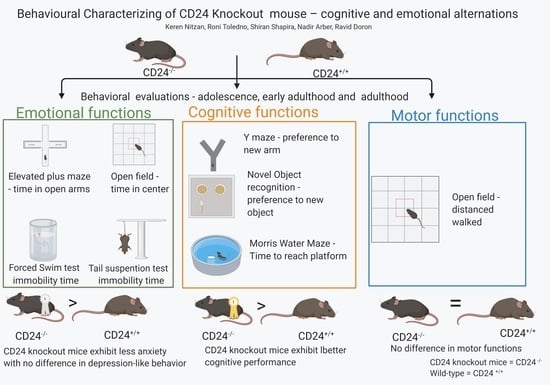
Behavioral Characterizing of CD24 Knockout Mouse—Cognitive and Emotional Alternations
Abstract
:1. Introduction
2. Materials and Methods
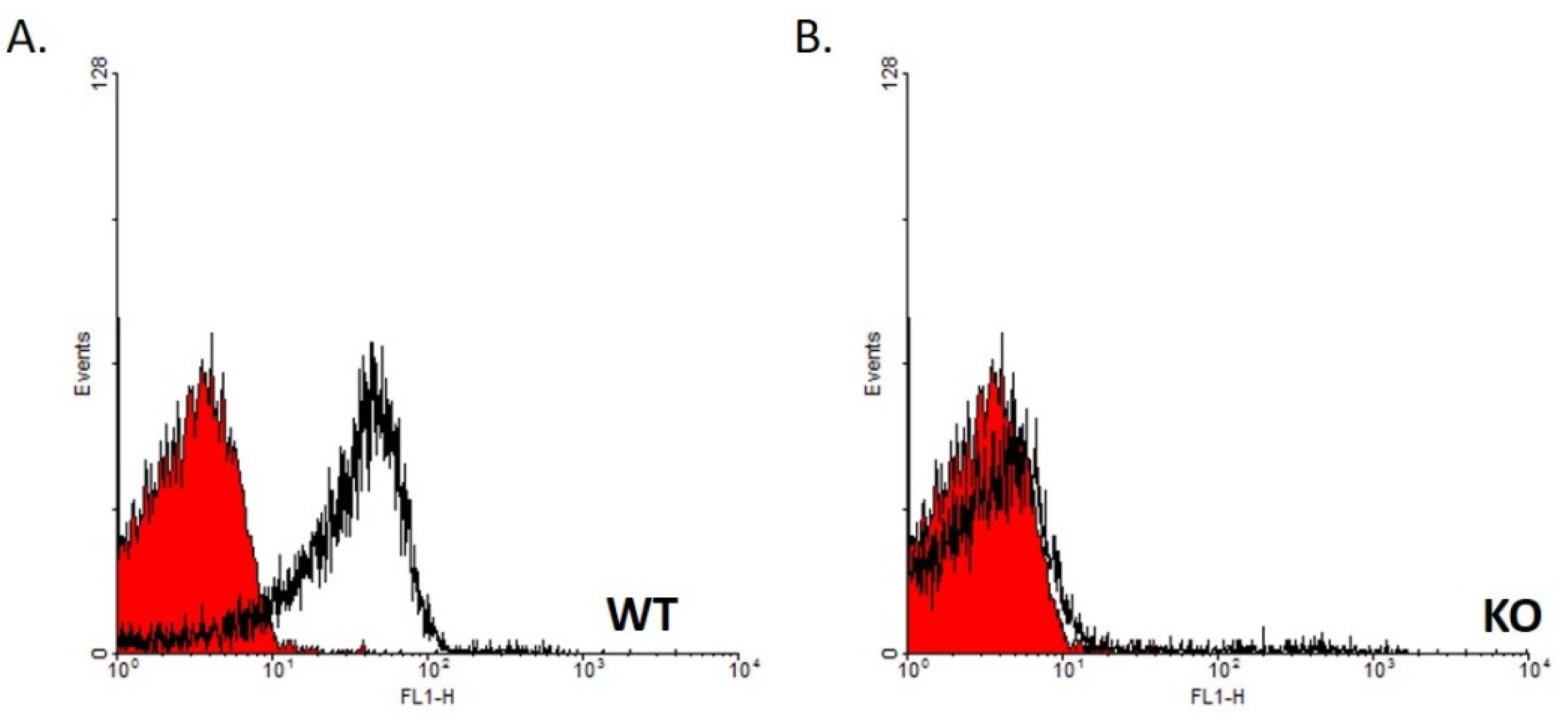
2.1. Genotype Verification by FACS Analysis
2.2. Behavioral and Cognitive Evaluations
2.3. Data Analysis
3. Results
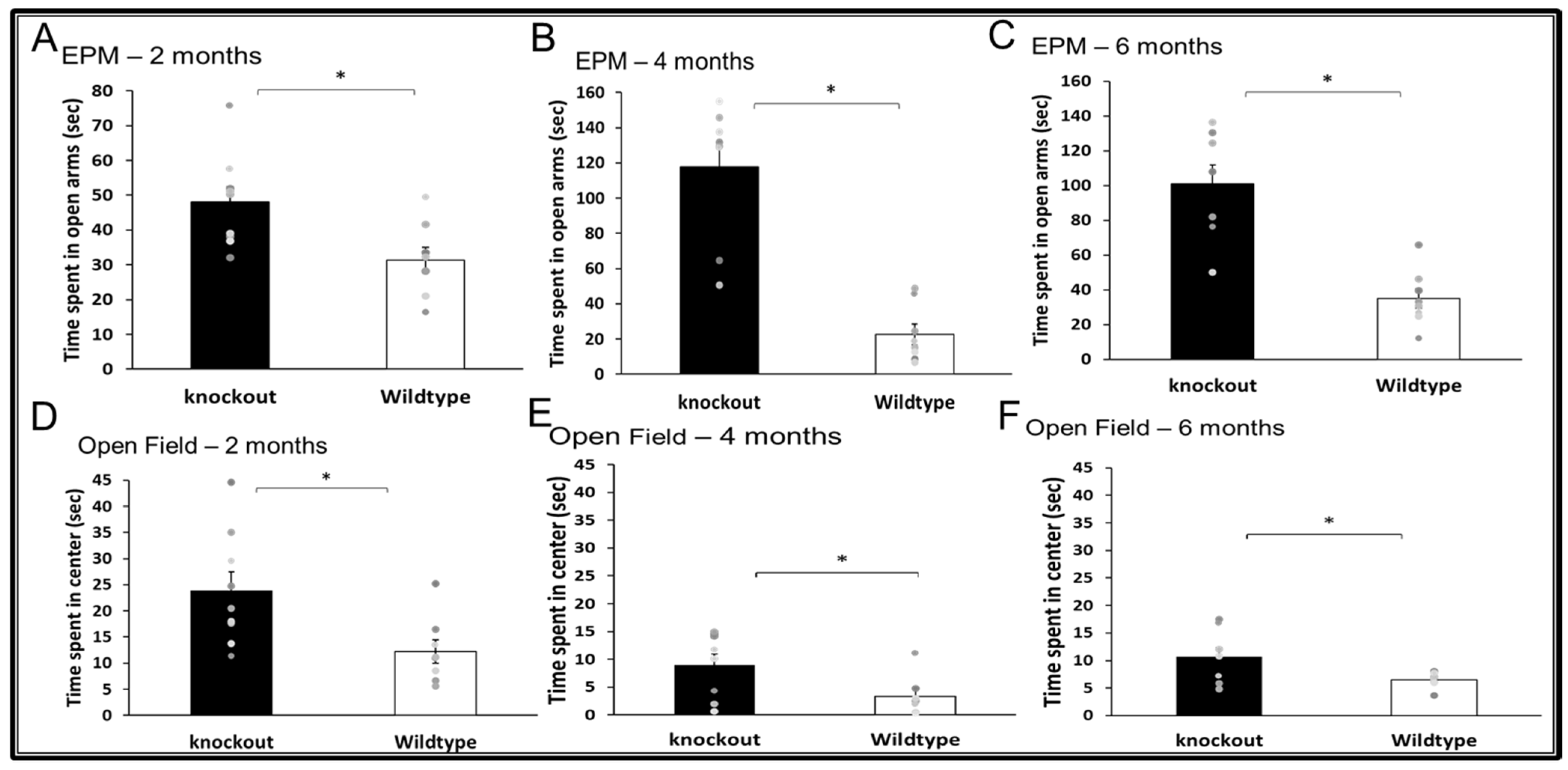
3.1. Anxiety-Like Behavior
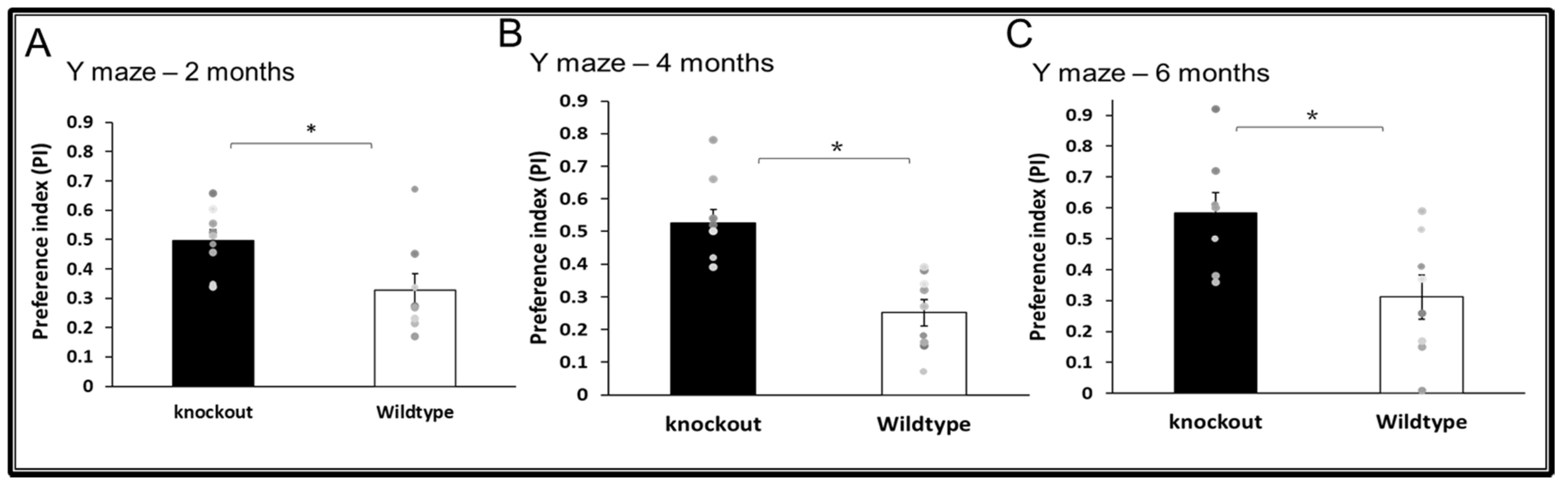
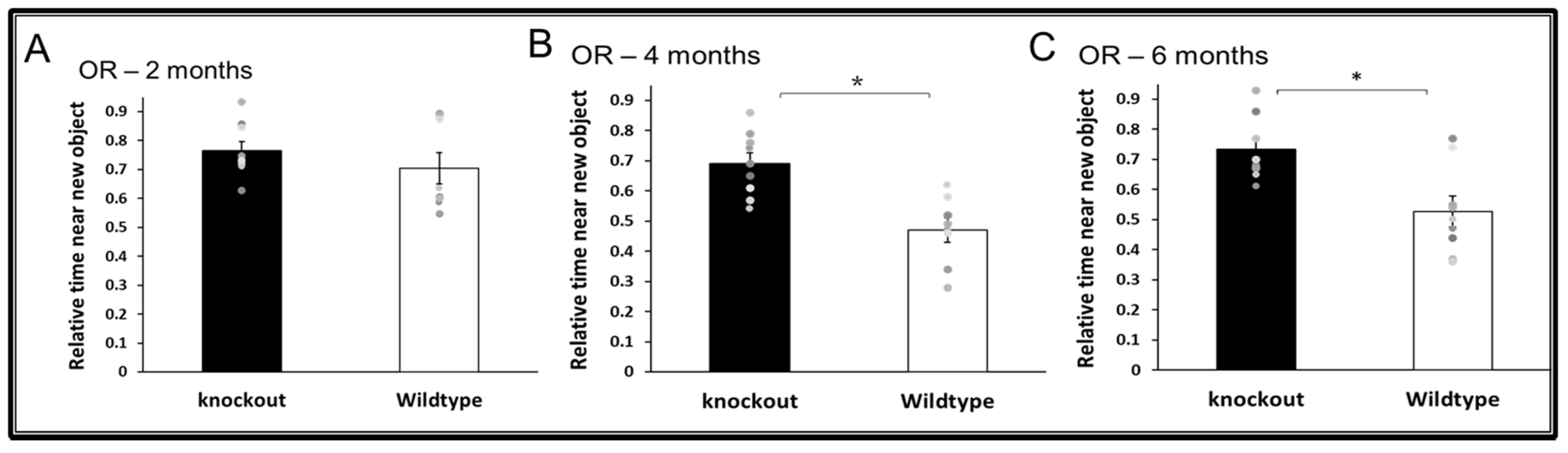
3.2. Cognitive Functions
3.3. Depression-Like Behavior
3.4. Motor Functions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kadmon, G.; Eckert, M.; Sammar, M.; Schachner, M.; Altevogt, P. Nectadrin, the heat-stable antigen, is a cell adhesion molecule. J. Cell Biol. 1992, 118, 1245–1258. [Google Scholar] [CrossRef] [Green Version]
