The Impact of Primary Tumor Location on Long-Term Oncological Outcomes in Patients with Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- (P)opulation: Studies that included patients with UTUC who underwent RNU.
- (I)nvestigated condition: Patients with tumors located in the ureter (UT group).
- (C)omparison condition: Patients with tumors located in the renal pelvis (RPT group).
- (O)utcome: The primary outcome was cancer-specific survival (CSS). The secondary outcomes were overall survival (OS) and disease-free survival (DFS). CSS was defined as the time from surgery to death from UTUC, while OS was defined as the time from surgery to death from any reason. DFS was defined as the time from the date of surgery to the date of documented relapse/recurrence at the surgery site, regional lymph nodes, and/or distant metastases.
- (S)tudy design: Randomized controlled trials (RCTs), nonrandomized observational cohorts, and population-based cohorts.
2.3. Data Extraction
2.4. Quality Assessment and Risk of Bias
2.5. Statistical Analysis
3. Results
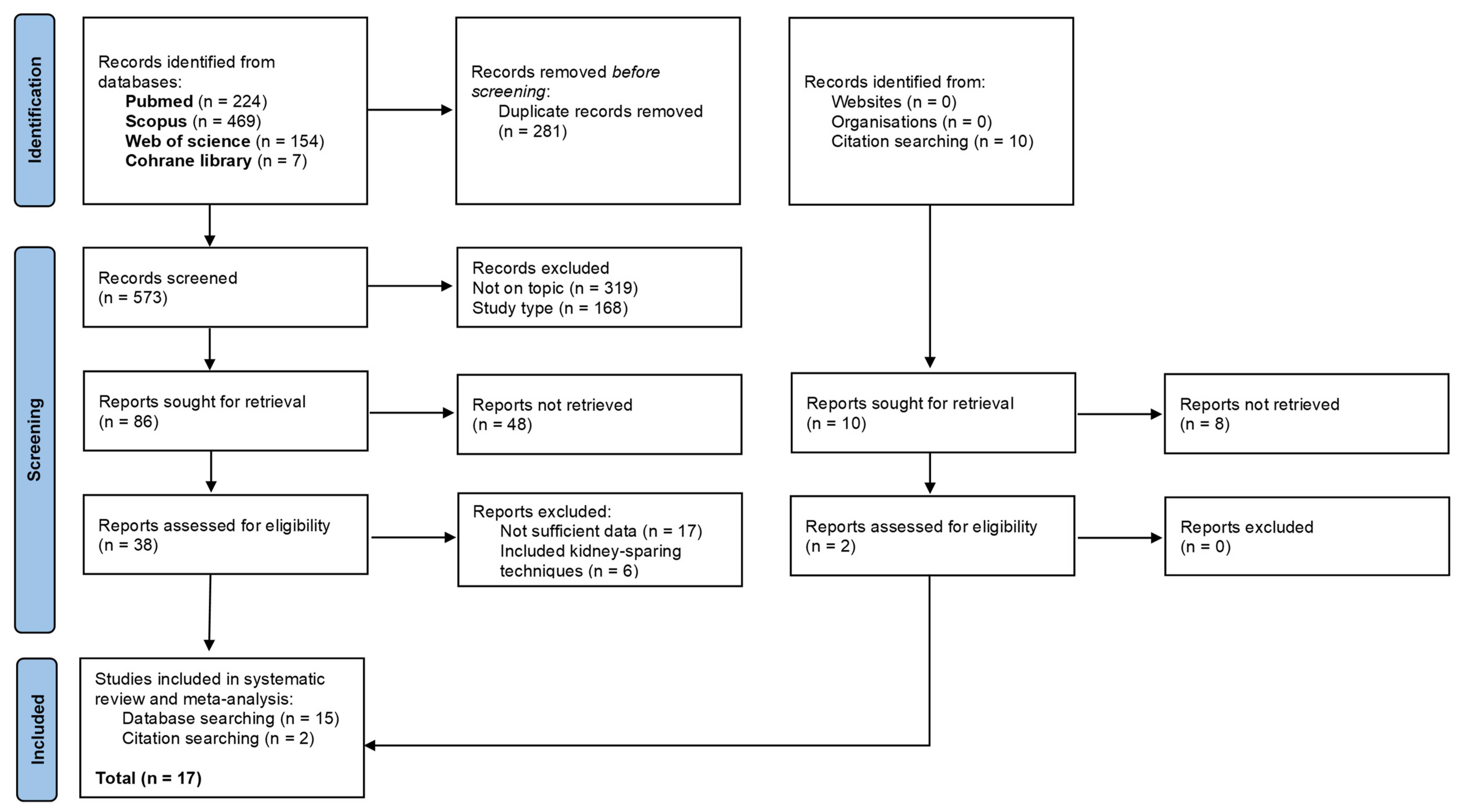
3.1. Literature Selection
3.2. Baseline Characteristics of Included Studies
3.3. Clinicopathological Characteristics of Included Studies
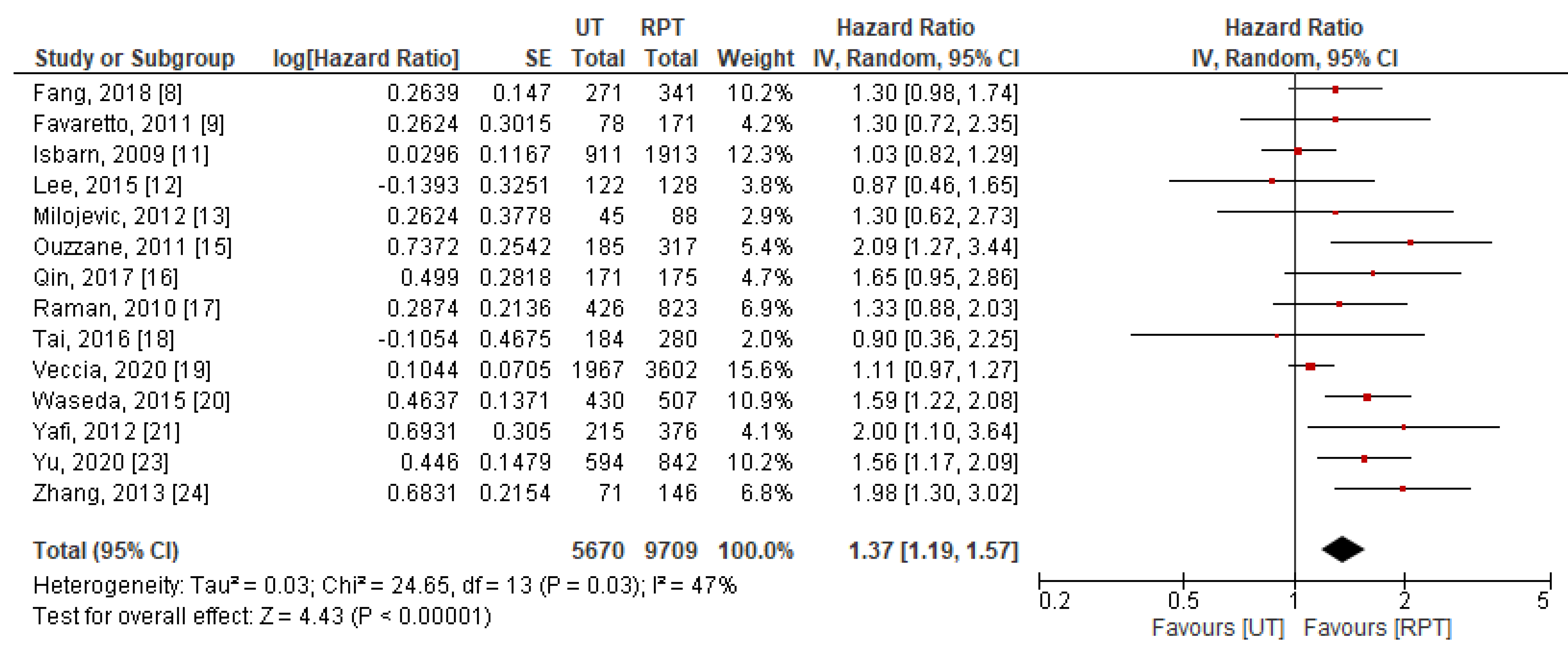
3.4. Meta-Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, Q.; Liu, L.; Han, P.; Wei, Q. The impact of tumor location and multifocality on prognosis for patients with upper tract urothelial carcinoma: A meta-analysis. Sci. Rep. 2014, 4, 6361. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, K.; Lemiński, A.; Gołąb, A.; Słojewski, M. Survival differences of patients with ureteral versus pelvicalyceal tumours: A systematic review and meta-analysis. Arch. Med. Sci. 2021, 17, 603–612. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020). Available online: www.training.cochrane.org/handbook (accessed on 1 October 2021).
