Salivary Micro-RNA and Oral Squamous Cell Carcinoma: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
- Patients diagnosed with OSCC;
- Minimum of 10 patients included;
- Study subjects included cancer patients and healthy controls;
- Possibility to extrapolate data for patients with OSCC (data regarding oral cancer in general or head and neck cancer were excluded).
2.2. Screening and Selection
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Bibliographic Search and Study Selection
3.2. Description of Included Studies
3.3. Excluded Studies
3.4. Quality Assessment of Included Studies
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Article Excluded | Reason for Exclusion |
|---|---|
| Dumache R et al. 2017 | Review article |
| Cristaldi M et al. 2019 | Review article |
| Gaba et al. 2018 | Review article |
| Salazar-Ruales et al. 2018 | Oral cancer in general |
| Lay YH et al. 2018 | In vitro study |
| Arantes et al. 2018 | Review article |
| Rapado-Gonzalez et al. 2019 | Review article |
| Wan Y et al. 2017 | Oral cancer in general |
| Greither T et al. 2017 | No control group |
| Fadhil et al. 2020 | Oral cancer in general |
| Yeh LY et al. 2015 | Oral cancer in general |
| Pentenero et al. 2019 | Review article |
| Coon J et al. 2020 | In vitro study |
| Brinkmann O et al. 2011 | Review article |
| Langevin S et al. 2017 | In vitro study |
| Shaidi M et al. 2017 | Oral lichen planus |
| Dharmawardana N et al. 2019 | Review article |
| Dumache R et al. 2015 | Review article |
| Peacock B et al. 2018 | Tissue sample |
| Patil S et al. 2019 | Review article |
| Li et al. 2018 | Tissue sample |
| Sun C et al. 2018 | Tissue sample |
| Salazar C et al. 2014 | Oral cancer in general |
| Gallo A et al. 2013 | Descriptive article |
| Chen M et al. 2020 | In vitro study |
| Yang et al. 2013 | Oral premalignant lesion |
| Al Makey et al. 2015 | Oral cancer in general |
| Hung et al. 2016 | Oral premalignant lesion |
| Maheswari et al. 2020 | Oral premalignant lesion |
| Petronacci et al. 2019 | Tissue sample |
| Wang Y et al. 2018 | Tissue sample |
| Pedersen et al. 2018 | Tissue sample and plasma |
| Gissi et al. 2018 | Tissue sample |
| Yang et al. 2017 | Tissue sample |
| Moratin et al. 2016 | Tissue sample |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Bugshan, A.; Farooq, I. Oral squamous cell carcinoma: Metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. F1000Research 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Sabiha, B.; Jan, H.U.; Haider, S.A.; Khan, A.A.; Ali, S.S. Genetic etiology of oral cancer. Oral Oncol. 2017, 70, 23–28. [Google Scholar] [CrossRef]
- Gigliotti, J.; Madathil, S.; Makhoul, N. Delays in oral cavity cancer. Int. J. Oral Maxillofac. Surg. 2019, 48, 1131–1137. [Google Scholar] [CrossRef]
- Fuller, C.; Camilon, R.; Nguyen, S.; Jennings, J.; Day, T.; Gillespie, M.B. Adjunctive diagnostic techniques for oral lesions of unknown malignant potential: Systematic review with meta-analysis. Head Neck 2015, 37, 755–762. [Google Scholar] [CrossRef]
- Wang, J.; Chang, S.; Li, G.; Sun, Y. Application of liquid biopsy in precision medicine: Opportunities and challenges. Front. Med. 2017, 11, 522–527. [Google Scholar] [CrossRef]
- Izzotti, A. Molecular medicine and development of cancer chemopreventive agents. Ann. N. Y. Acad. Sci. 2012, 1259, 26–32. [Google Scholar] [CrossRef]
