [Google Scholar] [CrossRef]
- Meselson, M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2063. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Anfinrud, P.; Stadnytskyi, V.; Bax, C.E.; Bax, A. Visualizing Speech-Generated Oral Fluid Droplets with Laser Light Scattering. N. Engl. J. Med. 2020, 382, 2061–2063. [Google Scholar] [CrossRef]
- Buitrago-Garcia, D.; Egli-Gany, D.; Counotte, M.J.; Hossmann, S.; Imeri, H.; Ipekci, A.M.; Salanti, G.; Low, N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020, 17, e1003346. [Google Scholar] [CrossRef]
- Asadi, S.; Wexler, A.S.; Cappa, C.D.; Barreda, S.; Bouvier, N.M.; Ristenpart, W.D. Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 2019, 9, 2348. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.A.; Man, J.C.; Brand, P.; Katstra, J.P.; Sommerer, K.; Stone, H.A.; Nardell, E.; Scheuch, G. Inhaling to mitigate exhaled bioaerosols. Proc. Natl. Acad. Sci. USA 2004, 101, 17383–17388. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.S.; Duchaine, C. SARS-CoV-2 and Health Care Worker Protection in Low-Risk Settings: A Review of Modes of Transmission and a Novel Airborne Model Involving Inhalable Particles. Clin. Microbiol. Rev. 2020, 34, e00184-20. [Google Scholar] [CrossRef]
- Yu, I.T.S.; Li, Y.; Wong, T.W.; Tam, W.; Chan, A.T.; Lee, J.H.W.; Leung, D.Y.C.; Ho, T. Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus. N. Engl. J. Med. 2004, 350, 1731–1739. [Google Scholar] [CrossRef] [Green Version]
- He, G.; Pan, Y.; Tanaka, T. The short-term impacts of COVID-19 lockdown on urban air pollution in China. Nat. Sustain. 2020, 3, 1005–1011. [Google Scholar] [CrossRef]
- Danchin, A.; Ng, T.W.; Turinici, G. A New Transmission Route for the Propagation of the SARS-CoV-2 Coronavirus. Biology 2021, 10, 10. [Google Scholar] [CrossRef]
- Todt, D.; Meister, T.L.; Tamele, B.; Howes, J.; Paulmann, D.; Becker, B.; Brill, F.H.; Wind, M.; Schijven, J.; Heinen, N.; et al. A realistic transfer method reveals low risk of SARS-CoV-2 transmission via contaminated euro coins and banknotes. IScience 2021, 24, 102908. [Google Scholar] [CrossRef]
- Wilson, A.M.; Weir, M.H.; Bloomfield, S.F.; Scott, E.A.; Reynolds, K.A. Modeling COVID-19 infection risks for a single hand-to-fomite scenario and potential risk reductions offered by surface disinfection. Am. J. Infect. Control. 2021, 49, 846–848. [Google Scholar] [CrossRef]
- Harvey, A.P.; Fuhrmeister, E.R.; Cantrell, M.E.; Pitol, A.K.; Swarthout, J.M.; Powers, J.E.; Nadimpalli, M.L.; Julian, T.R.; Pickering, A.J. Longitudinal Monitoring of SARS-CoV-2 RNA on High-Touch Surfaces in a Community Setting. Environ. Sci. Technol. Lett. 2021, 8, 168–175. [Google Scholar] [CrossRef]
- Pitol, A.K.; Julian, T.R. Community Transmission of SARS-CoV-2 by Surfaces: Risks and Risk Reduction Strategies. Environ. Sci. Technol. Lett. 2021, 8, 263–269. [Google Scholar] [CrossRef]
- Doung-Ngern, P.; Suphanchaimat, R.; Panjangampatthana, A.; Janekrongtham, C.; Ruampoom, D.; Daochaeng, N.; Eungkanit, N.; Pisitpayat, N.; Srisong, N.; Yasopa, O.; et al. Case-Control Study of Use of Personal Protective Measures and Risk for SARS-CoV 2 Infection, Thailand. Emerg. Infect. Dis. 2020, 26, 2607–2616. [Google Scholar] [CrossRef]
- Castaño, N.; Cordts, S.C.; Kurosu Jalil, M.; Zhang, K.S.; Koppaka, S.; Bick, A.D.; Paul, R.; Tang, S.K.Y. Fomite Transmission, Physicochemical Origin of Virus–Surface Interactions, and Disinfection Strategies for Enveloped Viruses with Applications to SARS-CoV-2. ACS Omega 2021, 6, 6509–6527. [Google Scholar] [CrossRef]
