Virtual Reality-Based Early Neurocognitive Stimulation in Critically Ill Patients: A Pilot Randomized Clinical Trial
Abstract
:1. Introduction
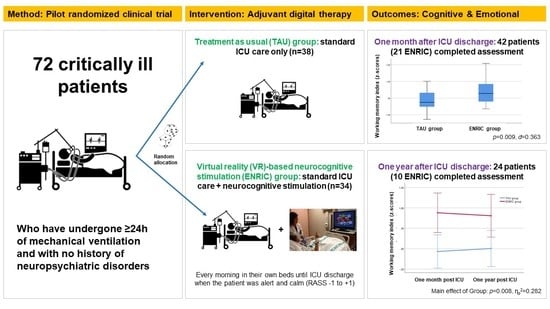
2. Materials and Methods
2.1. Study Design
2.2. Sample Size
2.3. ENRIC Technology Features
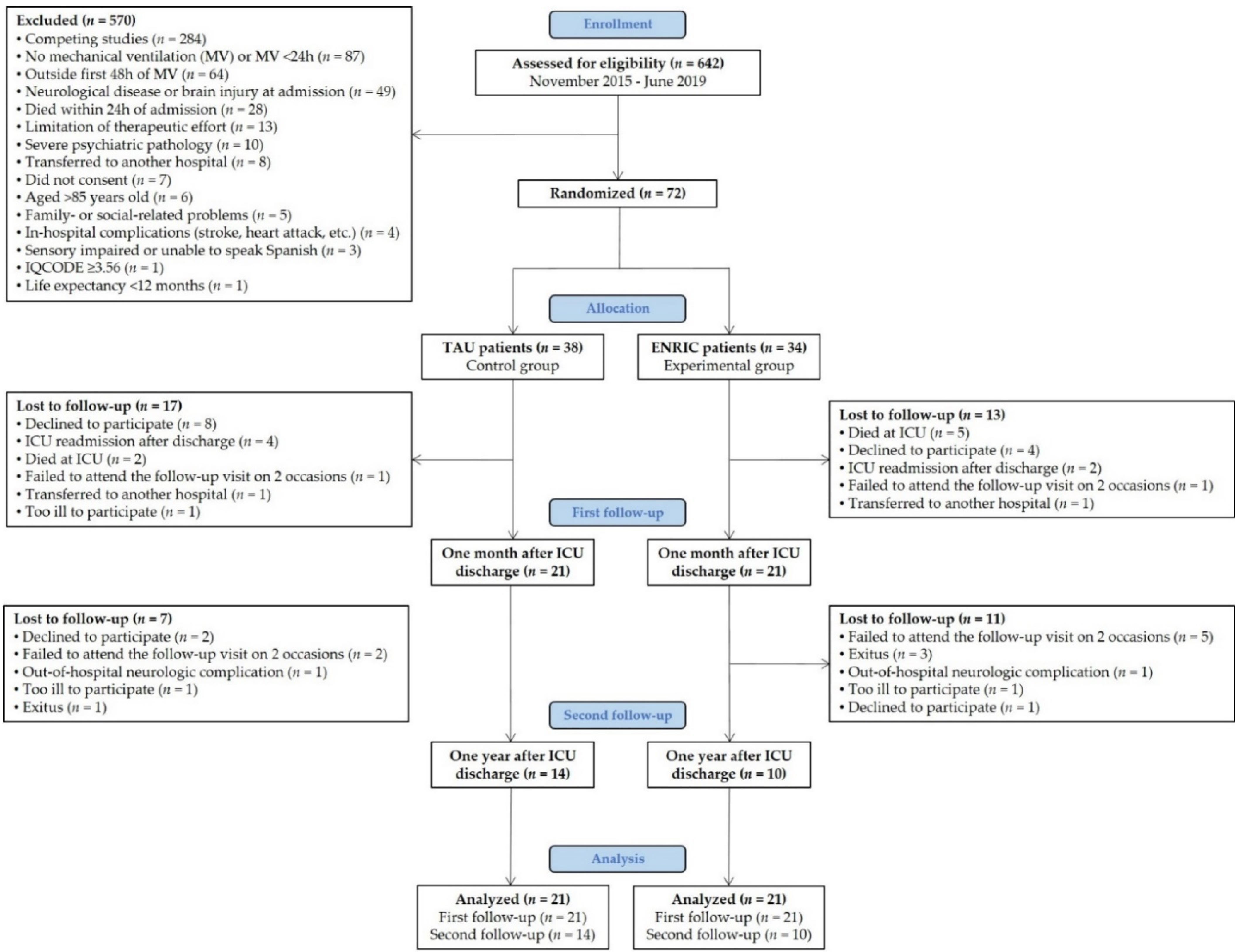
2.4. Participants and Procedure
2.5. Cognitive and Emotional Assessment
2.6. Primary and Secondary Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Characteristics of Early Neurocognitive Stimulation
3.3. Cognitive and Emotional Outcomes One Month after ICU Discharge
3.4. Change in Cognitive and Emotional Outcomes over a 12 Month Period
3.5. Impact of Demographic and Clinical Variables on Working Memory Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, S.; Hatakeyama, J.; Kondo, Y.; Hifumi, T.; Sakuramoto, H.; Kawasaki, T.; Taito, S.; Nakamura, K.; Unoki, T.; Kawai, Y.; et al. Post-intensive care syndrome: Its pathophysiology, prevention, and future directions. Acute Med. Surg. 2019, 6, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, J.C.; Mitchell, N.; Hopkins, R.O. Cognitive Functioning, Mental Health, and Quality of Life in ICU Survivors: An Overview. Psychiatr. Clin. N. Am. 2015, 38, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA 2010, 304, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, R.; Girard, T. Medical and Economic Implications of Cognitive and Psychiatric Disability of Survivorship. Semin. Respir. Crit. Care Med. 2012, 33, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-Term Cognitive Impairment after Critical Illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef] [Green Version]
- Schulte, P.J.; Warner, D.O.; Martin, D.P.; Deljou, A.; Mielke, M.M.; Knopman, D.S.; Petersen, R.C.; Weingarten, T.N.; Warner, M.A.; Rabinstein, A.A.; et al. Association Between Critical Care Admissions and Cognitive Trajectories in Older Adults. Crit. Care Med. 2019, 47, 1116–1124. [Google Scholar] [CrossRef]
- Fernández-Gonzalo, S.; Navarra-Ventura, G.; Bacardit, N.; Gomà Fernández, G.; de Haro, C.; Subirà, C.; López-Aguilar, J.; Magrans, R.; Sarlabous, L.; Aquino Esperanza, J.; et al. Cognitive phenotypes 1 month after ICU discharge in mechanically ventilated patients: A prospective observational cohort study. Crit. Care 2020, 24, 618. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Girard, T.D.; Jackson, J.C.; Pandharipande, P.P.; Pun, B.T.; Thompson, J.L.; Shintani, A.K.; Gordon, S.M.; Canonico, A.E.; Dittus, R.S.; Bernard, G.R.; et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit. Care Med. 2010, 38, 1513–1520. [Google Scholar] [CrossRef]