- Hwang, H.-W.; Mendell, J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 2006, 94, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Dellepiane, E.; Baldi, D.; Longobardi, M.G.; Pera, P.; Izzotti, A. Microarray expression in peri-implant tissue next to different titanium implant surfaces predicts clinical outcomes: A split-mouth study. Clin. Oral. Implant. Res. 2017, 28, e121–e134. [Google Scholar] [CrossRef]
- Menini, M.; Pesce, P.; Baldi, D.; Coronel Vargas, G.; Pera, P.; Izzotti, A. Prediction of Titanium Implant Success by Analysis of microRNA Expression in Peri-Implant Tissue. A 5-Year Follow-Up Study. J. Clin. Med. 2019, 8, 888. [Google Scholar] [CrossRef]
- Di Leva, G.; Garofalo, M.; Croce, C.M. MicroRNAs in cancer. Annu. Rev. Pathol. 2014, 9, 287–314. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Wang, J.; Lv, N.; Lu, X.; Yuan, R.; Chen, Z.; Yu, J. Diagnostic and therapeutic role of microRNAs in oral cancer. Oncol. Rep. 2020, 45, 58–64. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Lee, J.H.; Chen, H.C.; Yang, C.M.; Huang, S.J.; Liou, H.H.; Chi, C.-C.; Tsai, K.-W.; Ger, L.-P. The association between miR-499a polymorphism and oral squamous cell carcinoma progression. Oral Dis. 2015, 21, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Tsai, M.M.; Tu, H.F.; Lui, M.T.; Cheng, H.W.; Lin, S.C. miR196a overexpression and miR-196a2 gene polymorphism are prognostic predictors of oral carcinomas. Ann. Surg. Oncol. 2013, 20, S406–S414. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Routray, S. Assessing the analytical efficacy of TEX in diagnosing oral cancer using a systematic review approach. J. Oral Pathol. Med. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Patil, S.; Warnakulasuriya, S. Blood-based circulating microRNAs as potential biomarkers for predicting the prognosis of head and neck cancer-a systematic review. Clin. Oral Investig. 2020, 24, 3833–3841. [Google Scholar] [CrossRef]
- Dioguardi, M.; Caloro, G.A.; Laino, L.; Alovisi, M.; Sovereto, D.; Crincoli, V.; Aiuto, R.; Coccia, E.; Troiano, G.; Lo Muzio, L.; et al. Circulating miR-21 as a potential biomarker for the diagnosis of oral cancer: A systematic review with meta-analysis. Cancers 2020, 12, 936. [Google Scholar] [CrossRef]
- Lamichhane, S.R.; Thachil, T.; Gee, H.; Milic, N. Circulating MicroRNAs as prognostic molecular biomarkers in human head and neck cancer: A systematic review and meta-analysis. dis markers. Dis. Markers 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, C.; Devi, A.; Jayaraj, R. Prognostic value of microRNAs in head and neck cancers: A systematic review and meta-analysis protocol. Syst. Rev. 2018, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Arellano, M.; Boontheung, P.; Wang, J.; Zhou, H.; Jiang, J.; Elashoff, D.; Wei, R.; Loo, J.A.; Wong, D.T. Salivary proteomics for oral cancer biomarker discovery. Clin. Cancer Res. 2008, 14, 6246–6252. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; St John, M.A.; Zhou, X.; Kim, Y.; Sinha, U.; Jordan, R.C.; Eisele, D.; Abemayor, E.; Elashoff, D.; Park, N.H.; et al. Salivary transcriptome diagnostics for oral cancer detection. Clin. Cancer Res. 2004, 10, 8442–8450. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Zhou, X.; Yu, T.; Brinkman, B.M.; Zimmermann, B.G.; Palanisamy, V.; Wong, D.T. Characterization of salivary RNA by cDNA library analysis. Arch. Oral Biol. 2007, 52, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2009, 339, b2535. [Google Scholar]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef]