- Li, D.; Sangion, A.; Li, L. Evaluating consumer exposure to disinfecting chemicals against coronavirus disease 2019 (COVID-19) and associated health risks. Environ. Int. 2020, 145, 106108. [Google Scholar] [CrossRef]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. MSphere 2020, 5, e00441-20. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020, 1, e10. [Google Scholar] [CrossRef]
- Kratzel, A.; Steiner, S.; Todt, D.; V’Kovski, P.; Brueggemann, Y.; Steinmann, J.; Steinmann, E.; Thiel, V.; Pfaender, S. Temperature-dependent surface stability of SARS-CoV-2. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef]
- Firquet, S.; Beaujard, S.; Lobert, P.-E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Stein, R.A. Super-spreaders in infectious diseases. Int. J. Infect. Dis. 2011, 15, e510–e513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Murallidharan, J.S.; Agrawal, A.; Bhardwaj, R. Why coronavirus survives longer on impermeable than porous surfaces. Phys. Fluids 2021, 33, 021701. [Google Scholar] [CrossRef]
- Spurbeck, R.R.; Minard-Smith, A.T.; Catlin, L.A. Applicability of Neighborhood and Building Scale Wastewater-Based Genomic Epidemiology to Track the SARS-CoV-2 Pandemic and other Pathogens. MedRxiv 2021. [Google Scholar] [CrossRef]
- Kitajima, M.; Ahmed, W.; Bibby, K.; Carducci, A.; Gerba, C.P.; Hamilton, K.A.; Haramoto, E.; Rose, J.B. SARS-CoV-2 in wastewater: State of the knowledge and research needs. Sci. Total Environ. 2020, 739, 139076. [Google Scholar] [CrossRef]
- Michael-Kordatou, I.; Karaolia, P.; Fatta-Kassinos, D. Sewage analysis as a tool for the COVID-19 pandemic response and management: The urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020, 8, 104306. [Google Scholar] [CrossRef]
- Lu, D.; Huang, Z.; Luo, J.; Zhang, X.; Sha, S. Primary concentration—The critical step in implementing the wastewater based epidemiology for the COVID-19 pandemic: A mini-review. Sci. Total Environ. 2020, 747, 141245. [Google Scholar] [CrossRef]
- Fuschi, C.; Pu, H.; Negri, M.; Colwell, R.; Chen, J. Wastewater-Based Epidemiology for Managing the COVID-19 Pandemic. ACS EST Water 2021, 1, 1352–1362. [Google Scholar] [CrossRef]
- Saththasivam, J.; El-Malah, S.S.; Gomez, T.A.; Jabbar, K.A.; Remanan, R.; Krishnankutty, A.K.; Ogunbiyi, O.; Rasool, K.; Ashhab, S.; Rashkeev, S.; et al. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci. Total Environ. 2021, 774, 145608. [Google Scholar] [CrossRef] [PubMed]
- Hrudey, S.E.; Silva, D.S.; Shelley, J.; Pons, W.; Isaac-Renton, J.; Chik, A.H.-S.; Conant, B. Ethics Guidance for Environmental Scientists Engaged in Surveillance of Wastewater for SARS-CoV-2. Environ. Sci. Technol. 2021, 55, 8484–8491. [Google Scholar] [CrossRef] [PubMed]
- McKeigue, P.M.; Weir, A.; Bishop, J.; McGurnaghan, S.J.; Kennedy, S.; McAllister, D.; Robertson, C.; Wood, R.; Lone, N.; Murray, J.; et al. Rapid Epidemiological Analysis of Comorbidities and Treatments as risk factors for COVID-19 in Scotland (REACT-SCOT): A population-based case-control study. PLoS Med. 2020, 17, e1003374. [Google Scholar] [CrossRef]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef] [Green Version]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef]