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; He, S.; Xiong, G.; Singla, N.; Cao, Z.; Zhang, L.; Li, X.; Zhou, L. Comparison of clinicopathologic characteristics, epigenetic biomarkers and prognosis between renal pelvic and ureteral tumors in upper tract urothelial carcinoma. BMC Urol. 2018, 18, 22. [Google Scholar] [CrossRef] [Green Version]
- Favaretto, R.L.; Shariat, S.F.; Chade, D.C.; Godoy, G.; Adamy, A.; Kaag, M.; Bochner, B.H.; Coleman, J.; Dalbagni, G. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur. Urol. 2010, 58, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inamoto, T.; Matsuyama, H.; Komura, K.; Ibuki, N.; Fujimoto, K.; Shiina, H.; Sakano, S.; Nagao, K.; Mastumoto, H.; Miyake, M.; et al. Tumor Location Based Segmentation in Upper-Tract Urothelial Carcinoma Impacts on the Urothelial Recurrence-Free Survival: A Multi-Institutional Database Study. Curr. Urol. 2020, 14, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Isbarn, H.; Jeldres, C.; Shariat, S.F.; Liberman, D.; Sun, M.; Lughezzani, G.; Widmer, H.; Arjane, P.; Pharand, D.; Fisch, M.; et al. Location of the primary tumor is not an independent predictor of cancer specific mortality in patients with upper urinary tract urothelial carcinoma. J. Urol. 2009, 182, 2177–2181. [Google Scholar] [CrossRef]
- Lee, H.Y.; Li, C.C.; Huang, C.N.; Ke, H.L.; Li, W.M.; Liang, P.I.; Yang, S.F.; Tu, H.P.; Wu, W.J.; Yeh, H.C. Prognostic significance of lymphovascular invasion in upper urinary tract urothelial carcinoma is influenced by tumor location. Ann. Surg. Oncol. 2015, 22, 1392–1400. [Google Scholar] [CrossRef]
- Milojevic, B.; Djokic, M.; Sipetic-Grujicic, S.; Milenkovic-Petronic, D.; Vuksanovic, A.; Bumbasirevic, U.; Vukovic, I.; Dragicevic, D.; Tulic, C. Upper urinary tract transitional cell carcinoma: Location is not correlated with prognosis. BJU Int. 2012, 109, 1037–1042. [Google Scholar] [CrossRef]
- Mouracade, P.; Velten, M.; Gigante, M.; Alenda, O.; Ploussard, G.; Obadia, F.; Timsit, M.O.; Mejean, A. Factors impacting survival in patients with upper tract urothelial carcinoma undergoing radical nephroureterectomy. Can. J. Urol. 2012, 19, 6105–6110. [Google Scholar]
- Ouzzane, A.; Colin, P.; Xylinas, E.; Pignot, G.; Ariane, M.M.; Saint, F.; Hoarau, N.; Adam, E.; Azemar, M.D.; Bensadoun, H.; et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur. Urol. 2011, 60, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Liang, E.L.; Du, Z.Y.; Qiu, X.Y.; Tang, G.; Chen, F.R.; Zhang, B.; Tian, D.W.; Hu, H.L.; Wu, C.L. Prognostic significance of urothelial carcinoma with divergent differentiation in upper urinary tract after radical nephroureterectomy without metastatic diseases: A retrospective cohort study. Medicine 2017, 96, e6945. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.D.; Ng, C.K.; Scherr, D.S.; Margulis, V.; Lotan, Y.; Bensalah, K.; Patard, J.J.; Kikuchi, E.; Montorsi, F.; Zigeuner, R.; et al. Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur. Urol. 2010, 57, 1072–1079. [Google Scholar] [CrossRef]