- Wiklund, E.D.; Gao, S.; Hulf, T.; Sibbritt, T.; Nair, S.; Costea, D.E.; Villadsen, S.B.; Bakholdt, V.; Bramsen, J.B.; Sørensen, J.A.; et al. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS ONE 2011, 6, e27840. [Google Scholar] [CrossRef]
- Liu, C.J.; Lin, S.C.; Yang, C.C.; Cheng, H.W.; Chang, K.W. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck 2012, 34, 219–224. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Trachtenberg, A.J.; Kuo, W.P.; Cheng, Y.S. Genomewide study of salivary MicroRNAs for detection of oral cancer. J. Dent. Res. 2014, 93, 86S–93S. [Google Scholar] [CrossRef]
- Zahran, F.; Ghalwash, D.; Shaker, O.; Al-Johani, K.; Scully, C. Salivary microRNAs in oral cancer. Oral Dis. 2015, 21, 739–747. [Google Scholar] [CrossRef]
- Duz, M.B.; Karatas, O.F.; Guzel, E.; Turgut, N.F.; Yilmaz, M.; Creighton, C.J.; Ozen, M. Identification of miR-139–5p as a saliva biomarker for tongue squamous cell carcinoma: A pilot study. Cell Oncol. 2016, 39, 187–193. [Google Scholar] [CrossRef]
- Min, S.K.; Jung, S.Y.; Kang, H.K.; Park, S.A.; Lee, J.H.; Kim, M.J.; Min, B.M. Functional diversity of miR-146a-5p and TRAF6 in normal and oral cancer cells. Int. J. Oncol. 2017, 51, 1541–1552. [Google Scholar] [CrossRef]
- Yap, T.; Koo, K.; Cheng, L.; Vella, L.J.; Hill, A.F.; Reynolds, E.; Nastri, A.; Cirillo, N.; Seers, C.; McCullough, M. Predicting the presence of oral squamous cell carcinoma using commonly dysregulated MicroRNA in oral swirls. Cancer Prev. Res. 2018, 11, 491–502. [Google Scholar] [CrossRef]
- Mehdipour, M.; Shahidi, M.; Manifar, S.; Jafari, S.; Mashhadi Abbas, F.; Barati, M.; Mortazavi, H.; Shirkhoda, M.; Farzanegan, A.; Elmi Rankohi, Z.; et al. Diagnostic and prognostic relevance of salivary microRNA-21, -125a, -31 and -200a levels in patients with oral lichen planus-a short report. Cell. Oncol. 2018, 41, 329–334. [Google Scholar] [CrossRef]
- Gai, C.; Camussi, F.; Broccoletti, R.; Gambino, A.; Cabras, M.; Molinaro, L.; Carossa, S.; Camussi, G.; Arduino, P.G. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 2018, 18, 439. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.; Seers, C.; Koo, K.; Cheng, L.; Vella, L.J.; Hill, A.F.; Reynolds, E.; Nastri, A.; Cirillo, N.; McCullough, M.; et al. Non-invasive screening of a microRNA-based dysregulation signature in oral cancer and oral potentially malignant disorders. Oral Oncol. 2019, 96, 113–120. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ping, F.; Fan, Z.; Zhang, C.; Deng, M.; Cheng, B.; Xia, J. Salivary exosomal miR-24–3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed Pharmacother. 2020, 121, 109553. [Google Scholar] [CrossRef]
- Dumache, R. Early diagnosis of oral squamous cell carcinoma by salivary microRNAs. Clin. Lab. 2017, 63, 1771–1776. [Google Scholar] [CrossRef]
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Gaba, F.I.; Sheth, C.C.; Veses, V. Salivary biomarkers and their efficacies as diagnostic tools for oral squamous cell carcinoma: Systematic review and meta-analysis. J. Oral Pathol. Med. 2018. [Google Scholar] [CrossRef]