- Sanche, S.; Lin, Y.T.; Xu, C.; Romero-Severson, E.; Hengartner, N.; Ke, R. High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg. Infect. Dis. 2020, 26, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Eifan, S.A.; Nour, I.; Hanif, A.; Zamzam, A.M.M.; AlJohani, S.M. A pandemic risk assessment of middle east respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 1631–1638. [Google Scholar] [CrossRef]
- Bauch, C.T.; Lloyd-Smith, J.O.; Coffee, M.P.; Galvani, A.P. Dynamically Modeling SARS and Other Newly Emerging Respiratory Illnesses: Past, Present, and Future. Epidemiology 2005, 16, 791–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolai, L.A.; Meyer, C.G.; Kremsner, P.G.; Velavan, T.P. Asymptomatic SARS Coronavirus 2 infection: Invisible yet invincible. Int. J. Infect. Dis. 2020, 100, 112–116. [Google Scholar] [CrossRef]
- Prevalence of Asymptomatic SARS-CoV-2 Infection. Ann. Intern. Med. 2020, 173, 362–367. [CrossRef]
- Giordano, G.; Colaneri, M.; Di Filippo, A.; Blanchini, F.; Bolzern, P.; De Nicolao, G.; Sacchi, P.; Colaneri, P.; Bruno, R. Modeling vaccination rollouts, SARS-CoV-2 variants and the requirement for non-pharmaceutical interventions in Italy. Nat. Med. 2021, 27, 993–998. [Google Scholar] [CrossRef]
- Li, M.; Yuan, J.; Lv, G.; Brown, J.; Jiang, X.; Lu, Z.K. Myocarditis and Pericarditis following COVID-19 Vaccination: Inequalities in Age and Vaccine Types. J. Pers. Med. 2021, 11, 1106. [Google Scholar] [CrossRef]
- Tsang, T.K.; Cowling, B.J.; Fang, V.J.; Chan, K.-H.; Ip, D.K.M.; Leung, G.M.; Peiris, J.S.M.; Cauchemez, S. Influenza A Virus Shedding and Infectivity in Households. J. Infect. Dis. 2015, 212, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Paulo, A.C.; Correia-Neves, M.; Domingos, T.; Murta, A.G.; Pedrosa, J. Influenza Infectious Dose May Explain the High Mortality of the Second and Third Wave of 1918–1919 Influenza Pandemic. PLoS ONE 2010, 5, e11655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.-Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquès, M.; Domingo, J.L. Contamination of inert surfaces by SARS-CoV-2: Persistence, stability and infectivity. A review. Environ. Res. 2021, 193, 110559. [Google Scholar] [CrossRef]
- Corman, V.M.; Albarrak, A.M.; Omrani, A.S.; Albarrak, M.M.; Farah, M.E.; Almasri, M.; Muth, D.; Sieberg, A.; Meyer, B.; Assiri, A.M.; et al. Viral Shedding and Antibody Response in 37 Patients With Middle East Respiratory Syndrome Coronavirus Infection. Clin. Infect. Dis. 2015, 62, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet. Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.K.; Leong, D.; Munusamy, H.; Zenol Ariffin, N.H.; Kori, N.; Hod, R.; Periyasamy, P. The prevalence and clinical significance of Presymptomatic COVID-19 patients: How we can be one step ahead in mitigating a deadly pandemic. BMC Infect. Dis. 2021, 21, 249. [Google Scholar] [CrossRef]
- Seligman, B.; Ferranna, M.; Bloom, D.E. Social determinants of mortality from COVID-19: A simulation study using NHANES. PLoS Med. 2021, 18, e1003490. [Google Scholar] [CrossRef]
- Skopljanac, I.; Ivelja, M.P.; Barcot, O.; Brdar, I.; Dolic, K.; Polasek, O.; Radic, M. Role of Lung Ultrasound in Predicting Clinical Severity and Fatality in COVID-19 Pneumonia. J. Pers. Med. 2021, 11, 757. [Google Scholar] [CrossRef]