- Turon, M.; Fernández-Gonzalo, S.; de Haro, C.; Magrans, R.; López-Aguilar, J.; Blanch, L. Mechanisms involved in brain dysfunction in mechanically ventilated critically ill patients: Implications and therapeutics. Ann. Transl. Med. 2018, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalo, S.; Turon, M.; De Haro, C.; López-Aguilar, J.; Jodar, M.; Blanch, L. Do sedation and analgesia contribute to long-term cognitive dysfunction in critical care survivors? Med. Intensiva 2018, 42, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.C.; Pandharipande, P.P.; Girard, T.D.; Brummel, N.E.; Thompson, J.L.; Hughes, C.G.; Pun, B.T.; Vasilevskis, E.E.; Morandi, A.; Shintani, A.K.; et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: A longitudinal cohort study. Lancet Respir. Med. 2014, 2, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Kang, J.; Jeong, Y.J. Risk factors for post–intensive care syndrome: A systematic review and meta-analysis. Aust. Crit. Care 2020, 33, 287–294. [Google Scholar] [CrossRef]
- Navarra-Ventura, G.; López-Aguilar, J.; Blanch, L.; Fernández-Gonzalo, S. Characterization and management of cognitive and emotional alterations in COVID-19 critically ill patients after ICU discharge. Med. Intensiva 2020. [Google Scholar] [CrossRef]
- Albaiceta, G.M.; Brochard, L.; Dos Santos, C.C.; Fernández, R.; Georgopoulos, D.; Girard, T.; Jubran, A.; López-Aguilar, J.; Mancebo, J.; Pelosi, P.; et al. The central nervous system during lung injury and mechanical ventilation: A narrative review. Br. J. Anaesth. 2021, 127, 648–659. [Google Scholar] [CrossRef]
- Marra, A.; Ely, E.W.; Pandharipande, P.P.; Patel, M.B. The ABCDEF Bundle in Critical Care. Crit. Care Clin. 2017, 33, 225–243. [Google Scholar] [CrossRef] [Green Version]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef]
- Morris, P.E.; Goad, A.; Thompson, C.; Taylor, K.; Harry, B.; Passmore, L.; Ross, A.; Anderson, L.; Baker, S.; Sanchez, M.; et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit. Care Med. 2008, 36, 2238–2243. [Google Scholar] [CrossRef] [Green Version]
- Costigan, F.A.; Duffett, M.; Harris, J.E.; Baptiste, S.; Kho, M.E. Occupational Therapy in the ICU. Crit. Care Med. 2019, 47, e1014–e1021. [Google Scholar] [CrossRef]
- Brummel, N.E.; Girard, T.D.; Ely, E.W.; Pandharipande, P.P.; Morandi, A.; Hughes, C.G.; Graves, A.J.; Shintani, A.; Murphy, E.; Work, B.; et al. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: The Activity and Cognitive Therapy in ICU (ACT-ICU) trial. Intensive Care Med. 2014, 40, 370–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlroy, P.A.; King, R.S.; Garrouste-Orgeas, M.; Tabah, A.; Ramanan, M. The Effect of ICU Diaries on Psychological Outcomes and Quality of Life of Survivors of Critical Illness and Their Relatives. Crit. Care Med. 2019, 47, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Halpern, N.A.; Anderson, D.C.; Kesecioglu, J. ICU design in 2050: Looking into the crystal ball! Intensive Care Med. 2017, 43, 690–692. [Google Scholar] [CrossRef]
