Cardiac Remodeling According to the Nocturnal Fall of Blood Pressure in Hypertensive Subjects: The Whole Assessment of Cardiac Abnormalities in Non-Dipper Subjects with Arterial Hypertension (Wacanda) Study
Abstract
:1. Introduction
2. Materials and Methods
- -
- Diagnosis of secondary hypertension;
- -
- Record of an acute vascular event (ischemic or hemorrhagic stroke, acute myocardial infarction, acute limb ischemia) in the six months prior to the date of the evaluation;
- -
- Every condition contraindicating the reliability of the ABPM:
- Supraventricular arrhythmias (atrial flutter, paroxysmal, persistent or permanent atrial fibrillation);
- Clinical history or orthostatic hypotension, autonomic dysfunction or diabetic neuropathy;
- Body mass Index > 35 Kg/m2;
- History of sleep disturbance (including patients with obstructive sleep apnea) and/or night-workers;
- -
- dippers: mean reduction in night-time BP between 10 and 20% (night-day BP ratio > 0.8 and ≤0.9; this is commonly considered the physiological profile);
- -
- mild dippers: night-time drop in BP is between 0 and 10%, i.e., a night-day BP ratio > 0.9 and ≤1);
- -
- reverse dippers: paradoxical increase in BP during the night (night-day BP ratio > 1);
- -
- extreme dippers: night-time reduction in BP is >20% (night-day BP ratio ≤ 0.8);
- -
- Left ventricular end-diastolic volume measured in ml (LV-EDV). We considered reference values ranging from 42 and 58.4 mm for men and 37.8 and 52.2 mm for women.
- -
- Left ventricular mass and Ejection Fraction (LVM, EF%). The mass of the left ventricle was calculated in grams, using the following formula: 0.8 (1.04 [(LVIDd + IVS + PWT)3/LVID3]) + 0.6 g. where IVS is the end-diastolic interventricular septal thickness, LVID is the left ventricular end-diastolic diameter, and PWT is the posterior wall end-diastolic thickness. The procedure of our study included indexing of left ventricular mass (LVMI), since this system allows the comparison of ventricular masses of subjects having different body weights. We considered a normal left ventricle indexed mass in case of values between 49 and 115 g/m2 for men and 43 and 95 g/m2 for women. We speak of left ventricular hypertrophy (LVH) when LVMI values exceed 115 g/m2 in men and 95 g/m2 in women and considered normal a LVEF > of 52% for men and >54% for women.
- -
- Relative Wall Thickness (RWT).
- -
- Left atrium volume (LAV). The volume obtained by measuring atrial areas and diameters was indexed for body surface area (LAVi), and a left atrial volume of up to 34 mL/m2 was considered normal in both genders.
- -
- E/A ratio and E/e’ ratio. LV diastolic function was evaluated through the capture of Tissue Doppler images at mitral cusps level, obtained through a two-dimensional apical window of the four cardiac chambers, were used to measure the transmitral flow velocities; this was measured (in m/s) during the peak of early passive diastolic filling (early, E-wave) and during the late peak of the diastolic flow due to atrial contraction (A-wave). The E/A ratio was then calculated, which decrypts blood flow from the atria to the ventricles during ventricular diastole and provides information about the atrial contribution to ventricular filling. The lower the ratio, the greater the atrial contribution. The E/A ratio value in a subject with normal diastolic function is between 0.8 and 2, but the correct evaluation of the E/A ratio requires a broader framework of the data and, therefore, it is more correct to consider a normal pattern and three abnormal patterns: altered diastolic release (E/A < 1), pseudonormal pattern (E/A falsely in range) and restrictive pattern (E/A > 2).
- -
- Area of the right atrium. According to the Guidelines for the echocardiographic assessment of the right heart in adults [21] we considered normal a right atrium area < 18 cm2 regardless of sex.
- -
- Basal diameter of the right ventricle. It was measured at the basal third of the right ventricle and has been considered a normal range between 25–41 mm. The right ventricle is assumed to be dilated if the basal diameter is >41 mm.