- Hunte, B.E.; Capone, M.; Zlotnik, A.; Rennick, D.; Moore, T.A. Acquisition of CD24 expression by Lin-CD43+B220(low)ckit(hi) cells coincides with commitment to the B cell lineage. Eur. J. Immunol. 1998, 28, 3850–3856. [Google Scholar] [CrossRef]
- Altevogt, P.; Sammar, M.; Hüser, L.; Kristiansen, G. Novel insights into the function of CD24: A driving force in cancer. International Journal of Cancer. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/ijc.33249 (accessed on 13 October 2020).
- Wang, W.; Wang, X.; Peng, L.; Deng, Q.; Liang, Y.; Qing, H.; Jiang, B. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci. 2010, 101, 112–119. [Google Scholar] [CrossRef]
- Pei, Z.; Zhu, G.; Huo, X.; Gao, L.; Liao, S.; He, J.; Long, Y.; Yi, H.; Xiao, S.; Yi, W.; et al. CD24 promotes the proliferation and inhibits the apoptosis of cervical cancer cells in vitro. Oncol. Rep. 2016, 35, 1593–1601. [Google Scholar] [CrossRef]
- Lee, K.; Ju, J.; Jang, K.; Yang, W.; Yi, J.Y.; Noh, D.Y.; Shin, I. CD24 regulates cell proliferation and transforming growth factor β-induced epithelial to mesenchymal transition through modulation of integrin β1 stability. Cell. Signal. 2012, 24, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Shapira, A.; Starr, A.; Kazanov, D.; Kraus, S.; Benhar, I.; Arber, N. An Immunoconjugate of Anti-CD24 and Pseudomonas Exotoxin Selectively Kills Human Colorectal Tumors in Mice. Gastroenterology 2011, 140, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Dai, Z.; Jiang, Z.; He, Y.; Wang, L.; Liao, Q.; Sun, N.; Wang, Y.; Sun, S.; Lin, W.; et al. CD24: A marker of granulosa cell subpopulation and a mediator of ovulation. Cell Death Dis. 2019, 10, 791. [Google Scholar] [CrossRef]
- Karnan, S.; Ota, A.; Murakami, H.; Rahman, M.L.; Hasan, M.N.; Wahiduzzaman, M.; Hanamura, I.; Quang Vu, L.; Inoko, A.; Hyodo, T.; et al. Identification of CD24 as a potential diagnostic and therapeutic target for malignant pleural mesothelioma. Cell Death Discov. 