- Moreno, A.; Wall, K.J.; Thangavelu, K.; Craven, L.; Ward, E.; Dissanayaka, N.N. A systematic review of the use of virtual reality and its effects on cognition in individuals with neurocognitive disorders. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 834–850. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Reeve, S.; Robinson, A.; Ehlers, A.; Clark, D.; Spanlang, B.; Slater, M. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychol. Med. 2017, 47, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Blair, G.J.; Kapil, S.; Cole, S.P.; Rodriguez, S. Virtual reality use in adult ICU to mitigate anxiety for a patient on V-V ECMO. J. Clin. Anesth. 2019, 55, 26–27. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Rodriguez, R.A.; Gonzalez, M.; Bernardy, M.; Peña, R.; Beck, W.; Patterson, D.R.; Meyer, W.J. Immersive Virtual Reality as an Adjunctive Non-opioid Analgesic for Pre-dominantly Latin American Children with Large Severe Burn Wounds During Burn Wound Cleaning in the Intensive Care Unit: A Pilot Study. Front. Hum. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, S.M.; Jeitziner, M.-M.; Knobel, S.E.J.; Mosimann, U.P.; Müri, R.M.; Jakob, S.M.; Nef, T. Perception and Performance on a Virtual Reality Cognitive Stimulation for Use in the Intensive Care Unit: A Non-randomized Trial in Critically Ill Patients. Front. Med. 2019, 6, 287. [Google Scholar] [CrossRef]
- Lynch, C.; Jones, G. Feasibility and Potential Benefits of Immersive Virtual Reality in the Intensive Care Unit. ICU Manag. Pract. 2020, 20, 92–98. [Google Scholar]
- Turon, M.; Fernandez-Gonzalo, S.; Jodar, M.; Gomà, G.; Montanya, J.; Hernando, D.; Bailón, R.; de Haro, C.; Gomez-Simon, V.; Lopez-Aguilar, J.; et al. Feasibility and safety of virtual-reality-based early neurocognitive stimulation in critically ill patients. Ann. Intensive Care 2017, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Boutron, I.; Moher, D.; Altman, D.G.; Schulz, K.F.; Ravaud, P. Extending the CONSORT Statement to Randomized Trials of Nonpharmacologic Treatment: Explanation and Elaboration. Ann. Intern. Med. 2008, 148, 295. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A.; PAFS Consensus Group. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016, 2, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocks, K.; Torgerson, D.J. Sample size calculations for pilot randomized trials: A confidence interval approach. J. Clin. Epidemiol. 2013, 66, 197–201. [Google Scholar] [CrossRef]
- Fernandez-Gonzalo, S.; Turon, M.; Jodar, M.; Pousa, E.; Hernandez Rambla, C.; García, R.; Palao, D. A new computerized cognitive and social cognition training specifically designed for patients with schizophrenia/schizoaffective disorder in early stages of illness: A pilot study. Psychiatry Res. 2015, 228, 501–509. [Google Scholar] [CrossRef]
- Bogdanova, Y.; Yee, M.K.; Ho, V.T.; Cicerone, K.D. Computerized Cognitive Rehabilitation of Attention and Executive Function in Acquired Brain Injury. J. Head Trauma Rehabil. 2016, 31, 419–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorm, A.F. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): A review. Int. Psychogeriatrics 2004, 16, 275–293. [Google Scholar] [CrossRef]