- Salazar-Ruales, C.; Arguello, J.V.; López-Cortés, A.; Cabrera-Andrade, A.; García-Cárdenas, J.M.; Guevara-Ramírez, P.; Peralta, P.; Leone, P.E.; Paz-y-Miño, C. Salivary MicroRNAs for early detection of head and neck squamous cell carcinoma: A case-control study in the high altitude mestizo ecuadorian population. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Lai, Y.H.; Liu, H.; Chiang, W.F.; Chen, T.W.; Chu, L.J.; Yu, J.S.; Chen, S.J.; Chen, H.C.; Tan, B.C. MiR-31–5p-ACOX1 axis enhances tumorigenic fitness in oral squamous cell carcinoma via the promigratory prostaglandin E2. Theranostics 2018, 8, 486–504. [Google Scholar] [CrossRef]
- Arantes, L.M.R.B.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef]
- Rapado-González, O.; Martínez-Reglero, C.; Salgado-Barreira, A.; López-López, R.; Suárez-Cunqueiro, M.M.; Muinelo-Romay, L. miRNAs in liquid biopsy for oral squamous cell carcinoma diagnosis: Systematic review and meta-analysis. Oral Oncol. 2019, 99, 104465. [Google Scholar] [CrossRef]
- Wan, Y.; Vagenas, D.; Salazar, C.; Kenny, L.; Perry, C.; Calvopiña, D.; Punyadeera, C. Salivary miRNA panel to detect HPV-positive and HPV-negative head and neck cancer patients. Oncotarget 2017, 8, 99990–100001. [Google Scholar] [CrossRef]
- Greither, T.; Vorwerk, F.; Kappler, M.; Bache, M.; Taubert, H.; Kuhnt, T.; Hey, J.; Eckert, A.W. Salivary miR-93 and miR-200a as post-radiotherapy biomarkers in head and neck squamous cell carcinoma. Oncol. Rep. 2017, 38, 1268–1275. [Google Scholar] [CrossRef]
- Fadhil, R.S.; Wei, M.Q.; Nikolarakos, D.; Good, D.; Nair, R.G. Salivary microRNA miR-let-7a-5p and miR-3928 could be used as potential diagnostic bio-markers for head and neck squamous cell carcinoma. PLoS ONE 2020, 15, e0221779. [Google Scholar] [CrossRef]
- Yeh, L.Y.; Liu, C.J.; Wong, Y.K.; Chang, C.; Lin, S.C.; Chang, K.W. miR-372 inhibits p62 in head and neck squamous cell carcinoma In Vitro and In Vivo. Oncotarget 2015, 6, 6062–6075. [Google Scholar] [CrossRef]
- Pentenero, M.; Bowers, L.; Jayasinghe, R.; Cheong, S.C.; Farah, C.S.; Kerr, A.R.; Alevizos, I. World workshop on oral medicine VII: Functional pathways involving differentially expressed lncRNAs in oral squamous cell carcinoma. Oral Dis. 2019, 25, 79–87. [Google Scholar] [CrossRef]
- Coon, J.; Kingsley, K.; Howard, K.M. miR-365 (microRNA): Potential biomarker in oral squamous cell carcinoma exosomes and extracellular vesicles. Int. J. Mol. Sci. 2020, 21, 5317. [Google Scholar] [CrossRef]
- Brinkmann, O.; Wong, D.T. Salivary transcriptome biomarkers in oral squamous cell cancer detection. Adv. Clin. Chem. 2011, 55, 21–34. [Google Scholar] [PubMed]
- Langevin, S.; Kuhnell, D.; Parry, T.; Biesiada, J.; Huang, S.; Wise-Draper, T.; Casper, K.; Zhang, X.; Medvedovic, M.; Kasper, S.; et al. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget 2017, 8, 82459–82474. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, M.; Jafari, S.; Barati, M.; Mahdipour, M.; Gholami, M.S. Predictive value of salivary microRNA-320a, vascular endothelial growth factor receptor 2, CRP and IL-6 in Oral lichen planus progression. Inflammopharmacology 2017, 25, 577–583. [Google Scholar] [CrossRef]
- Dharmawardana, N.; Ooi, E.H.; Woods, C.; Hussey, D. Circulating microRNAs in head and neck cancer: A scoping review of methods. Clin. Exp. Metastasis 2019, 36, 291–302. [Google Scholar] [CrossRef]