- Tai, Y.S.; Chen, C.H.; Huang, C.Y.; Tai, H.C.; Wang, S.M.; Pu, Y.S. The effect of tumor location on oncologic outcomes in patients with upper urinary tract urothelial carcinoma stratified by pathologic stage. Urol. Oncol. 2016, 34, 4.e19–4.e25. [Google Scholar] [CrossRef] [PubMed]
- Veccia, A.; Antonelli, A.; Martini, A.; Falagario, U.; Carrieri, G.; Grob, M.B.; Guruli, G.; Simeone, C.; Wiklund, P.; Porpiglia, F.; et al. Ureteral location is associated with survival outcomes in upper tract urothelial carcinoma: A population-based analysis. Int. J. Urol. 2020, 27, 966–972. [Google Scholar] [CrossRef]
- Waseda, Y.; Saito, K.; Ishioka, J.; Matsuoka, Y.; Numao, N.; Fujii, Y.; Sakai, Y.; Koga, F.; Okuno, T.; Arisawa, C.; et al. Ureteral Involvement Is Associated with Poor Prognosis in Upper Urinary Tract Urothelial Carcinoma Patients Treated by Nephroureterectomy: A Multicenter Database Study. Eur. Urol. Focus 2016, 2, 296–302. [Google Scholar] [CrossRef]
- Yafi, F.A.; Novara, G.; Shariat, S.F.; Gupta, A.; Matsumoto, K.; Walton, T.J.; Fritsche, H.M.; El-Hakim, A.; Trischler, S.; Martínez-Salamanca, J.I.; et al. Impact of tumour location versus multifocality in patients with upper tract urothelial carcinoma treated with nephroureterectomy and bladder cuff excision: A homogeneous series without perioperative chemotherapy. BJU Int. 2012, 110, E7–E13. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; You, D.; Jeong, I.G.; Hong, B.; Hong, J.H.; Ahn, H.; Kim, C.S. Impact of Tumor Location on Local Recurrence after Nephroureterectomy for Upper Tract Urothelial Carcinoma: Implications for Adjuvant Radiotherapy. Clin. Genitourin. Cancer 2017, 15, e199–e204. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.; Chang, C.H.; Huang, C.P.; Huang, C.Y.; Hong, J.H.; Tai, T.Y.; Weng, H.Y.; Lo, C.W.; Tsai, C.Y.; Lee, Y.K.; et al. Prognostic Significance of Primary Tumor Location in Upper Tract Urothelial Carcinoma Treated with Nephroureterectomy: A Retrospective, Multi-Center Cohort Study in Taiwan. J. Clin. Med. 2020, 9, 3866. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Zhong, S.; Xu, T.; Shen, Z. Ureteral tumours showing a worse prognosis than renal pelvis tumours may be attributed to ureteral tumours more likely to have hydronephrosis and less likely to have haematuria. World J. Urol. 2013, 31, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Catto, J.W.; Yates, D.R.; Rehman, I.; Azzouzi, A.R.; Patterson, J.; Sibony, M.; Cussenot, O.; Hamdy, F.C. Behavior of urothelial carcinoma with respect to anatomical location. J. Urol. 2007, 177, 1715–1720. [Google Scholar] [CrossRef]