- Synowiec, A.; Szczepański, A.; Barreto-Duran, E.; Lie, L.K.; Pyrc, K. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Systemic Infection. Clin. Microbiol. Rev. 2021, 34, e00133-20. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Song, I.-A.; Oh, T.-K. Dementia Risk among Coronavirus Disease Survivors: A Nationwide Cohort Study in South Korea. J. Pers. Med. 2021, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Mon, M.; Ortega, M.A.; Gasulla, Ó.; Fortuny-Profitós, J.; Mazaira-Font, F.A.; Saurina, P.; Monserrat, J.; Plana, M.N.; Troncoso, D.; Moreno, J.S.; et al. A Predictive Model and Risk Factors for Case Fatality of COVID-19. J. Pers. Med. 2021, 11, 36. [Google Scholar] [CrossRef]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spreeuwenberg, P.; Kroneman, M.; Paget, J. Reassessing the Global Mortality Burden of the 1918 Influenza Pandemic. Am. J. Epidemiol. 2018, 187, 2561–2567. [Google Scholar] [CrossRef] [Green Version]
- Viboud, C.; Simonsen, L.; Fuentes, R.; Flores, J.; Miller, M.A.; Chowell, G. Global Mortality Impact of the 1957–1959 Influenza Pandemic. J. Infect. Dis. 2016, 213, 738–745. [Google Scholar] [CrossRef]
- Viboud, C.; Grais, R.F.; Lafont, B.A.; Miller, M.A.; Simonsen, L. Multinational impact of the 1968 Hong Kong influenza pandemic: Evidence for a smoldering pandemic. J. Infect. Dis. 2005, 192, 233–248. [Google Scholar] [CrossRef]
- Tchounga, B.; Ekouevi, D.K.; Balestre, E.; Dabis, F. Mortality and survival patterns of people living with HIV-2. Curr. Opin. HIV AIDS 2016, 11, 537–544. [Google Scholar] [CrossRef] [Green Version]
- CHAN-YEUNG, M.; XU, R.-H. SARS: Epidemiology. Respirology 2003, 8, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Kelly, H.; Ip, D.K.M.; Wu, J.T.; Leung, G.M.; Cowling, B.J. Case Fatality Risk of Influenza A (H1N1pdm09): A Systematic Review. Epidemiology 2013, 24, 830–841. [Google Scholar] [CrossRef] [Green Version]
- Al-Raddadi, R.M.; Shabouni, O.I.; Alraddadi, Z.M.; Alzalabani, A.H.; Al-Asmari, A.M.; Ibrahim, A.; Almarashi, A.; Madani, T.A. Burden of Middle East respiratory syndrome coronavirus infection in Saudi Arabia. J. Infect. Public Health 2020, 13, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Kucharski, A.J.; Funk, S.; Breman, J.; Piot, P.; Edmunds, W.J. Potential for large outbreaks of Ebola virus disease. Epidemics 2014, 9, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.J.; de Magalhães-Barbosa, M.C.; Lima-Setta, F.; Medronho, R.A.; Prata-Barbosa, A. Microcephaly Case Fatality Rate Associated with Zika Virus Infection in Brazil: Current Estimates. Pediatric Infect. Dis. J. 2017, 36, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Ghayda, R.A.; Lee, K.H.; Han, Y.J.; Ryu, S.; Hong, S.H.; Yoon, S.; Jeong, G.H.; Lee, J.; Lee, J.Y.; Yang, J.W.; et al. Estimation of global case fatality rate of coronavirus disease 2019 (COVID-19) using meta-analyses: Comparison between calendar date and days since the outbreak of the first confirmed case. Int. J. Infect. Dis. 2020, 100, 302–308. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kagaya, K.; Chowell, G. Early epidemiological assessment of the transmission potential and virulence of coronavirus disease 2019 (COVID-19) in Wuhan City: China, January–February, 2020. MedRxiv 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Auwaerter, P.M.D. Coronavirus COVID-19 (SARS-CoV-2). 2020. Available online: https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540747/all/Coronavirus_COVID_19__SARS_CoV_2_ (accessed on 15 October 2021).