- Dumache, R.; Rogobete, A.F.; Andreescu, N.; Puiu, M. Genetic and epigenetic biomarkers of molecular alterations in oral carcinogenesis. Clin. Lab. 2015, 61, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Peacock, B.; Rigby, A.; Bradford, J.; Pink, R.; Hunter, K.; Lambert, D.; Hunt, S. Extracellular vesicle microRNA cargo is correlated with HPV status in oropharyngeal carcinoma. J. Oral. Pathol. Med. 2018, 47, 954–963. [Google Scholar] [CrossRef]
- Patil, S.; Arakeri, G.; Alamir, A.W.H.; Awan, K.H.; Baeshen, H.; Ferrari, M.; Patil, S.; Fonseca, F.P.; Brennan, P.A. Role of salivary transcriptomics as potential biomarkers in oral cancer: A systematic review. J. Oral. Pathol. Med. 2019, 48, 871–879. [Google Scholar] [CrossRef]
- Li, Y.Y.; Tao, Y.W.; Gao, S.; Li, P.; Zheng, J.M.; Zhang, S.E.; Liang, J.; Zhang, Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. [Google Scholar] [CrossRef]
- Sun, C.; Li, J. Expression of MiRNA-137 in oral squamous cell carcinoma and its clinical significance. J. Buon. 2018, 23, 167–172. [Google Scholar]
- Salazar, C.; Nagadia, R.; Pandit, P.; Cooper-White, J.; Banerjee, N.; Dimitrova, N.; Coman, W.B.; Punyadeera, C. A novel saliva-based microRNA biomarker panel to detect head and neck cancers. Cell Oncol. 2014, 37, 331–338. [Google Scholar] [CrossRef]
- Gallo, A.; Alevizos, I. Isolation of circulating microRNA in saliva. Methods Mol. Biol. 2013, 1024, 183–190. [Google Scholar] [PubMed]
- Chen, M.; Luo, R.; Li, S.; Li, H.; Qin, Y.; Zhou, D.; Liu, H.; Gong, X.; Chang, J. Paper-based strip for ultrasensitive detection of OSCC-associated salivary MicroRNA via CRISPR/Cas12a coupling with is-primer amplification reaction. Anal. Chem. 2020, 92, 13336–13342. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.X.; Yang, X.; Jiang, L.; Zhou, Z.J.; Zhu, Y.Q. Progress risk assessment of oral premalignant lesions with saliva miRNA analysis. BMC Cancer 2013, 13, 129. [Google Scholar] [CrossRef]
- Al-Malkey, M.K.; Abbas, A.A.H. Original research article expression analysis of salivary microrna-31 in oral cancer patients. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 375–382. [Google Scholar]
- Hung, K.F.; Liu, C.J.; Chiu, P.C.; Lin, J.S.; Chang, K.W.; Shih, W.Y.; Kao, S.Y.; Tu, H.F. MicroRNA-31 upregulation predicts increased risk of progression of oral potentially malignant disorder. Oral Oncol. 2016, 53, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Uma Maheswari, T.N.; Nivedhitha, M.S.; Ramani, P. Expression profile of salivary microRNA-21 and 31 in oral potentially malignant disorders. Braz. Oral. Res. 2020, 34, e002. [Google Scholar] [CrossRef]
- Chamorro Petronacci, C.M.; Pérez-Sayáns, M.; Padín Iruegas, M.E.; Suárez Peñaranda, J.M.; Lorenzo Pouso, A.I.; Blanco Carrión, A.; García García, A. miRNAs expression of oral squamous cell carcinoma patients: Validation of two putative biomarkers. Medicine 2019, 98, e14922. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, B.W.; Sun, S.; Li, X.; Su, W.; Wang, Z.H.; Wang, F.; Zhang, W.; Yang, H.Y. Circular RNA expression in oral squamous cell carcinoma. Front. Oncol. 2018, 8, 398. [Google Scholar] [CrossRef]
- Pedersen, N.J.; Jensen, D.H.; Lelkaitis, G.; Kiss, K.; Charabi, B.W.; Ullum, H.; Specht, L.; Schmidt, A.Y.; Nielsen, F.C.; von Buchwald, C. MicroRNA-based classifiers for diagnosis of oral cavity squamous cell carcinoma in tissue and plasma. Oral Oncol. 2018, 83, 46–52. [Google Scholar] [CrossRef]
