Potential Neurophysiological Mechanisms of 1Hz-TMS to the Right Prefrontal Cortex for Depression: An Exploratory TMS-EEG Study in Healthy Participants
Abstract
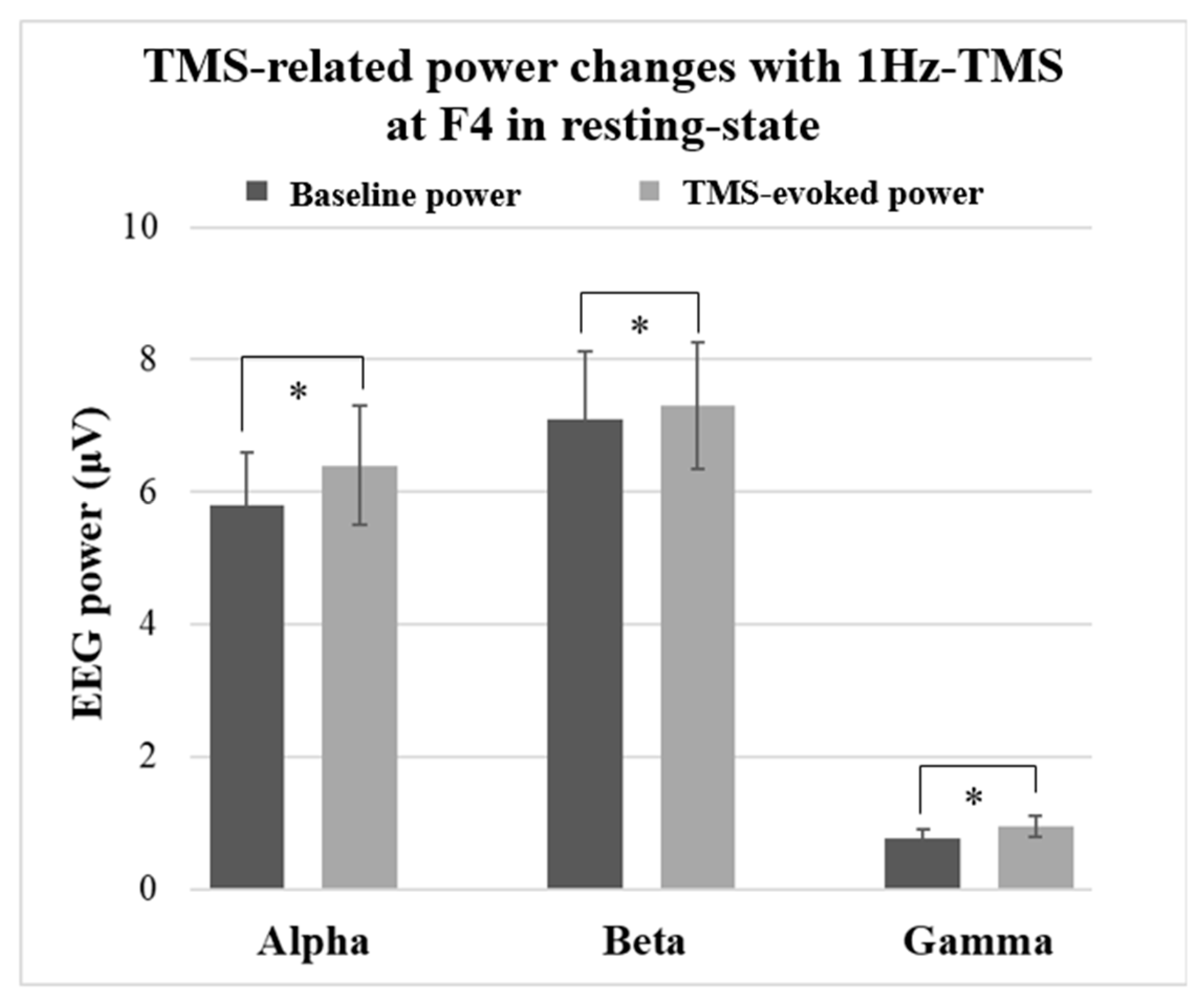
:- Low-frequency 1Hz-TMS to the right DLPFC in healthy participants resulted in a significant increase in spectral power in alpha, beta, and gamma bands.