2020, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zheng, P.; Tang, J.; Liu, Y. CD24: From A to Z. Cell Mol. Immunol. 2010, 7, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Stott, S.R.W.; Hayat, S.; Carnwath, T.; Garas, S.; Sleeman, J.P.; Barker, R.A. CD24 expression does not affect dopamine neuronal survival in a mouse model of Parkinson’s disease. PLoS ONE 2017, 12, e0171748. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Carl, J.W.; Joshi, P.S.; RayChaudhury, A.; Pu, X.-A.; Shi, F.-D.; Bai, X.-F. CD24 on the Resident Cells of the Central Nervous System Enhances Experimental Autoimmune Encephalomyelitis. J. Immunol. 2007, 178, 6227–6235. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-F.; Liu, J.-Q.; Liu, X.; Guo, Y.; Cox, K.; Wen, J.; Zheng, P.; Liu, Y. The heat-stable antigen determines pathogenicity of self-reactive T cells in experimental autoimmune encephalomyelitis. J. Clin. Invest. 2000, 105, 1227–1232. [Google Scholar] [CrossRef]
- Naboichtchikov, I.; Maharshak, N.; Shapira, S.; Fokra, A.; Arber, N.; Kraus, S. Mo1767 CD24 Plays a Role in the Pathogenesis of DSS-Induced Experimental Colitis in Mice. Gastroenterology 2015, 148, S-706. [Google Scholar] [CrossRef]
- Goris, A.; Maranian, M.; Walton, A.; Yeo, T.W.; Ban, M.; Gray, J.; Dubois, B.; Compston, A.; Sawcer, S. CD24 Ala/Val polymorphism and multiple sclerosis. J. Neuroimmunol. 2006, 175, 200–202. [Google Scholar] [CrossRef]
- Lisiansky, V.; Kraus, S.; Naumov, I.; Kazanov, D.; Nabiochtchikov, I.; Toledano, O.; Leshno, M.; Avivi, D.; Dotan, I.; Arber, N.; et al. Role of CD24 Polymorphisms in the Susceptibility to Inflammatory Bowel Disease: The International Journal of Biological Markers. Available online: https://journals.sagepub.com/doi/10.5301/jbm.5000072 (accessed on 14 October 2020).