- Colin, P.; Ouzzane, A.; Yates, D.R.; Audenet, F.; Pignot, G.; Arvin-Berod, A.; Merigot de Treigny, O.; Laurent, G.; Valeri, A.; Irani, J.; et al. Influence of positive surgical margin status after radical nephroureterectomy on upper urinary tract urothelial carcinoma survival. Ann. Surg. Oncol. 2012, 19, 3613–3620. [Google Scholar] [CrossRef]
- Rouprêt, M.; Smyth, G.; Irani, J.; Guy, L.; Davin, J.L.; Saint, F.; Pfister, C.; Wallerand, H.; Rozet, F. Oncological risk of laparoscopic surgery in urothelial carcinomas. World J. Urol. 2009, 27, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, R.; Nowak, Ł.; Krajewski, W.; Chorbińska, J.; Poletajew, S.; Moschini, M.; Kaliszewski, K.; Zdrojowy, R. Oncological outcomes of laparoscopic versus open nephroureterectomy for the treatment of upper tract urothelial carcinoma: An updated meta-analysis. World J. Surg. Oncol. 2021, 19, 129. [Google Scholar] [CrossRef]
- Milojevic, B.; Bumbasirevic, U.; Santric, V.; Kajmakovic, B.; Dragicevic, D.; Radisavcevic, D.; Sretenovic, M.; Grujicic, S.S. Prognostic significance of tumor multifocality on outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy: A cohort study. Curr. Probl Cancer 2021, 45, 100747. [Google Scholar] [CrossRef]
- Soualhi, A.; Rammant, E.; George, G.; Russell, B.; Enting, D.; Nair, R.; Van Hemelrijck, M.; Bosco, C. The incidence and prevalence of upper tract urothelial carcinoma: A systematic review. BMC Urol. 2021, 21, 110. [Google Scholar] [CrossRef]
- Grollman, A.P. Aristolochic acid nephropathy: Harbinger of a global iatrogenic disease. Environ. Mol. Mutagen. 2013, 54, 1–7. [Google Scholar] [CrossRef]
- Leow, J.J.; Chong, Y.L.; Chang, S.L.; Valderrama, B.P.; Powles, T.; Bellmunt, J. Neoadjuvant and Adjuvant Chemotherapy for Upper Tract Urothelial Carcinoma: A 2020 Systematic Review and Meta-analysis, and Future Perspectives on Systemic Therapy. Eur. Urol. 2021, 79, 635–654. [Google Scholar] [CrossRef] [PubMed]






| First Author, Year [Reference] | Journal | Country | Study Design | Recruitment Period, Years | No. of Patients, n RPT/UT | Follow Up, Months RPT/UT | Reported Outcomes | Methodological Quality (NOS) |
|---|---|---|---|---|---|---|---|---|
| Fang, 2018 [8] | BMC Urology | China | R, single-center | 1999–2011 | 341/271 | Median: 64 | CSS, OS | 8 |
| Favaretto, 2011 [9] | European Urology | United States | R, single-center | 1995–2008 | 171/78 | Median: 48 | CSS, DFS | 8 |
| Inamoto, 2020 [10] | Current Urology | Japan | R, multi-center | 1994–2009 | 475/359 a | Median: 34 | DFS | 7 |