- Harrison, S.L.; Fazio-Eynullayeva, E.; Lane, D.A.; Underhill, P.; Lip, G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020, 17, e1003321. [Google Scholar] [CrossRef]
- Hauser, A.; Counotte, M.J.; Margossian, C.C.; Konstantinoudis, G.; Low, N.; Althaus, C.L.; Riou, J. Estimation of SARS-CoV-2 mortality during the early stages of an epidemic: A modeling study in Hubei, China, and six regions in Europe. PLoS Med. 2020, 17, e1003189. [Google Scholar] [CrossRef]
- Bassett, M.T.; Chen, J.T.; Krieger, N. Variation in racial/ethnic disparities in COVID-19 mortality by age in the United States: A cross-sectional study. PLoS Med. 2020, 17, e1003402. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, Y.; Shen, M.; Fairley, C.K.; Guo, Y. Can self-imposed prevention measures mitigate the COVID-19 epidemic? PLoS Med. 2020, 17, e1003240. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.-D.; Novoselov, K.S. Sustainable Personal Protective Clothing for Healthcare Applications: A Review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef] [PubMed]
- Goldman, N.; Pebley, A.R.; Lee, K.; Andrasfay, T.; Pratt, B. Racial and ethnic differentials in COVID-19-related job exposures by occupational standing in the US. Medrxiv Prepr. Serv. Health Sci. 2021, 16, e0256085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Li, B.-R.; Ning, B.-T. The Comparative Immunological Characteristics of SARS-CoV, MERS-CoV, and SARS-CoV-2 Coronavirus Infections. Front. Immunol. 2020, 11, 2033. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, P.; Kuśnierz, S.; Sosna, P.; Mauer, J.; Maj, D. Disposal of Personal Protective Equipment during the COVID-19 Pandemic Is a Challenge for Waste Collection Companies and Society: A Case Study in Poland. Resources 2020, 9, 116. [Google Scholar] [CrossRef]
- Berardi, A.; Perinelli, D.R.; Merchant, H.A.; Bisharat, L.; Basheti, I.A.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. Hand sanitisers amid CoViD-19: A critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int. J. Pharm. 2020, 584, 119431. [Google Scholar] [CrossRef]
- Schrank, C.L.; Minbiole, K.P.C.; Wuest, W.M. Are Quaternary Ammonium Compounds, the Workhorse Disinfectants, Effective against Severe Acute Respiratory Syndrome-Coronavirus-2? ACS Infect. Dis. 2020, 6, 1553–1557. [Google Scholar] [CrossRef]
- Bavel, J.J.V.; Baicker, K.; Boggio, P.S.; Capraro, V.; Cichocka, A.; Cikara, M.; Crockett, M.J.; Crum, A.J.; Douglas, K.M.; Druckman, J.N.; et al. Using social and behavioural science to support COVID-19 pandemic response. Nat. Hum. Behav. 2020, 4, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, N.J.; Milton, D.K. Applying the Hierarchy of Controls: What Occupational Safety Can Teach us About Safely Navigating the Next Phase of the Global COVID-19 Pandemic. Front. Public Health 2021, 9, 7894. [Google Scholar] [CrossRef] [PubMed]
- Köppen, J.; Hartl, K.; Maier, C.B. Health workforce response to Covid-19: What pandemic preparedness planning and action at the federal and state levels in Germany?: Germany’s health workforce responses to Covid-19. Int. J. Health Plan. Manag. 2021, 36, 71–91. [Google Scholar] [CrossRef]
- Dighe, A.; Cattarino, L.; Cuomo-Dannenburg, G.; Skarp, J.; Imai, N.; Bhatia, S.; Gaythorpe, K.A.M.; Ainslie, K.E.C.; Baguelin, M.; Bhatt, S.; et al. Response to COVID-19 in South Korea and implications for lifting stringent interventions. BMC Med. 2020, 18, 321. [Google Scholar] [CrossRef]
- Hannan, M.M.; Azadian, B.S.; Gazzard, B.G.; Hawkins, D.A.; Hoffman, P.N. Hospital infection control in an era of HIV infection and multi-drug resistant tuberculosis. J. Hosp. Infect. 2000, 44, 5–11. [Google Scholar] [CrossRef]