- -
- Diameter of the inferior vena cava. According to the recommendations, a VCI diameter < 21 mm associated with inspiratory collapse > 50% suggests an atrial pressure of 3 mmHg (range 0–5 mmHg), whereas a VCI diameter > 21 mm with inspiratory collapse < 50% suggests an elevated atrial pressure, approximately 15 mm Hg (range 10–20 mmHg). In indeterminate cases, in which the VCI did not meet the two profiles, an intermediate value of 8 mmHg (range 5–10 mmHg) was considered.
- -
- Tricuspid annular plane excursion (TAPSE). It is measured in M-Mode by placing the cursor on the lateral tricuspid annulus from the apical 4-chamber projection. TAPSE quantifies the systolic excursion of the tricuspid annulus along the longitudinal plane thus assessing the efficiency of contraction; it is, therefore, a reliable index of right ventricular systolic function demonstrating a good correlation with other parameters such as myocardial scintigraphy and 2D estimation of right ventricular ejection fraction. The greater the excursion, the better the performance of the right ventricle. A value > 17 mm was considered normal in both genders.
- -
- Estimated systolic pulmonary artery pressure. Right ventricular systolic pressure was estimated through the velocity of tricuspid regurgitation using the simplified Bernoulli equation (dP = 4V2), which allows measurement of the right ventricular-atrial pressure gradient. Adding this value to the right atrial pressure estimated by assessing the diameter and respiratory excursions of the inferior vena cava will yield an estimate of pulmonary arterial pressure. The maximum tricuspid regurgitation velocity was considered normal if ≤2.8 m/s, whereas the maximum normal trans-tricuspid gradient was considered for values ≤ 36 mmHg.
Statistical Analysis
3. Results
4. Discussion
- (1)
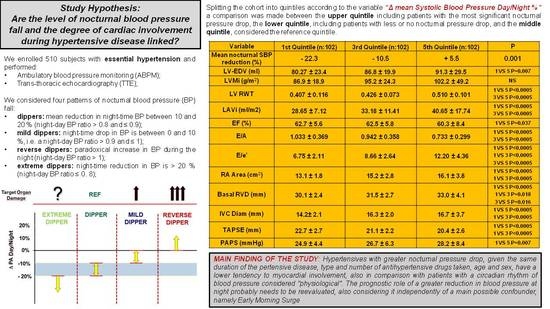
- Considering a large cohort of 510 subjects with essential hypertension divided into quintiles with reference to the level of nocturnal BP drop, it is possible to highlight significant differences in the epidemiology, clinic, organ damage, level of comorbidity, and finally cardiac involvement.
- (2)
- Mild and reverse dipper hypertensives, as already reported by several authors, are those burdened by a worse clinical, laboratory, instrumental, comorbidity, and organ damage profile.
- (3)
- Extreme dipper hypertensives, on the contrary, even when compared with the dipper profile (i.e., compared with subjects with a normal amount of blood pressure reduction during the night) represent the population of subjects with the lowest systemic involvement as well as the lowest level of organ damage.
- (4)
- The cardiac involvement during hypertensive disease is different in relation to the nocturnal BP profile, to an extent that seems to go beyond the epidemiological and clinical differences between the groups. A higher nocturnal reduction of SBP seems to be linked to a less marked cardiac involvement;
- (5)
- Our data, obtained in groups of subjects with superimposable age, type and mean number of antihypertensive drugs taken daily, duration of hypertensive disease, and day-time BP load, adjusted for the main confounder, 24-h SBP, reaffirms the prognostic relevance of night-time compared with day-time BP.
- (1)
- Retrograde transmission of the increased afterload through the dysfunctional LV and thus of the dilated LA associated with a possible increase in pulmonary resistance [32]. This mechanism would justify a greater involvement of the right sections in patients with higher mean BP values or in subjects with a highly impaired nocturnal BP fall [33] as we have found in our study.
- (2)
- Peculiar susceptibility of the pulmonary circulation of the hypertensive patient to catecholaminergic stress resulting in prolonged vasoconstriction and overload on the RV [34].
- (3)
- As a consequence of interventricular septum remodeling induced by chronic BP overload, there was a demonstrated direct mechanical transmission of parietal stress from left to right [35].