- Gissi, D.B.; Morandi, L.; Gabusi, A.; Tarsitano, A.; Marchetti, C.; Cura, F.; Palmieri, A.; Montebugnoli, L.; Asioli, S.; Foschini, M.P.; et al. A noninvasive test for MicroRNA expression in oral squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 1789. [Google Scholar] [CrossRef]
- Yang, X.; Ruan, H.; Hu, X.; Cao, A.; Song, L. miR-381–3p suppresses the proliferation of oral squamous cell carcinoma cells by directly targeting FGFR. Am. J. Cancer Res. 2017, 7, 913–922. [Google Scholar]
- Moratin, J.; Hartmann, S.; Brands, R.; Brisam, M.; Mutzbauer, G.; Scholz, C.; Seher, A.; Müller-Richter, U.; Kübler, A.C.; Linz, C.; et al. Evaluation of miRNA-expression and clinical tumour parameters in oral squamous cell carcinoma (OSCC). J. Craniomaxillofac Surg. 2016, 44, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Carozzo, S.; Pulliero, A.; Zhabayeva, D.; Ravetti, J.L.; Bersimbaev, R. Extracellular MicroRNA in liquid biopsy: Applicability in cancer diagnosis and prevention. Am. J. Cancer Res. 2016, 6, 1461–1493. [Google Scholar] [PubMed]
- Lousada-Fernandez, F.; Rapado-Gonzalez, O.; Lopez-Cedrun, J.L.; Lopez-Lopez, R.; Muinelo-Romay, L.; Suarez-Cunqueiro, M.M. Liquid biopsy in oral cancer. Int. J. Mol. Sci. 2018, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; GarciaGodoy, F.; Wong, D.T.W. Saliva diagnostics-current views and directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; Majem, B.; Muinelo-Romay, L.; Álvarez-Castro, A.; Santamaría, A.; Gil-Moreno, A.; López-López, R.; Suárez-Cunqueiro, M.M. Human salivary microRNAs in cancer. J. Cancer 2018, 9, 638–649. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar] [CrossRef]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef]
- Endzeliņš, E.; Berger, A.; Melne, V.; Bajo-Santos, C.; Sobolevska, K.; Ābols, A.; Rodriguez, M.; Šantare, D.; Rudņickiha, A.; Lietuvietis, V.; et al. Detection of circulating miRNAs: Comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer 2017, 17, 730. [Google Scholar] [CrossRef]

| Authors (Year) | Cases (OSCC Patients) | Controls (Healthy Subjects) | Tumor Histological Stage or Grade | miRNAs Detection Method | Salivary miRNAs—Disregulation in OSCC Patients | Application | Follow-Up | Main Outcomes |
|---|---|---|---|---|---|---|---|---|
| Park et al. 2009 [25]. | 50 OSCC | 50 | 10 patients were at tumor stage I, 14 were at stage II, 16 were at stage III, 10 were at stage IV | RT-PCR preampqPCR Saliva miRNA stability assay | miR142-3p miR200a miR125a miR-93 Downregulation | Diagnosis | ND | miRNAs are present in both whole saliva and supernatant saliva. miR-125a and miR-200a, are downregulated in the saliva of OSCC patients compared to healthy controls |
| Wiklund et al. 2011 [26]. | 15 OSCC | 7 | ND | TaqManH low density array qRT-PCR | miR-34b miR-137 miR-155 miR-200c-141 miR-203 mir-205 miR-375 mir-410 Aberrant expression and DNA hypermethylation | Diagnosis | ND | Compared to healthy subjects, OSCC patients had deregulated miRNAs with associated DNA methylation patterns. Particularly, repression of miR-375 and methylation on miR-137, miR-200c-141, and miR-200 s/miR-205 loci were found in OSCC patients vs. healthy patients, being promising candidates to develop OSCC-specific miRNA signatures. |