- Huang, S.; Sun, C.; Hou, Y.; Tang, Y.; Zhu, Z.; Zhang, Z.; Zhang, Y.; Wang, L.; Zhao, Q.; Chen, M.-G.; et al. A comprehensive bioinformatics analysis on multiple Gene Expression Omnibus datasets of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Sci. Rep. 2018, 8, 7630. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.; del Rey, A.; Sorkin, E.; Dinarello, C. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 1986, 233, 652–654. [Google Scholar] [CrossRef]
- Wolf, G.; Gabay, E.; Tal, M.; Yirmiya, R.; Shavit, Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain 2006, 120, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Shavit, Y.; Fridel, K.; Beilin, B. Postoperative Pain Management and Proinflammatory Cytokines: Animal and Human Studies. J. Neuroimmune Pharm. 2006, 1, 443–451. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.; Pinteaux, E. The Interleukin-1 System: An Attractive and Viable Therapeutic Target in Neurodegenerative Disease. CDTCNSND 2003, 2, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Duman, R.S. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr. Opin. Investig. Drugs 2009, 10, 664–671. [Google Scholar]
- Calaora, V.; Chazal, G.; Nielsen, P.J.; Rougon, G.; moreau, H. mCD24 expression in the developing mouse brain and in zones of secondary neurogenesis in the adult. Neuroscience 1996, 73, 581–594. [Google Scholar] [CrossRef]
- Bai, X.-F.; Li, O.; Zhou, Q.; Zhang, H.; Joshi, P.S.; Zheng, X.; Liu, Y.; Wang, Y.; Zheng, P.; Liu, Y. CD24 Controls Expansion and Persistence of Autoreactive T Cells in the Central Nervous System during Experimental Autoimmune Encephalomyelitis. J. Exp. Med. 2004, 200, 447–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doron, R.; Lotan, D.; Rak-Rabl, A.; Raskin-Ramot, A.; Lavi, K.; Rehavi, M. Anxiolytic effects of a novel herbal treatment in mice models of anxiety. Life Sci. 2012, 90, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Doron, R.; Lotan, D.; Versano, Z.; Benatav, L.; Franko, M.; Armoza, S.; Kately, N.; Rehavi, M. Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PLoS ONE 2014, 9, e91455. [Google Scholar] [CrossRef] [PubMed]
- Sarne, Y.; Toledano, R.; Rachmany, L.; Sasson, E.; Doron, R. Reversal of age-related cognitive impairments in mice by an extremely low dose of tetrahydrocannabinol. Neurobiol. Aging 2018, 61, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Doron, R.; Lotan, D.; Einat, N.; Yaffe, R.; Winer, A.; Marom, I.; Meron, G.; Kately, N.; Rehavi, M. A novel herbal treatment reduces depressive-like behaviors and increases BDNF levels in the brain of stressed mice. Life Sci. 2014, 94, 151–157. [Google Scholar] [CrossRef]
- Kleene, R.; Yang, H.; Kutsche, M.; Schachner, M. The Neural Recognition Molecule L1 Is a Sialic Acid-binding Lectin for CD24, Which Induces Promotion and Inhibition of Neurite Outgrowth. J. Biol. Chem. 2001, 276, 21656–21663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.J.; Munchow, A.H.; Rios, L.M.; Zhang, G.; Ásgeirsdóttir, H.N.; Stackman, R.W. The Rodent Hippocampus Is Essential for Nonspatial Object Memory. Curr. Biol. 2013, 23, 1685–1690. [Google Scholar] [CrossRef] [Green Version]
- Jarrard, L.E. On the role of the hippocampus in learning and memory in the rat. Behav. Neural Biol. 1993, 60, 9–26. [Google Scholar] [CrossRef]
- Baptista, P.; Andrade, J.P. Adult Hippocampal Neurogenesis: Regulation and Possible Functional and Clinical Correlates. Front. Neuroanat. 2018, 12, 44. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Belvindrah, R.; Rougon, G.; Chazal, G. Increased Neurogenesis in Adult mCD24-Deficient Mice. J. Neurosci. 2002, 22, 3594–3607. [Google Scholar] [CrossRef]
- Conrad, C.D.; Galea, L.A.M.; Kuroda, Y.; McEwen, B.S. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996, 110, 1321–1334. [Google Scholar] [CrossRef]