- de Haro, C.; Magrans, R.; López-Aguilar, J.; Montanyà, J.; Lena, E.; Subirà, C.; Fernandez-Gonzalo, S.; Gomà, G.; Fernández, R.; Albaiceta, G.M.; et al. Effects of sedatives and opioids on trigger and cycling asynchronies throughout mechanical ventilation: An observational study in a large dataset from critically ill patients. Crit. Care 2019, 23, 245. [Google Scholar] [CrossRef] [Green Version]
- Blanch, L.; Sales, B.; Montanya, J.; Lucangelo, U.; Garcia-Esquirol, O.; Villagra, A.; Chacon, E.; Estruga, A.; Borelli, M.; Burgueño, M.J.; et al. Validation of the Better Care® system to detect ineffective efforts during expiration in mechanically ventilated patients: A pilot study. Intensive Care Med. 2012, 38, 772–780. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale, 3rd Edition: WAIS-III; TEA Ediciones: Barcelona, Spain, 1999. [Google Scholar]
- Wechsler, D. Wechsler Memory Scale, 3rd Edition: WMS-III; TEA Ediciones: Barcelona, Spain, 1997. [Google Scholar]
- Strauss, E.; Sherman, E.M.S.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Daniel, T. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Gomar, J.J.; Ortiz-Gil, J.; McKenna, P.J.; Salvador, R.; Sans-Sansa, B.; Sarró, S.; Guerrero, A.; Pomarol-Clotet, E. Validation of the Word Accentuation Test (TAP) as a means of estimating premorbid IQ in Spanish speakers. Schizophr. Res. 2011, 128, 175–176. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Davidson, J.R.T.; Tharwani, H.M.; Connor, K.M. Davidson Trauma Scale (DTS): Normative scores in the general population and effect sizes in placebo-controlled SSRI trials. Depress. Anxiety 2002, 15, 75–78. [Google Scholar] [CrossRef]
- Blanch, L.; Quintel, M. Lung–brain cross talk in the critically ill. Intensive Care Med. 2017, 43, 557–559. [Google Scholar] [CrossRef]
- Zanni, J.M.; Korupolu, R.; Fan, E.; Pradhan, P.; Janjua, K.; Palmer, J.B.; Brower, R.G.; Needham, D.M. Rehabilitation therapy and outcomes in acute respiratory failure: An observational pilot project. J. Crit. Care 2010, 25, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Ballester, B.R.; Ward, N.S.; Brander, F.; Maier, M.; Kelly, K.; Verschure, P.F.M.J. Relationship between intensity and recovery in post-stroke rehabilitation: A retrospective analysis. J. Neurol. Neurosurg. Psychiatry 2021. [Google Scholar] [CrossRef]
- Chai, W.J.; Abd Hamid, A.I.; Abdullah, J.M. Working Memory from the Psychological and Neurosciences Perspectives: A Review. Front. Psychol. 2018, 9, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baddeley, A. Working memory. Science 1992, 255, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, A.C.; Moreira, C.S.; Magalhães, R.; Magalhães, C.; Pereira, D.R.; Leite, J.; Carvalho, S.; Sampaio, A. Reviewing working memory training gains in healthy older adults: A meta-analytic review of transfer for cognitive outcomes. Neurosci. Biobehav. Rev. 2019, 103, 163–177. [Google Scholar] [CrossRef]