- (4)
- The activation of bio-humoral mechanisms related to cardiac remodeling in the hypertensive patient (for example the renin-angiotensin-aldosterone system and the atrial natriuretic peptide system) act as much on the left as on the right chambers [33].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Millar-Craig, M.W.; Bishop, C.N.; Raftery, E.B. Circadian variation of blood-pressure. Lancet 1978, 1, 795–797. [Google Scholar] [CrossRef]
- Salles, G.F.; Reboldi, G.; Fagard, R.H.; Cardoso, C.R.; Pierdomenico, S.D.; Verdecchia, P.; Eguchi, K.; Kario, K.; Hoshide, S.; Polonia, J.; et al. ABC-H Investigators. Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients: The Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) Meta-Analysis. Hypertension 2016, 67, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Stanton, A.; Thijs, L.; Hinedi, K.; Atkins, N.; McClory, S.; Den Hond, E.; McCormack, P.; Staessen, J.A.; O’Brien, E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension 2005, 46, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Ben-Dov, I.Z.; Kark, J.D.; Ben-Ishay, D.; Mekler, J.; Ben-Arie, L.; Bursztyn, M. Predictors of all-cause mortality in clinical ambulatory monitoring: Unique aspects of blood pressure during sleep. Hypertension 2007, 49, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, D.; Musiari, G.; Pinto, A. Nocturnal blood pressure patterns and cardiac damage: There is still much to learn. Hypertens. Res. 2020, 43, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Y.; Melgarejo, J.D.; Thijs, L.; Zhang, Z.Y.; Boggia, J.; Wei, F.F.; Hansen, T.W.; Asayama, K.; Ohkubo, T.; Jeppesen, J.; et al. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators. Association of Office and Ambulatory Blood Pressure With Mortality and Cardiovascular Outcomes. JAMA 2019, 322, 409–420. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Akiguchi, I.; Oiwa, K.; Hayashi, M.; Ohara, T.; Ozasa, K. The relationship between 24-hour blood pressure readings, subcortical ischemic lesions, and vascular dementia. Cerebrovasc. Dis. 2005, 19, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.W.; Jeppesen, J.; Rasmussen, S.; Ibsen, H.; Torp-Pedersen, C. Ambulatory blood pressure and mortality: A population-based study. Hypertension 2005, 45, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.W.; Li, Y.; Boggia, J.; Thijs, L.; Richart, T.; Staessen, J.A. Predictive role of the nighttime blood pressure. Hypertension 2011, 57, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A.L.; Pasanisi, F.; Crivaro, M.; Guida, L.; Palmieri, V.; Gaeta, I.; Iannuzzi, R.; Celentano, A. Cardiovascular abnormalities in never-treated hypertensives according to nondipper status. Am. J. Hypertens. 1998, 11, 1352–1357. [Google Scholar] [CrossRef] [Green Version]
- Hoshide, S.; Kario, K.; Hoshide, Y.; Umeda, Y.; Hashimoto, T.; Kunii, O.; Ojima, T.; Shimada, K. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am. J. Hypertens. 2003, 16, 434–438. [Google Scholar] [CrossRef] [Green Version]