| Liu et al. 2012 [27]. | 45 OSCC | 24 | 21 stage I-II 24 stage III-IV | qRT-PCR | miR-31 Upregulation | Diagnosis and follow-up | 4–6 weeks after surgery | Salivary miR-31 was significantly increased in patients with OSCC at all clinical stages, including very early stages. In addition, it was shown to be more abundant in saliva than in plasma, and after tumor surgical removal its expression was reduced. |
| Momen-Heravi et al. 2014 [28]. | 9 OSCC (before treatment), 8 OSCC-r (in remission) | 9 | ND | RT-qPCR | miR136 miR147 miR1250 miR148a miR632 miR646 miR668 miR877 miR503 miR220a miR323-5p underexpressed miR-24 miR27b overexpressed | Diagnosis | ND | miRNA profiles derived from OSCC, OSCC-r, and healthy controls were distinctively different. In particular, overexpression of miRNA-27b was found in OSCC saliva samples and not in the saliva of the other two groups |
| Zarhan et al. 2015 [29]. | 100 Oral cancer (20 OSCC) | 20 | Grade III (high grade) LN involvement: 7 Grade II, LN involvement (2 LN): 1 Grade II, no LN involvement: 7 No record available: 3 | qRT-PCR | miR-21 upregulation miR184 upregulation miR145 downregulation | Diagnosis | ND | Salivary miRNA-21, miRNA-145, and miRNA-184 were differentially expressed in OSCC and healthy saliva samples, with miRNA-184 having the best diagnostic value |
| Duz et al. 2016 [30]. | 25 OSCC | 25 | 2 grade 1 16 grade 2 4 grade 3 3 ND | qRT-PCR Microarray-based miRNA | miR139-5p downregulation | Diagnosis and follow-up | 4–6 weeks after surgery | Salivary miR-139-5p was significantly reduced in TSCC patients compared to controls, and its level turned back to normal after surgery. |
| Seung-Ki Min et al. 2017 [31]. | 18 OSCC | ND | ND | RT-qPCR miRNA Microarray | miR-146a-5p upregulated | Diagnosis | ND | miR-146a-5p expression was highly upregulated in OSCC patients. |
| Yap T et al. 2018 [32]. | 30 OSCC | 30 | 14 stage 1 3 stage 2 3 stage 3 10 stage 4 | RT-qPCR | miR-24 miR-21 miR-31 upregulation miR-99a let-7c miR-100 downregulation | Diagnosis | ND | Upregulation of miR-31 and miR-21 and downregulation of miR-99a, let-7c, miR-125b, and miR-100 were found in OSCC and controls in both FFPE and fresh-frozen samples. These miRNAs were studied in oral swirls to develop a dysregulation score with the classification tree identifying 100% (15/15) of OSCC and 67% (10/15) of controls. |
| Mehdipour et al. 2018 [33]. | 30 OLP 15 OSCC | 15 | ND | qRT-PCR | miR-21 upregulation miR-125a downregulation miR-31 upregulation miR-200a downregulation | Diagnosis | ND | miR-21 levels were significantly increased in saliva samples derived from patients with OLP, dysplastic OLP and OSCC, compared to those from healthy controls. Conversely, significant decreases in miR-125a levels were found in the OLP, dysplastic OLP and OSCC samples, compared to those from healthy controls. Significant increases in miR-31 levels were found in samples derived from dysplastic OLP and OSCC patients, but not in nondysplastic OLP, compared to healthy controls. miR-200a levels were significantly decreased only in samples derived from OSCC patients |
| Gai C et al. 2018 [34]. | 21 OSCC | 11 | T1 (n = 7), T2 (n = 8), T3 (n = 3), T4 (n = 3) | qRT-PCR array qRT-PCR | miR-412- 3p, miR-489-3p, miR-512-3p, miR-597-5p, and miR-603 upregulated miR-193b-3p, miR-30e3p, miR-376c-3p, miR-484, miR-720, and miR-93-3pdownregulated | Diagnosis | ND | miR-302b-3p and miR-517b-3p were expressed only in EVs from OSCC patients and miR-512-3p and miR-412-3p were up-regulated in salivary EVs from OSCC patients compared to controls with the ROC curve showing a good discrimination power for OSCC diagnosis |