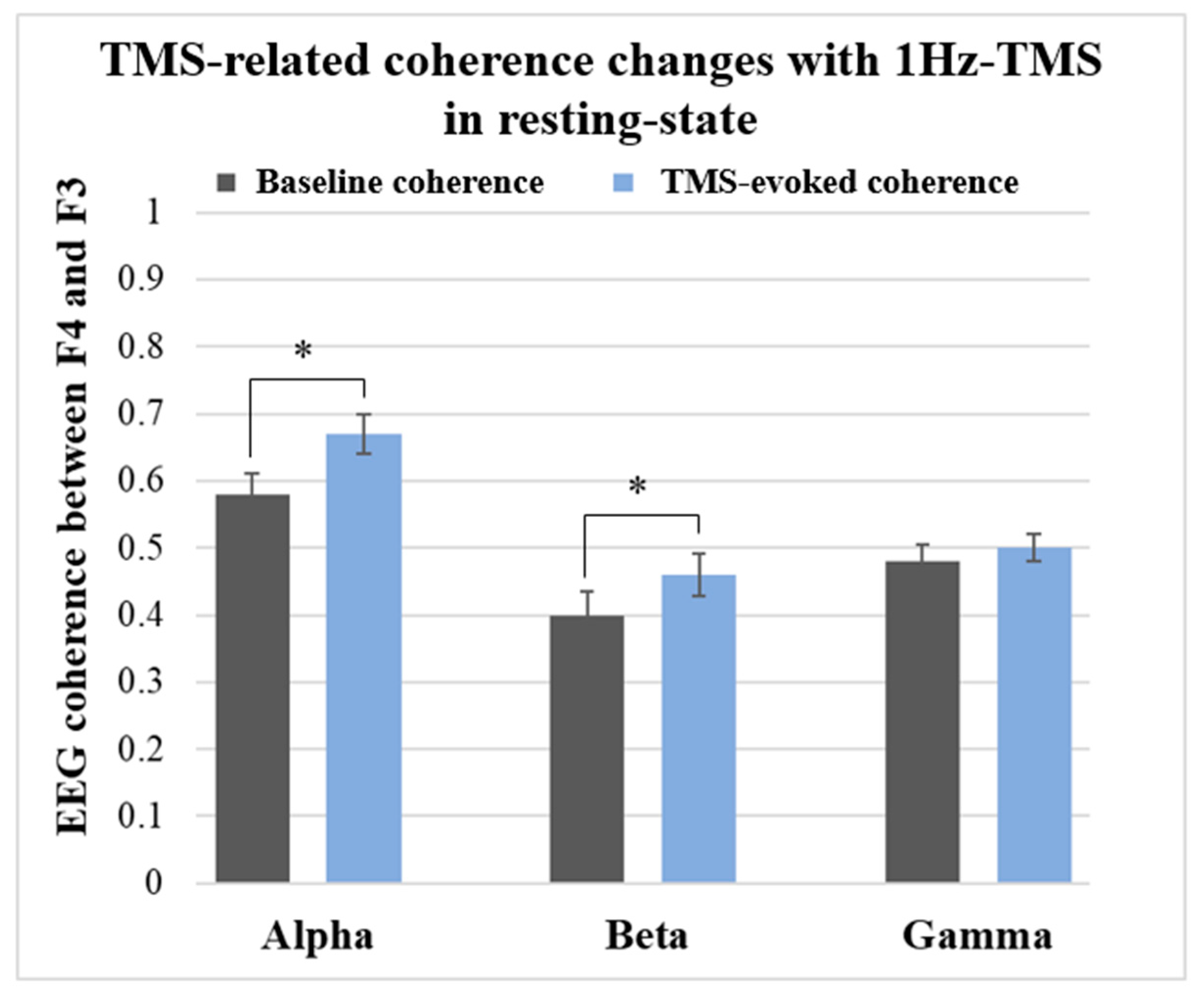
- The same 1Hz-TMS induced a significant increase in alpha and beta coherences between the right and left DLPFC in healthy participants.
- These findings suggest parts of the therapeutic mechanisms of 1Hz-rTMS to the right DLPFC which may exert in patients with depression.