- Christopherson, D.A.; Yao, W.C.; Lu, M.; Vijayakumar, R.; Sedaghat, A.R. High-Efficiency Particulate Air Filters in the Era of COVID-19: Function and Efficacy. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2020, 163, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Huang, L.; Chen, X.; Wang, J.; Wu, W.; Yin, S.; Chen, W.; Zhan, J.; Yan, L.; Ma, L.; et al. Ventilation of wards and nosocomial outbreak of severe acute respiratory syndrome among healthcare workers. Chin. Med. J. 2003, 116, 1293–1297. [Google Scholar]
- Smieszek, T.; Lazzari, G.; Salathé, M. Assessing the Dynamics and Control of Droplet- and Aerosol-Transmitted Influenza Using an Indoor Positioning System. Sci. Rep. 2019, 9, 2185. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Li, Y.; Xu, P.; Cowling, B.J. Evaluation of intervention strategies in schools including ventilation for influenza transmission control. Build. Simul. 2012, 5, 29–37. [Google Scholar] [CrossRef]
- Escombe, A.R.; Oeser, C.C.; Gilman, R.H.; Navincopa, M.; Ticona, E.; Pan, W.; Martínez, C.; Chacaltana, J.; Rodríguez, R.; Moore, D.A.J.; et al. Natural Ventilation for the Prevention of Airborne Contagion. PLoS Med. 2007, 4, e68. [Google Scholar] [CrossRef] [Green Version]
- Morawska, L.; Milton, D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar] [CrossRef]
- Morawska, L.; Allen, J.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.J.; Floto, A.; Franchimon, F.; et al. A paradigm shift to combat indoor respiratory infection. Science 2021, 372, 689–691. [Google Scholar] [CrossRef]
- Allen, J.G.; Ibrahim, A.M. Indoor Air Changes and Potential Implications for SARS-CoV-2 Transmission. JAMA 2021, 325, 2112–2113. [Google Scholar] [CrossRef]
- Sharma, P. Personal Protective Equipment. BDJ Pract. 2021, 34, 38. [Google Scholar] [CrossRef]
- O’Dowd, K.; Nair, K.M.; Forouzandeh, P.; Mathew, S.; Grant, J.; Moran, R.; Bartlett, J.; Bird, J.; Pillai, S.C. Face Masks and Respirators in the Fight Against the COVID-19 Pandemic: A Review of Current Materials, Advances and Future Perspectives. Materials 2020, 13, 3363. [Google Scholar] [CrossRef]
- Elkington, P.T.; Dickinson, A.S.; Mavrogordato, M.N.; Spencer, D.C.; Gillams, R.J.; De Grazia, A.; Rosini, S.; Garay-Baquero, D.J.; Diment, L.E.; Mahobia, N.; et al. A Personal Respirator to Improve Protection for Healthcare Workers Treating COVID-19 (PeRSo). Front. Med. Technol. 2021, 3, 4259. [Google Scholar] [CrossRef]
- Parlin, A.F.; Stratton, S.M.; Culley, T.M.; Guerra, P.A. A laboratory-based study examining the properties of silk fabric to evaluate its potential as a protective barrier for personal protective equipment and as a functional material for face coverings during the COVID-19 pandemic. PLoS ONE 2020, 15, e0239531. [Google Scholar] [CrossRef]
- Singh, N.; Tang, Y.; Ogunseitan, O.A. Environmentally Sustainable Management of Used Personal Protective Equipment. Environ. Sci. Technol. 2020, 54, 8500–8502. [Google Scholar] [CrossRef]
- Uddin, M.A.; Afroj, S.; Hasan, T.; Carr, C.; Novoselov, K.S.; Karim, N. Environmental Impacts of Personal Protective Clothing Used to Combat COVID-19. Adv. Sustain. Syst. 2021, 2100176. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-C.; Lin, S.-Y.; Chen, S.-C.; Chiu, Y.-W.; Chang, J.-M.; Hwang, S.-J. COVID-19 Vaccines in Patients with Maintenance Hemodialysis. J. Pers. Med. 2021, 11, 789. [Google Scholar] [CrossRef]
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matrajt, L.; Eaton, J.; Leung, T.; Dimitrov, D.; Schiffer, J.T.; Swan, D.A.; Janes, H. Optimizing vaccine allocation for COVID-19 vaccines shows the potential role of single-dose vaccination. Nat. Commun. 2021, 12, 3449. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A. Core Concept: Herd immunity is an important—and often misunderstood—public health phenomenon. Proc. Natl. Acad. Sci. USA 2021, 118, e2107692118. [Google Scholar] [CrossRef]
- Forman, R.; Shah, S.; Jeurissen, P.; Jit, M.; Mossialos, E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy 2021, 125, 553–567. [Google Scholar] [CrossRef]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Cohen, D.; Muhsen, K.; Chodick, G.; Patalon, T. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: Reinfections versus breakthrough infections. MedRxiv 2021. [Google Scholar] [CrossRef]