- Acar, G.; Bulut, M.; Arslan, K.; Alizade, E.; Ozkan, B.; Alici, G.; Tanboğa, I.; Yazıcıoğlu, M.; Akçakoyun, M.; Esen, A. Comparison of left atrial mechanical function in nondipper versus dipper hypertensive patients: A speckle tracking study. Echocardiography 2013, 30, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Kang, T.S.; Park, S.; Choi, E.Y.; Ko, Y.G.; Choi, D.; Ha, J.; Rim, S.-J.; Chung, N. Non-dippers are associated with adverse cardiac remodelling and dysfunction. Int. J. Cardiol. 2006, 112, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Macca, G.; Sampieri, L.; Fusi, V.; Severgnini, B.; Michev, I.; Salerno, M.; Magrini, F.; Zanchetti, A. Target organ damage and non-dipping pattern defined by two sessions of ambulatory blood pressure monitoring in recently diagnosed essential hypertensive patients. J. Hypertens. 2001, 19, 1539–1545. [Google Scholar] [CrossRef]
- Ivanovic, B.A.; Tadic, M.V.; Celic, V.P. To dip or not to dip? The unique relationship between different blood pressure patterns and cardiac function and structure. J. Hum. Hypertens. 2013, 27, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadic, M.V.; Ivanovic, B.A.; Celic, V.P. Does a nondipping pattern impact the right ventricle in hypertensive patients? Blood Press. Monit. 2012, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Parati, G.; Stergiou, G.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension Position paper on ambulatory blood pressure monitoring. J. Hypertens. 2013, 31, 1731–1768. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Tas k Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Fagard, R.H. Dipping pattern of nocturnal blood pressure in patients with hypertension. Expert Rev. Cardiovasc. Ther. 2009, 7, 599–605. [Google Scholar] [CrossRef]
- Fagard, R.H.; Thijs, L.; Staessen, J.A.; Clement, D.L.; De Buyzere, M.L.; De Bacquer, D.A. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J. Hum. Hypertens. 2009, 23, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokmen, G.; Sokmen, A.; Aksu, E.; Koroglu, S.; Suner, A.; Tuncer, C. The influence of ambulatory blood pressure profile on global and regional functions of the left and the right ventricles in orderly treated hypertensive patients. Echocardiography 2008, 25, 465–472. [Google Scholar] [CrossRef]
- Kario, K.; Pickering, T.G.; Matsuo, T.; Hoshide, S.; Schwartz, J.E.; Shimada, K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001, 38, 852–857. [Google Scholar] [CrossRef] [Green Version]
- Yano, Y.; Kario, K. Possible difference in the sympathetic activation on extreme dippers with or without exaggerated morning surge. Hypertension 2009, 53, e1, author reply e2. [Google Scholar] [CrossRef] [Green Version]
- Ohkubo, T.; Imai, Y.; Tsuji, I.; Nagai, K.; Watanabe, N.; Minami, N.; Kato, J.; Kikuchi, N.; Nishiyama, A.; Aihara, A.; et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am. J. Hypertens. 1997, 10, 1201–1207. [Google Scholar] [CrossRef] [Green Version]
- Boggia, J.; Li, Y.; Thijs, L.; Hansen, T.W.; Kikuya, M.; Björklund-Bodegård, K.; Richart, T.; Ohkubo, T.; Kuznetsova, T.; Torp-Pedersen, C.; et al. International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators. Prognostic accuracy of day versus night ambulatory blood pressure: A cohort study. Lancet 2007, 370, 1219–1229. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; Cardoso, C.R.; Salles, G.F. Prognostic value of nocturnal blood pressure reduction in resistant hypertension. Arch. Intern. Med. 2009, 169, 874–880. [Google Scholar] [CrossRef] [Green Version]