1. Introduction
2. Material and Methods
2.1. Participants
2.2. TMS and EEG System
2.3. TMS Experimental Procedure
2.4. TMS-EEG Experiment during the Verbal Fluency Task (VFT)
2.5. EEG Signal Processing
2.6. ICA Technique to Remove TMS-Related Artifacts
2.7. Data Analysis
2.8. Statistical Analysis
3. Results
3.1. Recruitment Curve of TEPs
3.2. TMS-Related Power Changes at the F4 Electrode Site
3.2.1. Results in Resting-State
3.2.2. Results during VFTs
3.3. TMS-Related Coherence Changes between F4 and F3 Electrode Sites
3.3.1. Results in Resting-State
3.3.2. Results during VFTs
4. Discussion
4.1. Recruitment Curve of TEPs
4.2. TMS-Related Power Changes
4.3. TMS-Related Coherence Changes
4.4. Limitations
4.5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, M.S.; Lisanby, S.H.; Avery, D.; McDonald, W.M.; Durkalski, V.; Pavlicova, M.; Anderson, B.; Nahas, Z.; Bulow, P.; Zarkowski, P.; et al. Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch. Gen. Psychiatry 2010, 67, 507–516. [Google Scholar] [CrossRef] [Green Version]
- McDonald, W.M.; Durkalski, V.; Ball, E.R.; Holtzheimer, P.E.; Pavlicova, M.; Lisanby, S.H.; George, M.S. Improving the antidepressant efficacy of transcranial magnetic stimulation: Maximizing the number of stimulations and treatment location in treatment-resistant depression. Depress. Anxiety 2011, 28, 973–980. [Google Scholar] [CrossRef] [Green Version]
- Pallanti, S.; Di Rollo, A.; Antonini, S.; Cauli, G.; Hollander, E.; Quercioli, L. Low-frequency rTMS over right dorsolateral prefrontal cortex in the treatment of resistant depression: Cognitive improvement is independent from clinical response, resting motor threshold is related to clinical response. Neuropsychobiology 2012, 65, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Li, C.T.; Wang, S.J.; Hirvonen, J.; Hsieh, J.C.; Bai, Y.M.; Hong, C.J.; Su, T.P. Antidepressant mechanism of add-on repetitive transcranial magnetic stimulation in medication-resistant depression using cerebral glucose metabolism. J. Affect. Disord. 2010, 127, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Pell, G.S.; Roth, Y.; Zangen, A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms. Prog. Neurobiol. 2011, 93, 59–98. [Google Scholar] [CrossRef] [PubMed]
- Fuggetta, G.; Noh, N.A. A neurophysiological insight into the potential link between transcranial magnetic stimulation, thalamocortical dysrhythmia and neuropsychiatric disorders. Exp. Neurol. 2013, 245, 87–95. [Google Scholar] [CrossRef]
- Strens, L.H.; Oliviero, A.; Bloem, B.R.; Gerschlager, W.; Rothwell, J.C.; Brown, P. The effects of subthreshold 1Hz repetitive TMS on cortico-cortical and interhemispheric coherence. Clin. Neurophysiol. 2002, 113, 1279–1285. [Google Scholar] [CrossRef]
- Oliviero, A.; Strens, L.H.; Di Lazzaro, V.; Tonali, P.A.; Brown, P. Persistent effects of high frequency repetitive TMS on the coupling between motor areas in the human. Exp. Brain Res. 2003, 149, 107–113. [Google Scholar] [CrossRef]
- Chen, W.H.; Mima, T.; Siebner, H.R.; Oga, T.; Hara, H.; Satow, T.; Begum, T.; Nagamine, T.; Shibasaki, H. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin. Neurophysiol. 2003, 114, 1628–1637. [Google Scholar] [CrossRef]
- Fuggetta, G.; Pavone, E.F.; Fiaschi, A.; Manganotti, P. Acute modulation of cortical oscillatory activities during short trains of high-frequency repetitive transcranial magnetic stimulation of the human motor cortex: A combined EEG and TMS study. Hum. Brain Mapp. 2008, 29, 1–13. [Google Scholar] [CrossRef]
- Azila Noh, N.; Fuggetta, G. Human cortical theta reactivity to high-frequency repetitive transcranial magnetic stimulation. Hum. Brain Mapp. 2012, 33, 2224–2237. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Takigawa, M. Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin. Neurophysiol. 2000, 111, 1620–1631. [Google Scholar] [CrossRef]
- Grimm, S.; Beck, J.; Schuepbach, D.; Hell, D.; Boesiger, P.; Bermpohl, F.; Niehaus, L.; Boeker, H.; Northoff, G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol. Psychiatry 2008, 63, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, S.; Bermpohl, F.; Rigonatti, S.P.; Pascual-Leone, A.; Paulo S Boggio, P.; Fregni, F. Impaired interhemispheric interactions in patients with major depression. J. Nerv. Ment. Dis. 2008, 196, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Hecht, D. Depression and the hyperactive right-hemisphere. Neurosci. Res. 2010, 68, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.; Ruohonen, J.; Näätänen, R.; Ilmoniemi, R.J. Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation. Med. Biol. Eng. Comput. 1999, 37, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Thut, G.; Ives, J.R.; Kampmann, F.; Pastor, M.A.; Pascual-Leone, A. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J. Neurosci. Methods 2005, 141, 207–217. [Google Scholar] [CrossRef]