- Gupta, R.K. Will SARS-CoV-2 variants of concern affect the promise of vaccines? Nat. Rev. Immunol. 2021, 21, 340–341. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef]
- Buckner, J.H.; Chowell, G.; Springborn, M.R. Dynamic prioritization of COVID-19 vaccines when social distancing is limited for essential workers. Proc. Natl. Acad. Sci. USA 2021, 118, e2025786118. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Estella, Á.; Cantón, M.L.; Muñoz, L.; Higueras, I.R.; Recuerda Núñez, M.; Tejero Aranguren, J.; Zaya, B.; Gómez, C.; Amaya, R.; Hurtado Martinez, Á.; et al. Vaccinated Patients Admitted in ICU with Severe Pneumonia Due to SARS-CoV-2: A Multicenter Pilot Study. J. Pers. Med. 2021, 11, 1086. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, N.; Thakur, N.; Bhatia, S.K.; Saratale, G.D.; Ghodake, G.; Mistry, B.M.; Alavilli, H.; Kishor, D.S.; Du, X.; et al. A Comprehensive Overview on the Production of Vaccines in Plant-Based Expression Systems and the Scope of Plant Biotechnology to Combat against SARS-CoV-2 Virus Pandemics. Plants 2021, 10, 1213. [Google Scholar] [CrossRef] [PubMed]
- Giubilini, A.; Savulescu, J.; Wilkinson, D. Queue questions: Ethics of COVID-19 vaccine prioritization. Bioethics 2021, 35, 348–355. [Google Scholar] [CrossRef]
- Cromer, D.; Juno, J.A.; Khoury, D.; Reynaldi, A.; Wheatley, A.K.; Kent, S.J.; Davenport, M.P. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat. Rev. Immunol. 2021, 21, 395–404. [Google Scholar] [CrossRef]
- Lv, G.; Yuan, J.; Xiong, X.; Li, M. Mortality Rate and Characteristics of Deaths Following COVID-19 Vaccination. Front. Med. 2021, 8, 370. [Google Scholar] [CrossRef]
- Bottino, F.; Tagliente, E.; Pasquini, L.; Napoli, A.D.; Lucignani, M.; Figà-Talamanca, L.; Napolitano, A. COVID Mortality Prediction with Machine Learning Methods: A Systematic Review and Critical Appraisal. J. Pers. Med. 2021, 11, 893. [Google Scholar] [CrossRef] [PubMed]



| Year | Contagion | Disease | Worldwide Cases | Worldwide Deaths | Fatality Ratio | References |
|---|---|---|---|---|---|---|
| 1918 | Influenza A (H1N1) | Influenza | 500 million | >17.4 million | >2.54% | [122] |
| 1957–1959 | Influenza A (H2N2) | Influenza | unidentified | 1.1 million | <0.11% | [123] |
| 1968 | Influenza A (H3N2) | Influenza | unidentified | 1.0 million | <0.52% | [124] |
| 1981 | HIV | HIV/AIDS | 75 million | 32 million | 99.98% | [125] |
| 2002 | SARS | SARS | 8422 | 916 | 11.4% | [126] |
| 2009 | Influenza A (H1N1) | Influenza | 12,700 | 4700 | 0.1–5% | [127] |
| 2012 | MERS | MERS | 2494 | 11,325 | 34% | [128] |
| 2014–2016 | Ebola virus | Ebola | 28,652 | 13,562 | 40% | [129] |
| 2016 | Zika virus | Zika | 41,300 | --- | 8.3% | [130] |
| 2019 | SARS-CoV-2 | COVID-19 | 101,561,219 | 2,196,944 | 2.1 | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-Y.; Shinde, S.K.; Lone, S.; Palem, R.R.; Ghodake, G.S. COVID-19 Pandemic: Public Health Risk Assessment and Risk Mitigation Strategies. J. Pers. Med. 2021, 11, 1243. https://doi.org/10.3390/jpm11121243
Kim D-Y, Shinde SK, Lone S, Palem RR, Ghodake GS. COVID-19 Pandemic: Public Health Risk Assessment and Risk Mitigation Strategies. Journal of Personalized Medicine. 2021; 11(12):1243. https://doi.org/10.3390/jpm11121243
Chicago/Turabian StyleKim, Dae-Young, Surendra Krushna Shinde, Saifullah Lone, Ramasubba Reddy Palem, and Gajanan Sampatrao Ghodake. 2021. "COVID-19 Pandemic: Public Health Risk Assessment and Risk Mitigation Strategies" Journal of Personalized Medicine 11, no. 12: 1243. https://doi.org/10.3390/jpm11121243
APA StyleKim, D. -Y., Shinde, S. K., Lone, S., Palem, R. R., & Ghodake, G. S. (2021). COVID-19 Pandemic: Public Health Risk Assessment and Risk Mitigation Strategies. Journal of Personalized Medicine, 11(12), 1243. https://doi.org/10.3390/jpm11121243