- Cuspidi, C.; Meani, S.; Valerio, C.; Fusi, V.; Catini, E.; Sala, C.; Zanchetti, A. Ambulatory blood pressure, target organ damage and left atrial size in nevertreated essential hypertensive individuals. J. Hypertens. 2005, 23, 1589–1595. [Google Scholar] [CrossRef]
- Tigen, K.; Karaahmet, T.; Fotbolcu, H.; Gürel, E.; Cevik, C.; Geçmen, C.; Bitigen, A.; Mutlu, B.; Başaran, Y. The influence of dipper and nondipper blood pressure patterns on left ventricular functions in hypertensive patients: A tissue Doppler study. Turk. Kardiyol. Dern. Ars. 2009, 37, 101–106. [Google Scholar]
- Tumuklu, M.M.; Erkorkmaz, U.; Ocal, A. The impact of hypertension and hypertension-related left ventricle hypertrophy on right ventricle function. Echocardiography 2007, 24, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Pencic, B.; Ivanovic, B.; Scepanovic, R.; Marjanovic, T.; Jozika, L.; Celic, V. Circadian blood pressure pattern and right ventricular and right atrial mechanics: A two- and three-dimensional echocardiographic study. J. Am. Soc. Hypertens. 2014, 8, 45–53. [Google Scholar] [CrossRef]
- Guazzi, M.D.; De Cesare, N.; Fiorentini, C.; Galli, C.; Montorsi, P.; Pepi, M.; Tamborini, G. Pulmonary vascular super-sensitivity to catecholamines in systemic high blood pressure. J. Am. Coll. Cardiol. 1986, 8, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Jurcut, R.; Giusca, S.; La Gerche, A.; Vasile, S.; Ginghina, C.; Voigt, J.U. The echocardiographic assessment of the right ventricle: What to do in 2010? Eur. J. Echocardiogr. 2010, 11, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Kohara, K.; Nishida, W.; Maguchi, M.; Hiwada, K. Autonomic nervous function in non-dipper essential hypertensive subjects. Evaluation by power spectral analysis of heart rate variability. Hypertension 1995, 26, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.F.; Ribeiro, F.M.; Guimarães, G.M.; Muxfeldt, E.S.; Cardoso, C.R. A reduced heart rate variability is independently associated with a blunted nocturnal blood pressure fall in patients with resistant hypertension. J. Hypertens. 2014, 32, 644–651. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Miceli, G.; Casuccio, A.; Tuttolomondo, A.; Buttà, C.; Zappulla, V.; Schimmenti, C.; Musiari, G.; Pinto, A. Does sympathetic overactivation feature all hypertensives? Differences of sympathovagal balance according to night/day blood pressure ratio in patients with essential hypertension. Hypertens. Res. 2016, 39, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Cavelaars, M.; Tulen, J.H.; Van Bemmel, J.H.; Van den Meiracker, A.H. Physical activity, dipping and haemodynamics. J. Hypertens. 2004, 22, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- El-Gharbawy, A.H.; Nadig, V.S.; Kotchen, J.M.; Grim, C.E.; Sagar, K.B.; Kaldunski, M.; Hamet, P.; Pausova, Z.; Gaudet, D.; Gossard, F.; et al. Arterial pressure, left ventricular mass, and aldosterone in essential hypertension. Hypertension 2001, 37, 845–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slight, S.H.; Joseph, J.; Ganjam, V.K.; Weber, K.T. Extra-adrenal mineralocorticoids and cardiovascular tissue. J. Mol. Cell Cardiol. 1999, 31, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Alonso, N.; Ruiz Arzalluz, M.V.; Garcia-Alvarez, A.; de Quincoces, D.F.-F.; Grandes, G. Reproducibility study of nocturnal blood pressure dipping in patients with high cardiovascular risk. J. Clin. Hypertens. 2021, 23, 1041–1050. [Google Scholar] [CrossRef] [PubMed]