- Larson, M.J.; Weaver, L.K.; Hopkins, R.O. Cognitive sequelae in acute respiratory distress syndrome patients with and without recall of the intensive care unit. J. Int. Neuropsychol. Soc. 2007, 13, 595–605. [Google Scholar] [CrossRef]
- Tow, A.; Holtzer, R.; Wang, C.; Sharan, A.; Kim, S.J.; Gladstein, A.; Blum, Y.; Verghese, J. Cognitive Reserve and Postoperative Delirium in Older Adults. J. Am. Geriatr. Soc. 2016, 64, 1341–1346. [Google Scholar] [CrossRef] [Green Version]
- Colangeli, S.; Boccia, M.; Verde, P.; Guariglia, P.; Bianchini, F.; Piccardi, L. Cognitive Reserve in Healthy Aging and Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dementiasr 2016, 31, 443–449. [Google Scholar] [CrossRef]
- Habib, S.; Khan, A.; Afridi, M.; Saeed, A.; Jan, A.; Amjad, N. Frequency and predictors of cognitive decline in patients undergoing coronary artery bypass graft surgery. J. Coll. Physicians Surg. Pak. 2014, 24, 543–548. [Google Scholar] [PubMed]
- Hope, A.A.; Morrison, R.S.; Du, Q.; Wallenstein, S.; Nelson, J.E. Risk Factors for Long-Term Brain Dysfunction after Chronic Critical Illness. Ann. Am. Thorac. Soc. 2013, 10, 315–323. [Google Scholar] [CrossRef] [PubMed]
| All Patients | TAU Group | ENRIC Group | p | |
|---|---|---|---|---|
| n | 42 | 21 | 21 | |
| Age, years | 68.8 [35.7–85.9] | 67.7 [36.6–85.3] | 69.1 [35.7–85.9] | 0.414 b |
| Education, years | 7.9 (4.6) | 7.7 (4.9) | 8.1 (4.5) | 0.767 a |
| Female gender, n (%) | 25 (59.5) | 12 (57.1) | 13 (61.9) | 0.753 c |
| Cognitive reserve, standard score | 98.5 (11.1) | 97.7 (11.7) | 99.3 (10.7) | 0.646 a |
| Diagnosis, n (%) | 0.779 c | |||
| Medical | 24 (57.1) | 11 (52.4) | 13 (61.9) | |
| Acute respiratory failure | 10 | 5 | 5 | |
| Septic shock | 6 | 4 | 2 | |
| Pneumonia | 4 | 1 | 3 | |
| Pancreatitis | 3 | 1 | 2 | |
| Toxic intake | 1 | 0 | 1 | |
| Unplanned surgery | 12 (28.6) | 7 (33.3) | 5 (23.8) | |
| Peritonitis | 3 | 0 | 3 | |
| Multiple trauma | 3 | 1 | 2 | |
| Abdominal aortic aneurism | 3 | 3 | 0 | |
| Intestinal perforation | 1 | 1 | 0 | |
| Intestinal ischemia | 1 | 1 | 0 | |
| Esophageal perforation | 1 | 1 | 0 | |
| Planned surgery | 6 (14.3) | 3 (14.3) | 3 (14.3) | |
| Hemorrhagic shock | 2 | 0 | 2 | |
| Tumor | 4 | 3 | 1 | |
| CCI | 4 [0–8] | 3 [0–8] | 4 [0–7] | 0.148 b |
| APACHE-II | 20.9 (7.7) | 20.2 (7.2) | 21.7 (8.4) | 0.542 a |
| SOFA at admission | 8.3 (3.8) | 7.4 (3.6) | 9.1 (3.8) | 0.131 a |
| Length of ICU stay, days | 13 [5–76] | 10 [5–73] | 16 [6–76] | 0.252 b |
| Length of hospital stay, days | 28 [7–169] | 19 [9–169] | 28 [7–103] | 0.588 b |
| Destination at hospital discharge, n (%) | 0.240 c | |||
| Home | 29 (69.0) | 15 (71.4) | 14 (66.7) | |
| Home hospitalization | 2 (4.8) | 2 (9.5) | 0 (0.0) | |
| Social-health center | 11 (26.2) | 4 (19.1) | 7 (33.3) | |