| Isbarn, 2009 [11] | Journal of Urology | United States | R, SEER database | 1988–2004 | 1913/911 | Median: 45/40 | CSS | 7 |
| Lee, 2015 [12] | Annals of Surgical Oncology | Taiwan | R, single-center | 2004–2010 | 128/122 | Median: 41 | CSS, DFS | 8 |
| Milojevic, 2012 [13] | BJU International | Serbia | R, single-center | 1999–2009 | 88/45 | Median: 35 | CSS, DFS | 7 |
| Mouracade, 2012 [14] | The Canadian Journal of Urology | France | R, multi-center | 1985–2005 | 161/108 | Median: 70.3 | OS | 8 |
| Ouzzane, 2011 [15] | European Urology | France | R, multi-center | 1995–2010 | 317/185 | Median: 32/30 | CSS, OS, DFS | 8 |
| Qin, 2017 [16] | Medicine (United States) | China | R, single-center | 2012–2016 | 175/171 | Median: 21 | CSS, OS | 7 |
| Raman, 2010 [17] | European Urology | Multinational | R, multi-center | 1987–2007 | 823/426 | Median: 49 | CSS, DFS | 8 |
| Tai, 2016 [18] | Urologic Oncology: Seminars and Original Investigations | Taiwan | R, single-center | 1996–2009 | 280/184 | Median: 52 | CSS, OS, DFS | 8 |
| Veccia, 2020 [19] | International Journal of Urology | United States | R, SEER database | 2005–2015 | 3602 b/1967 b | Median: 29 | CSS, OS | 7 |
| Waseda, 2015 [20] | European Urology Focus | Japan | R, multi-center | 1995–2013 | 507/430 | Median: 40 | CSS | 8 |
| Yafi, 2012 [21] | BJU International | Multinational | R, multi-center | 1990–2010 | 376/215 | Median: 37/38 | CSS, DFS | 8 |
| Yoo, 2017 [22] | Clinical Genitourinary Cancer | Korea | R, single-center | 1998–2012 | 192/161 | Mean: 73 | DFS | 8 |
| Yu, 2020 [23] | Journal of Clinical Medicine | Taiwan | R, multi-center | 1988–2019 | 842/594 | Median: 33.6 | CSS, OS, DFS | 9 |
| Zhang, 2013 [24] | World Journal of Urology | China | R, single-center | 2000–2010 | 146/71 | Median: 53/48 | CSS, DFS | 8 |
| First Author, Year [Reference] | Age RPT/UT | Male Gender, % RPT/UT | History of BC, % RPT/UT | Bladder Cuff Excision (%) RPT/UT | Pathological Stage ≥pT3, % RPT/UT | Pathological Grade (G3 or HG), % RPT/UT | LNI, % RPT/UT | Concomitant CIS, % RPT/UT | LVI, % RPT/UT | NAC, % RPT/UT | AC, % RPT/UT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fang, 2018 [8] | 65.3/68.1 a * | 54.8/56.5 | 9.4/12.9 | All patients | pT2–T4: 66.9/65.7 | G3: 34.9/51.7 * | 8.5/4.4 | 1.5/4.1 | NR | Excluded | NA |
| Favaretto, 2011 [9] | 71/73 b | 60.8/66.7 | 30.4/39.7 | All patients | 28.1/21.8 | HG: 76.0/76.9 | 8.8/10.3 | 24.0/37.2 * | NR | Excluded | NA |
| Inamoto, 2020 [10] | 72 a | 71.8 | NR | All patients | 32.7 | G3: 47.0 | 4.3 | 13.1 | 27.7 | Excluded | Excluded |
| Isbarn, 2009 [11] | 71/72 b * | 57.3/62.6 * | NR | 62.5/83.0 * | 57.9/38.3 * | NA | 9.8/6.0 * | NR | NR | NR | NR |
| Lee, 2015 [12] | ≥68 y: 50.0/61.5 | 42.2/44.3 | Excluded | NR | 37.5/29.5 | HG: 76.6/79.5 | 7.8/6.6 | NR | 32.0/15.6 * | Excluded | 16.8 |