| Yap T et al. 2019 [35]. | 53 OSCC | 54 | 24 T1 10 T2 3 T3 15 T4a 1 not specified | RT-qPCR | miR-24-3p, miR-21-5p, let-7c-5p, miR-99a-5p, miR-100-5p | Diagnosis | ND | MicroRNAs can be predictably isolated from oral swirls. A high-risk dysregulation signature was found to be accurate in indicating the presence of OSCC with 86.8% sensitivity and 81.5% specificity |
| He L et al. 2020 [36]. | 49 OSCC | 14 | ND | Microarray analysis qRT-PCR | miR-7975, miR-1246 and miR-24-3p upregulated | Diagnosis | ND | The authors identified a significant increase of miR-24-3p in the salivary exosomes from 45 preoperative OSCC patients compared to healthy individuals |
| Study | Selection | Comparability | Outcome/Exposure | NOS Score |
|---|---|---|---|---|
| Park et al. 2009 | 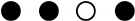 |  | 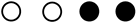 | 6 |
| Wiklund et al. 2011 | 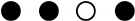 |  | 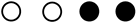 | 6 |
| Liu et al. 2012 | 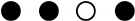 |  | 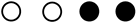 | 7 |
| Momen-Heravi et al. 2014 | 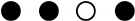 |  | 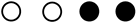 | 6 |
| Zarhan et al. 2015 | 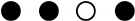 |  | 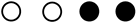 | 6 |
| Duz et al. 2016 | 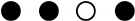 |  | 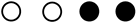 | 7 |
| Yap T et al. 2018 | 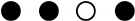 |  | 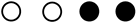 | 6 |
| Mehdipour et al. 2018 | 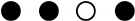 |  | 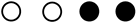 | 6 |
| Gai C et al. 2018 | 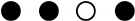 |  | 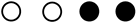 | 7 |
| Yap T et al. 2019 | 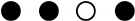 |  | 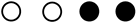 | 6 |
| He L et al. 2020 | 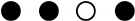 |  | 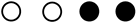 | 7 |
| Seung-Ki Min et al. 2017 | 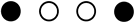 |  | 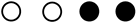 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menini, M.; De Giovanni, E.; Bagnasco, F.; Delucchi, F.; Pera, F.; Baldi, D.; Pesce, P. Salivary Micro-RNA and Oral Squamous Cell Carcinoma: A Systematic Review. J. Pers. Med. 2021, 11, 101. https://doi.org/10.3390/jpm11020101
Menini M, De Giovanni E, Bagnasco F, Delucchi F, Pera F, Baldi D, Pesce P. Salivary Micro-RNA and Oral Squamous Cell Carcinoma: A Systematic Review. Journal of Personalized Medicine. 2021; 11(2):101. https://doi.org/10.3390/jpm11020101
Chicago/Turabian StyleMenini, Maria, Emanuele De Giovanni, Francesco Bagnasco, Francesca Delucchi, Francesco Pera, Domenico Baldi, and Paolo Pesce. 2021. "Salivary Micro-RNA and Oral Squamous Cell Carcinoma: A Systematic Review" Journal of Personalized Medicine 11, no. 2: 101. https://doi.org/10.3390/jpm11020101
APA StyleMenini, M., De Giovanni, E., Bagnasco, F., Delucchi, F., Pera, F., Baldi, D., & Pesce, P. (2021). Salivary Micro-RNA and Oral Squamous Cell Carcinoma: A Systematic Review. Journal of Personalized Medicine, 11(2), 101. https://doi.org/10.3390/jpm11020101