| Duration of MV, days | 7 [2–71] | 7 [2–51] | 8 [3–71] | 0.331 b |
| Duration of delirium, days | 0.5 [0–8] | 1 [0–8] | 0 [0–6] | 0.655 b |
| ARDS, n (%) | 2 (4.8) | 0 (0.0) | 2 (9.5) | 0.147 c |
| Septic shock, n (%) | 17 (40.5) | 7 (33.3) | 10 (47.6) | 0.346 c |
| Cardiac arrest, n (%) | 1 (2.4) | 0 (0.0) | 1 (4.8) | 0.311 c |
| Morphine equivalents (mg/kg/day) | 1.6 [0.1–12.2] | 1.6 [0.1–8.4] | 1.9 [0.1–12.2] | 0.498 b |
| Midazolam equivalents (mg/kg/day) | 3.8 [0.1–77.1] | 3.2 [0.2–77.1] | 5.3 [0.1–37.8] | 0.361 b |
| TAU (n = 21) | ENRIC (n = 21) | t | p | 95% CI | d | |
|---|---|---|---|---|---|---|
| Cognitive outcomes, z-score | ||||||
| Attention | −0.06 (0.68) | 0.26 (0.74) | −1.453 | 0.154 | −0.76 to 0.12 | 0.190 |
| Working memory ** | −0.17 (0.60) | 0.44 (0.81) | −2.741 | 0.009 | −1.05 to −0.16 | 0.363 |
| Learning and memory | −0.86 (1.23) | −0.90 (0.91) | 0.137 | 0.891 | −0.63 to 0.72 | 0.019 |
| Memory retrieval | −1.03 (1.49) | −0.57 (0.94) | −1.223 | 0.230 | −1.24 to 0.31 | 0.209 |
| Executive functions | −1.06 (1.05) | −1.11 (1.09) | 0.146 | 0.764 | −0.62 to 0.72 | 0.024 |
| Processing speed | −0.99 (1.00) | −0.77 (1.35) | −0.579 | 0.566 | −0.96 to 0.53 | 0.102 |
| Global neurocognition | −0.78 (0.79) | −0.57 (0.81) | −0.852 | 0.339 | −0.71 to 0.29 | 0.118 |
| Emotional outcomes, raw score | ||||||
| HADS Anxiety | 4.00 (4.16) | 3.00 (4.56) | 0.021 | 0.983 | −0.28 to 0.28 | 0.240 |
| HADS Depression | 2.79 (2.96) | 3.29 (3.92) | −1.128 | 0.270 | −0.40 to 0.12 | 0.135 |
| Davidson Trauma Scale | 11.94 (12.32) | 8.94 (21.91) | 1.075 | 0.292 | −0.17 to 0.52 | 0.363 |
| TAU (n = 14) | ENRIC (n = 10) | Group | Time | Group–Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One Month | One Year | One Month | One Year | F | p | ηp2 | F | p | ηp2 | F | p | ηp2 | |
| Cognitive outcomes, z-score | |||||||||||||
| Attention | 0.09 (0.75) | −0.04 (0.75) | 0.49 (0.75) | 0.33 (1.10) | 1.389 | 0.251 | 0.059 | 1.340 | 0.259 | 0.057 | 0.020 | 0.889 | 0.001 |
| Working memory ** | −0.07 (0.62) | 0.01 (0.65) | 0.88 (0.88) | 0.81 (0.98) | 8.661 | 0.008 | 0.282 | 0.000 | 0.998 | 0.000 | 0.410 | 0.528 | 0.018 |
| Learning and memory | −0.95 (1.22) | −1.02 (1.14) | −0.80 (0.99) | −1.00 (1.39) | 0.037 | 0.850 | 0.002 | 0.447 | 0.511 | 0.020 | 0.111 | 0.742 | 0.005 |
| Memory retrieval | −0.85 (1.15) | −0.92 (1.11) | −0.30 (1.00) | −0.84 (1.33) | 0.562 | 0.461 | 0.025 | 1.798 | 0.194 | 0.076 | 1.041 | 0.319 | 0.045 |
| Executive functions | −0.95 (1.09) | −1.22 (1.60) | −1.12 (1.31) | −1.54 (1.38) | 0.239 | 0.630 | 0.011 | 1.979 | 0.173 | 0.083 | 0.096 | 0.760 | 0.004 |
| Processing speed | −1.06 (1.11) | −0.88 (1.35) | −0.02 (0.84) | −0.28 (0.99) | 2.961 | 0.101 | 0.129 | 0.067 | 0.799 | 0.003 | 2.226 | 0.151 | 0.100 |
| Global neurocognition | −0.71 (0.77) | −0.73 (0.88) | −0.27 (0.84) | −0.47 (0.87) | 1.164 | 0.292 | 0.050 | 0.869 | 0.361 | 0.038 | 0.504 | 0.485 | 0.022 |