- Bonato, C.; Miniussi, C.; Rossini, P.M. Transcranial magnetic stimulation and cortical evoked potentials: A TMS/EEG co-registration study. Clin. Neurophysiol. 2006, 117, 1699–1707. [Google Scholar] [CrossRef]
- Komssi, S.; Kahkonen, S. The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Res. Rev. 2006, 52, 183–192. [Google Scholar] [CrossRef]
- Veniero, D.; Bortoletto, M.; Miniussi, C. TMS-EEG co-registration: On TMS-induced artifact. Clin. Neurophysiol. 2009, 120, 1392–1399. [Google Scholar] [CrossRef] [Green Version]
- Ilmoniemi, R.J.; Kicic, D. Methodology for combined TMS and EEG. Brain Topogr. 2010, 22, 233–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaieb, L.; Paulus, W.; Antal, A. Evaluating aftereffects of short-duration transcranial random noise stimulation on cortical excitability. Neural. Plast 2011, 2011, 105927. [Google Scholar] [CrossRef] [PubMed]
- Thut, G.; Pascual-Leone, A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010, 22, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, M.S.; Ketter, T.A.; Parekh, P.I.; Horwitz, B.; Herscovitch, P.; Post, R.M. Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry 1995, 152, 341–351. [Google Scholar] [PubMed]
- Liotti, M.; Mayberg, H.S.; Brannan, S.K.; McGinnis, S.; Jerabek, P.; Fox, P.T. Differential limbic--cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biol. Psychiatry 2000, 48, 30–42. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 224–231. [Google Scholar]
- Otsubo, T.; Tanaka, K.; Koda, R.; Shinoda, J.; Sano, N.; Tanaka, S.; Aoyama, H.; Mimura, M.; Kamijima, K. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin. Neurosci. 2005, 59, 517–526. [Google Scholar] [CrossRef]
- Rossini, P.M.; Barker, A.T.; Berardelli, A.; Caramia, M.D.; Caruso, G.; Cracco, R.Q.; Dimitrijević, M.R.; Hallett, M.; Katayama, Y.; Lücking, C.H.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Noda, Y.; Cash, R.F.H.; Zomorrodi, R.; Garcia Dominguez, L.; Farzan, F.; Rajji, T.K.; Barr, M.S.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. A combined TMS-EEG study of short-latency afferent inhibition in the motor and dorsolateral prefrontal cortex. J. Neurophysiol. 2016, 116, 938–948. [Google Scholar] [CrossRef] [Green Version]
- Cash, R.F.H.; Noda, Y.; Zomorrodi, R.; Radhu, N.; Farzan, F.; Rajji, T.K.; Fitzgerald, P.B.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Characterization of Glutamatergic and GABAA-Mediated Neurotransmission in Motor and Dorsolateral Prefrontal Cortex Using Paired-Pulse TMS-EEG. Neuropsychopharmacology 2017, 42, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Garcia, M.; Atienza, M.; Cantero, J.L. Muscle artifact removal from human sleep EEG by using independent component analysis. Ann. Biomed. Eng. 2008, 36, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Slagter, H.A.; Tononi, G.; Postle, B.R. Brain responses evoked by high-frequency repetitive transcranial magnetic stimulation: An event-related potential study. Brain Stimul. 2010, 3, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Pavon, J.C.; Metsomaa, J.; Mutanen, T.; Stenroos, M.; Mäki, H.; Ilmoniemi, R.J.; Sarvas, J. Uncovering neural independent components from highly artifactual TMS-evoked EEG data. J. Neurosci. Methods 2012, 209, 144–157. [Google Scholar] [CrossRef]
- Iwahashi, M.; Arimatsu, T.; Ueno, S.; Iramina, K. Differences in evoked EEG by transcranial magnetic stimulation at various stimulus points on the head. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 2570–2573. [Google Scholar]
- Ilmoniemi, R.J.; Virtanen, J.; Ruohonen, J.; Karhu, J.; Aronen, H.J.; Näätänen, R.; Katila, T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport 1997, 8, 3537–3540. [Google Scholar] [CrossRef] [Green Version]
- Valiulis, V.; Gerulskis, G.; Dapšys, K.; Vištartaite, G.; Šiurkute, A.; Mačiulis, V. Electrophysiological differences between high and low frequency rTMS protocols in depression treatment. Acta Neurobiol. Exp. 2012, 72, 283–295. [Google Scholar]