| Variable | Extreme Dipper (n:102) | Dipper (n:102) | Reverse Dipper (n:102) | p |
|---|---|---|---|---|
| M/F, n (%) | 57/45 | 57/45 | 56/46 | NS |
| Mean nocturnal reduction of SBP (%) | −22.3 | −10.5 | +5.5 | 0.001 |
| Age (yrs) | 66.4 ± 18.3 | 68.0 ± 14.3 | 69.7 ± 13.0 | NS |
| Duration of hypertension (yrs) | 12.0 ± 9.1 | 11.6 ± 8.8 | 12.5 ± 9.3 | NS |
| Family history of hypertension (n, %) | 70 (68.6) | 76 (74.5) | 72 (70.6) | NS |
| Number of drugs taken/day, n * | 1.89 ± 1.2 | 2.12 ± 1.2 | 2.0 ± 1.5 | NS |
| Fasting Glucose (mg/dL) | 102.3 ± 33.7 | 102.8 ± 30.3 | 124.5 ± 42.0 | 1 vs. 5 p < 0.0005 3 vs. 5 p < 0.0005 |
| Oral Hypoglycemic Drugs (n, %) | 6 (5.9) | 2 1 (20.6) | 25 (24.5) | 1 vs. 3,5 p = 0.002 |
| Statins (n, %) | 26 (25.5) | 30 (29.4) | 30 (29.4) | NS |
| BMI (Kg/m2) | 27.7 ± 4.0 | 28.5 ± 4.8 | 29.5 ± 5.7 | NS |
| Waist circonference (cm) | 99.5 ±11.9 | 102.7 ±13.8 | 103.4 ± 12.9 | NS |
| Total Cholesterol (mg/dL) | 200.8 ± 40.3 | 192.0 ± 42.0 | 181.3 ± 51.5 | 1 vs. 5 p = 0.022 |
| HDL Cholesterol (mg/dL) | 53.1 ± 13.1 | 49.7 ± 13.4 | 48.4 ± 15.0 | NS |
| Triglycerides (mg/dL) | 115.5 ± 62.2 | 134.4 ± 65.0 | 128.0 ± 70.9 | NS |
| Creatinin (mg/dL) | 0.97 ± 1.0 | 0.98 ± 0.68 | 1.07 ± 0.61 | NS |
| Cr Cl (ml/min) ** | 102.0 ± 35.8 | 97.5 ± 39.5 | 68.8 ± 35.0 | 1 vs. 5 p < 0.0005 3 vs. 5 p < 0.0005 |
| MACE (number of events) *** | 18 | 17 | 35 | 1, 3 vs. 5 p = 0.033 |
| COPD (n, %) | 3 (2.9) | 2 (1.9) | 23 (22.5) | 1, 3 vs. 5 p < 0.0001 |
| Current smokers (n, %) | 20 (19.6) | 16 (15.7) | 13 (12.7) | 0.290 |
| Past smokers (n, %) | 28 (27.4) | 30 (29.4) | 28 (27.4) | 0.894 |
| White blood cells count (mm3) | 7028.0 ± 1855 | 7313.3 ± 1903 | 7694.6 ± 2981 | NS |
| hs C-Reactive protein (mg/dL) | 0.58 ± 1.5 | 1.69 ± 6.6 | 2.21 ±5.4 | NS |
| Fibrinogen (mg/dL) | 308.4 ± 81.0 | 316.9 ± 83.0 | 338.5 ± 98.1 | NS |
| Variable | Extreme Dipper (n:102) | Dipper (n:102) | Reverse Dipper (n:102) | p * |
|---|---|---|---|---|
| Mean nocturnal SBP reduction (%) | −22.3 | −10.5 | +5.5 | 0.001 |
| 24-h SBP (mmHg) | 131.1 ± 12.1 | 133.9 ± 11.6 | 140.0 ± 17.5 | 1 vs. 5 p < 0.0005 3 vs. 5 p = 0.019 |
| 24-h DBP (mmHg) | 78.0 ± 7.9 | 78.2 ± 8.4 | 76.1 ± 11.7 | NS |
| 24-h HR (bpm) | 74.2 ± 8.7 | 72.4 ± 8.2 | 69.9 ± 8.9 | 1 vs. 5 p = 0.007 |
| Day SBP (mmHg) | 139.2 ± 13.2 | 137.8 ± 12.0 | 137.9 ± 17.5 | NS |
| Day DBP (mmHg) | 82.1 ± 8.6 | 80.9 ± 8.6 | 78.8 ± 11.8 | NS |
| Day HR (bpm) | 77.2 ± 9.5 | 75.2 ± 8.8 | 71.4 ± 9.1 | 1 vs. 5 p = 0.001 |
| Night SBP (mmHg) | 108.2 ± 10.6 | 123.4 ± 11.1 | 145.3 ± 18.5 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 3 vs. 5 p < 0.0005 |
| Night DBP (mmHg) | 63.9 ± 7.9 | 71.1 ± 8.6 | 76.7 ± 12.1 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 3 vs. 5 p < 0.0005 |
| Night HR (bpm) | 65.2 ± 8.5 | 65.2 ± 7.4 | 66.0 ± 10.1 | NS |
| Morning surge SBP (mmHg) | +37.4 | +25.0 | +9.5 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 3 vs. 5 p < 0.0005 |
| Morning surge DBP (mmHg) | +23.3 | +16.7 | +7.0 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 3 vs. 5 p < 0.0005 |
| Variable | Extreme Dipper (n:102) | Dipper (n:102) | Reverse Dipper (n:102) | Unadjusted p | Adjusted p * |