| Milojevic, 2012 [13] | 66.7/66.6 a | 59.1/55.5 | 14.7/35.6 * | All patients | 73.8/46.6 * | G3: 72.7/51.1 * | 4.5/2.3 | NR | 70.5/35.6 * | Excluded | NA |
| Mouracade, 2012 [14] | 67/66 a | 60.7/39.3 | NR | All patients | 31.7/36.1 | G3: 31.0/28.7 | 8.1/8.3 | NR | NR | NR | 12.5/37.8 * |
| Ouzzane, 2011 [15] | 69/71 b | 66.6/68.1 | Excluded | NR | 39.4/29.7 | G3: 53.0/55.7 | 8.2/8.1 | NR | 20.5/15.1 | Excluded | NR |
| Qin, 2017 [16] | ≥68 y: 45.7/48.0 | 66.3/52.6 * | 5.1/11.7 * | NR | pT2–T4: 29.1/21.6 * | HG: 82.9/83.0 | Excluded | NR | NR | Excluded | 54.3/43.3 |
| Raman, 2010 [17] | 68/69 b | 66.0/71.1 | NR | All patients | 38.2/21.8 * | HG: 58.9/62.4 | 6.7/4.9 | NR | NR | NA | NA |
| Tai, 2016 [18] | 67/69 b | 52.5/45.1 | Excluded | All patients | 37.5/20.2 * | HG: 50.7/64.1 * | Excluded | NR | 17.5/17.4 | Excluded | 0/0 |
| Veccia, 2020 [19] | NA | NA | NR | NR | NA | NA | NR | NR | NR | NR | NR |
| Waseda, 2015 [20] | 69/70 b | 75.9/65.8 | NR | NR | 57.0/44.0 * | G3: 20.7/36.5 * | 7.5/9.3 | 5.1/12.1 * | 34.9/46.5 * | Excluded | NR |
| Yafi, 2012 [21] | 68/69 b | 66.2/69.3 | NR | All patients | 31.1/20.5 * | G3: 54.5/54.9 | 3.7/2.3 | 10.1/12.6 | 18.1/10.7 * | Excluded | Excluded |
| Yoo, 2017 [22] | 62.2/65.9 a * | 76.6/70.2 | NR | All patients | 23.4/24.8 | HG: 34.4/57.9 * | 3.1/7.5 | 8.3/18.6 * | 12.0/24.2 * | NR | Excluded |
| Yu, 2020 [23] | 69.2/69.8 a | 43.0/40.6 | 5.3/5.1 | NR | 39.2/28.6 * | G3: 78.7/77.8 | 4.9/3.3 | NR | 22.4/15.3 * | NR | NR |
| Zhang, 2013 [24] | 63/72 b | 58.2/63.4 | NR | NR | 43.2/60.6 * | G3: 70.5/49.3 | 6.8/12.7 | NR | 35.6/67.6 * | Excluded | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewski, W.; Nowak, Ł.; Małkiewicz, B.; Chorbińska, J.; Kiełb, P.; Poterek, A.; Sporniak, B.; Sut, M.; Moschini, M.; Lonati, C.; et al. The Impact of Primary Tumor Location on Long-Term Oncological Outcomes in Patients with Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1363. https://doi.org/10.3390/jpm11121363
Krajewski W, Nowak Ł, Małkiewicz B, Chorbińska J, Kiełb P, Poterek A, Sporniak B, Sut M, Moschini M, Lonati C, et al. The Impact of Primary Tumor Location on Long-Term Oncological Outcomes in Patients with Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2021; 11(12):1363. https://doi.org/10.3390/jpm11121363
Chicago/Turabian StyleKrajewski, Wojciech, Łukasz Nowak, Bartosz Małkiewicz, Joanna Chorbińska, Paweł Kiełb, Adrian Poterek, Bartłomiej Sporniak, Michał Sut, Marco Moschini, Chiara Lonati, and et al. 2021. "The Impact of Primary Tumor Location on Long-Term Oncological Outcomes in Patients with Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 11, no. 12: 1363. https://doi.org/10.3390/jpm11121363