- Noda, Y.; Zomorrodi, R.; Saeki, T.; Rajji, T.K.; Blumberger, D.M.; Daskalakis, Z.J.; Nakamura, M. Resting-state EEG gamma power and theta-gamma coupling enhancement following high-frequency left dorsolateral prefrontal rTMS in patients with depression. Clin. Neurophysiol. 2017, 128, 424–432. [Google Scholar] [CrossRef]
- Fink, A.; Neubauer, A.C. EEG alpha oscillations during the performance of verbal creativity tasks: Differential effects of sex and verbal intelligence. Int. J. Psychophysiol. 2006, 62, 46–53. [Google Scholar] [CrossRef]
- Hwang, G.; Jacobs, J.; Geller, A.; Danker, J.; Sekuler, R.; Kahana, M.J. EEG correlates of verbal and nonverbal working memory. Behav. Brain Funct. 2005, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Veniero, D.; Brignani, D.; Thut, G.; Miniussi, C. Alpha-generation as basic response-signature to transcranial magnetic stimulation (TMS) targeting the human resting motor cortex: A TMS/EEG co-registration study. Psychophysiology 2011, 48, 1381–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosanova, M.; Casali, A.; Bellina, V.; Resta, F.; Mariotti, M.; Massimini, M. Natural frequencies of human corticothalamic circuits. J. Neurosci. 2009, 29, 7679–7685. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Haenschel, C.; Nikolić, D.; Singer, W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 2008, 34, 927–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlhaas, P.J.; Pipa, G.; Neuenschwander, S.; Wibral, M.; Singer, W. A new look at gamma? High- (>60Hz) gamma-band activity in cortical networks: Function, mechanisms and impairment. Prog. Biophys. Mol. Biol. 2011, 105, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Singer, W. The development of neural synchrony and large-scale cortical networks during adolescence: Relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr. Bull. 2011, 37, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J.; Watson, B.O. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl. Psychiatry 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Palva, S.; Palva, J.M. New vistas for alpha-frequency band oscillations. Trends. Neurosci. 2007, 30, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Benedek, M. EEG alpha synchronization is related to top-down processing in convergent and divergent thinking. Neuropsychologia 2011, 49, 3505–3511. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y. Electroencephalographic differences between depressed and control subjects: An aspect of interdependence analysis. Brain Res. Bull. 2008, 76, 559–564. [Google Scholar] [CrossRef]
- Wei, L. Brain functional connectivity in depression during emotion processing. J. Biomed. Eng. 2010, 27, 961–967. [Google Scholar]
- Li, Y. Abnormal functional connectivity of EEG gamma band in patients with depression during emotional face processing. Clin. Neurophysiol. 2015, 126, 2078–2089. [Google Scholar] [CrossRef]
- Harris, J.A.; Clifford, C.W.; Miniussi, C. The functional effect of transcranial magnetic stimulation: Signal suppression or neural noise generation? J. Cogn. Neurosci. 2008, 20, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Siebner, H.R. How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex 2009, 45, 1035–1042. [Google Scholar] [PubMed] [Green Version]
- Noda, Y. Toward the establishment of neurophysiological indicators for neuropsychiatric disorders using transcranial magnetic stimulation-evoked potentials: A systematic review. Psychiatry Clin. Neurosci. 2020, 74, 12–34. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noda, Y. Potential Neurophysiological Mechanisms of 1Hz-TMS to the Right Prefrontal Cortex for Depression: An Exploratory TMS-EEG Study in Healthy Participants. J. Pers. Med. 2021, 11, 68. https://doi.org/10.3390/jpm11020068
Noda Y. Potential Neurophysiological Mechanisms of 1Hz-TMS to the Right Prefrontal Cortex for Depression: An Exploratory TMS-EEG Study in Healthy Participants. Journal of Personalized Medicine. 2021; 11(2):68. https://doi.org/10.3390/jpm11020068
Chicago/Turabian StyleNoda, Yoshihiro. 2021. "Potential Neurophysiological Mechanisms of 1Hz-TMS to the Right Prefrontal Cortex for Depression: An Exploratory TMS-EEG Study in Healthy Participants" Journal of Personalized Medicine 11, no. 2: 68. https://doi.org/10.3390/jpm11020068
APA StyleNoda, Y. (2021). Potential Neurophysiological Mechanisms of 1Hz-TMS to the Right Prefrontal Cortex for Depression: An Exploratory TMS-EEG Study in Healthy Participants. Journal of Personalized Medicine, 11(2), 68. https://doi.org/10.3390/jpm11020068