|---|---|---|---|---|---|
| LV-EDV (mL) | 80.27 ± 23.4 | 86.8 ± 19.9 | 91.3 ± 29.5 | 1 vs. 5 p = 0.007 | 1 vs. 5 p = 0.027 |
| LVMi (g/m2) | 86.9 ± 18.9 | 95.2 ± 24.3 | 102.2 ± 49.2 | 0.506 | 0.380 |
| LV RWT | 0.407 ± 0.116 | 0.426 ± 0.073 | 0.510 ± 0.101 | 1 vs. 5 p < 0.0005 3 vs. 5 p < 0.0005 | 1 vs. 5 p < 0.0005 3 vs. 5 p < 0.0005 |
| LAVi (mL/m2) | 28.65 ± 7.12 | 33.18 ± 11.41 | 40.65 ± 17.74 | 1 vs. 5 p < 0.0005 3 vs. 5 p < 0.0005 | 1 vs. 5 p < 0.0005 3 vs. 5 p < 0.0005 |
| EF (%) | 62.7 ± 5.6 | 62.5 ± 5.8 | 60.3 ± 8.4 | 1 vs. 5 p = 0.037 | 1 vs. 5 p = 0.004 3 vs. 5 p = 0.012 |
| E/A | 1.033 ± 0.369 | 0.942 ± 0.358 | 0.733 ± 0.299 | 1 vs. 5 p < 0.0005 3 vs. 5 p < 0.0005 | 1 vs. 5 p < 0.0005 3 vs. 5 p < 0.0005 |
| E/e’ | 6.75 ± 2.11 | 8.66 ± 2.64 | 12.20 ± 4.36 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 3 vs. 5 p < 0.0005 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.001 3 vs. 5 p < 0.0005 |
| RA Area (cm2) | 13.1 ± 1.8 | 15.2 ± 2.8 | 16.1 ± 3.8 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 |
| Basal RVD (mm) | 30.1 ± 2.4 | 31.5 ± 2.7 | 33.0 ± 4.1 | 1 vs. 5 p < 0.0005 1 vs. 3 p = 0.018 3 vs. 5 p = 0.016 | 1 vs. 5 p < 0.0005 1 vs. 3 p = 0.05 3 vs. 5 p = 0.032 |
| IVC Diam (mm) | 14.2± 2.1 | 16.3 ± 2.0 | 16.7 ± 3.7 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 |
| TAPSE (mm) | 22.7 ± 2.7 | 21.1 ± 2.2 | 20.4 ± 2.6 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 | 1 vs. 5 p < 0.0005 1 vs. 3 p < 0.0005 |
| PAPS (mmHg) | 24.9 ± 4.4 | 26.7 ± 6.3 | 28.2 ± 8.4 | 1 vs. 5 p = 0.007 | 1 vs. 5 p = 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Raimondo, D.; Musiari, G.; Casuccio, A.; Colomba, D.; Rizzo, G.; Pirera, E.; Pinto, A.; Tuttolomondo, A. Cardiac Remodeling According to the Nocturnal Fall of Blood Pressure in Hypertensive Subjects: The Whole Assessment of Cardiac Abnormalities in Non-Dipper Subjects with Arterial Hypertension (Wacanda) Study. J. Pers. Med. 2021, 11, 1371. https://doi.org/10.3390/jpm11121371
Di Raimondo D, Musiari G, Casuccio A, Colomba D, Rizzo G, Pirera E, Pinto A, Tuttolomondo A. Cardiac Remodeling According to the Nocturnal Fall of Blood Pressure in Hypertensive Subjects: The Whole Assessment of Cardiac Abnormalities in Non-Dipper Subjects with Arterial Hypertension (Wacanda) Study. Journal of Personalized Medicine. 2021; 11(12):1371. https://doi.org/10.3390/jpm11121371
Chicago/Turabian StyleDi Raimondo, Domenico, Gaia Musiari, Alessandra Casuccio, Daniela Colomba, Giuliana Rizzo, Edoardo Pirera, Antonio Pinto, and Antonino Tuttolomondo. 2021. "Cardiac Remodeling According to the Nocturnal Fall of Blood Pressure in Hypertensive Subjects: The Whole Assessment of Cardiac Abnormalities in Non-Dipper Subjects with Arterial Hypertension (Wacanda) Study" Journal of Personalized Medicine 11, no. 12: 1371. https://doi.org/10.3390/jpm11121371
APA StyleDi Raimondo, D., Musiari, G., Casuccio, A., Colomba, D., Rizzo, G., Pirera, E., Pinto, A., & Tuttolomondo, A. (2021). Cardiac Remodeling According to the Nocturnal Fall of Blood Pressure in Hypertensive Subjects: The Whole Assessment of Cardiac Abnormalities in Non-Dipper Subjects with Arterial Hypertension (Wacanda) Study. Journal of Personalized Medicine, 11(12), 1371. https://doi.org/10.3390/jpm11121371