- Cohen, S.J.; Stackman, R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015, 285, 105–117. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.-M.; Xu, W.-D.; Tao, T.; Liu, G.-J.; Gao, Y.-Y.; Lu, Y.; Wu, L.-Y.; Yu, Z.; Yuan, B.; et al. Inhibition of Elevated Hippocampal CD24 Reduces Neurogenesis in Mice with Traumatic Brain Injury. J. Surg. Res. 2020, 245, 321–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Salani, F.; Sterbini, V.; Sacchinelli, E.; Garramone, M.; Bossù, P. Is Innate Memory a Double-Edge Sword in Alzheimer’s Disease? A Reappraisal of New Concepts and Old Data. Front. Immunol. 2019, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Revest, J.-M.; Dupret, D.; Koehl, M.; Funk-Reiter, C.; Grosjean, N.; Piazza, P.-V.; Abrous, D.N. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry 2009, 14, 959–967. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Tang, J.; Zheng, P.; Liu, Y. CD24 and Siglec-10 Selectively Repress Tissue Damage-Induced Immune Responses. Science 2009, 323, 1722–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ling, H.-P.; You, W.-C.; Liu, H.-D.; Sun, Q.; Zhou, M.-L.; Shen, W.; Zhao, J.-B.; Zhu, L.; Hang, C.-H. Elevated Cerebral Cortical CD24 Levels in Patients and Mice with Traumatic Brain Injury: A Potential Negative Role in Nuclear Factor Kappa B/Inflammatory Factor Pathway. Mol. Neurobiol. 2014, 49, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Russo, S.J.; Ferguson, D.; Nestler, E.J.; Duman, R.S. Nuclear factor- B is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. 2010, 107, 2669–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Q.-Q.; Huang, H.-J.; Wang, Y.-L.; Yang, L.; Pilot, A.; Zhu, X.-C.; Yu, R.; Wang, J.; Chen, X.-R.; Liu, Q.; et al. Ghrelin exhibited antidepressant and anxiolytic effect via the p38-MAPK signaling pathway in hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 11–20. [Google Scholar] [CrossRef]
- Kwatra, M.; Ahmed, S.; Gawali, B.; Panda, S.R.; Naidu, V. Hesperidin alleviates chronic restraint stress and lipopolysaccharide-induced Hippocampus and Frontal cortex damage in mice: Role of TLR4/NF-κB, p38 MAPK/JNK, Nrf2/ARE signaling. Neurochem. Int. 2020, 140, 104835. [Google Scholar] [CrossRef]
- Dai, H.; Hu, W.; Jiang, L.; Li, L.; Gaung, X.; Xiao, Z. p38 MAPK Inhibition Improves Synaptic Plasticity and Memory in Angiotensin II-dependent Hypertensive Mice. Sci. Rep. 2016, 6, 27600. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Gilhotra, R.; Dhingra, D.; Gilhotra, N. Possible Underlying Influence of p38MAPK and NF-κB in the Diminished Anti-anxiety Effect of Diazepam in Stressed Mice. J. Pharmacol. Sci. 2011, 116, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Gates, P.; Gough, K.; Dhillon, H.; Wilson, C.; Hawkes, E.; Dore, V.; Perchyonok, Y.; Rowe, C.C.; Walker, A.K.; Vardy, J.L.; et al. Longitudinal exploration of cancer-related cognitive impairment in patients with newly diagnosed aggressive lymphoma: Protocol for a feasibility study. BMJ Open 2020, 10, e038312. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [Green Version]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, S.F. Bi-directional immune–brain communication: Implications for understanding stress, pain, and cognition. Brain Behav. Immun. 2003, 17, 69–85. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitzan, K.; Toledano, R.; Shapira, S.; Arber, N.; Doron, R. Behavioral Characterizing of CD24 Knockout Mouse—Cognitive and Emotional Alternations. J. Pers. Med. 2021, 11, 105. https://doi.org/10.3390/jpm11020105
Nitzan K, Toledano R, Shapira S, Arber N, Doron R. Behavioral Characterizing of CD24 Knockout Mouse—Cognitive and Emotional Alternations. Journal of Personalized Medicine. 2021; 11(2):105. https://doi.org/10.3390/jpm11020105
Chicago/Turabian StyleNitzan, Keren, Roni Toledano, Shiran Shapira, Nadir Arber, and Ravid Doron. 2021. "Behavioral Characterizing of CD24 Knockout Mouse—Cognitive and Emotional Alternations" Journal of Personalized Medicine 11, no. 2: 105. https://doi.org/10.3390/jpm11020105