| Emotional outcomes, raw score | |||||||||||||
| HADS Anxiety | 4.17 (4.90) | 3.75 (4.73) | 4.78 (5.65) | 4.56 (3.05) | 0.604 | 0.450 | 0.041 | 0.507 | 0.488 | 0.035 | 0.294 | 0.596 | 0.021 |
| HADS Depression | 2.58 (2.91) | 2.75 (3.08) | 4.78 (4.66) | 6.22 (5.22) | 0.371 | 0.082 | 0.030 | 0.553 | 0.471 | 0.044 | 0.403 | 0.538 | 0.032 |
| Davidson Trauma Scale | 11.92 (14.25) | 7.75 (10.02) | 10.78 (17.00) | 7.00 (9.12) | 0.922 | 0.632 | 0.093 | 0.235 | 0.640 | 0.025 | 4.278 | 0.949 | 0.322 |
| Bivariate Analyses | Multiple Linear Regression | |||
|---|---|---|---|---|
| B (95% CI) | p | B (95% CI) | p | |
| Group * | 0.602 (0.16 to 1.05) | 0.009 | 0.464 (0.05 to 0.88) | 0.029 |
| Age | −0.002 (−0.02 to 0.02) | 0.809 | ||
| Gender | −0.147 (−0.64 to 0.34) | 0.548 | ||
| Cognitive reserve * | 0.030 (0.01 to 0.05) | 0.005 | 0.026 (0.01 to 0.05) | 0.009 |
| Diagnosis | 0.101 (−0.17 to 0.37) | 0.458 | ||
| CCI | −0.065 (−0.17 to 0.04) | 0.206 | ||
| APACHE-II | −0.006 (−0.04 to 0.03) | 0.687 | ||
| Length of ICU stay | 0.005 (−0.01 to 0.02) | 0.528 | ||
| Length of hospital stay | 0.003 (−0.01 to 0.01) | 0.478 | ||
| Duration of MV | 0.007 (−0.01 to 0.02) | 0.393 | ||
| Duration of delirium | −0.036 (−0.15 to 0.08) | 0.520 | ||
| Morphine equivalents | 0.097 (0.02 to 0.18) | 0.019 | 0.058 (−0.02 to 0.13) | 0.117 |
| Midazolam equivalents | 0.008 (−0.01 to 0.03) | 0.392 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarra-Ventura, G.; Gomà, G.; de Haro, C.; Jodar, M.; Sarlabous, L.; Hernando, D.; Bailón, R.; Ochagavía, A.; Blanch, L.; López-Aguilar, J.; et al. Virtual Reality-Based Early Neurocognitive Stimulation in Critically Ill Patients: A Pilot Randomized Clinical Trial. J. Pers. Med. 2021, 11, 1260. https://doi.org/10.3390/jpm11121260
Navarra-Ventura G, Gomà G, de Haro C, Jodar M, Sarlabous L, Hernando D, Bailón R, Ochagavía A, Blanch L, López-Aguilar J, et al. Virtual Reality-Based Early Neurocognitive Stimulation in Critically Ill Patients: A Pilot Randomized Clinical Trial. Journal of Personalized Medicine. 2021; 11(12):1260. https://doi.org/10.3390/jpm11121260
Chicago/Turabian StyleNavarra-Ventura, Guillem, Gemma Gomà, Candelaria de Haro, Mercè Jodar, Leonardo Sarlabous, David Hernando, Raquel Bailón, Ana Ochagavía, Lluís Blanch, Josefina López-Aguilar, and et al. 2021. "Virtual Reality-Based Early Neurocognitive Stimulation in Critically Ill Patients: A Pilot Randomized Clinical Trial" Journal of Personalized Medicine 11, no. 12: 1260. https://doi.org/10.3390/jpm11121260
APA StyleNavarra-Ventura, G., Gomà, G., de Haro, C., Jodar, M., Sarlabous, L., Hernando, D., Bailón, R., Ochagavía, A., Blanch, L., López-Aguilar, J., & Fernández-Gonzalo, S. (2021). Virtual Reality-Based Early Neurocognitive Stimulation in Critically Ill Patients: A Pilot Randomized Clinical Trial. Journal of Personalized Medicine, 11(12), 1260. https://doi.org/10.3390/jpm11121260