Current and Future Treatments for Classic Galactosemia
Abstract
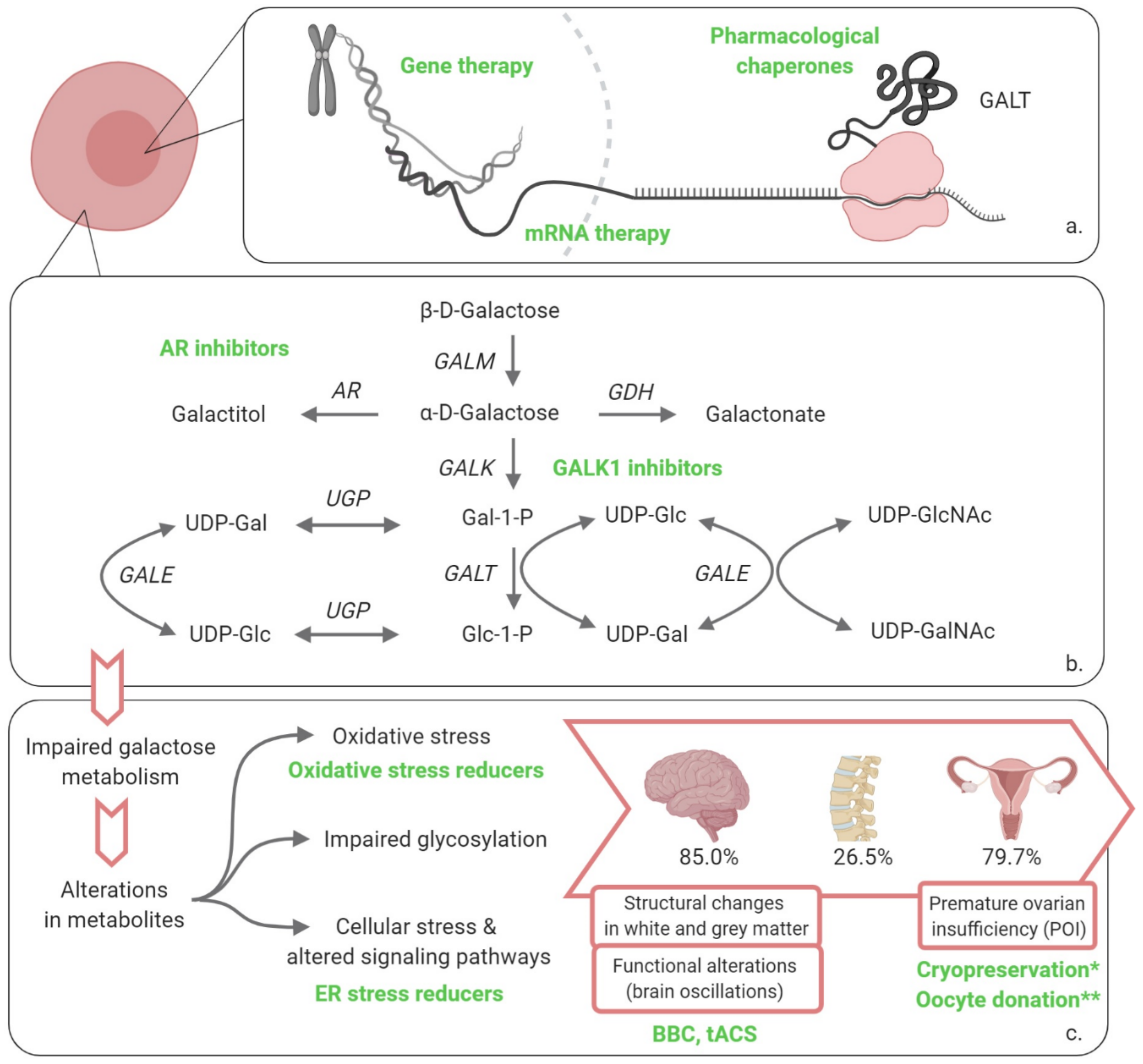
1. Introduction
2. Potential Therapies
2.1. Restoring GALT Activity
2.1.1. Gene Therapy
2.1.2. mRNA Therapy
2.1.3. Pharmacological Chaperones
2.2. Influence the Cascade of Events
2.2.1. Galactokinase 1 (GALK1) Inhibitors
2.2.2. Aldose Reductase (AR) Inhibitors
2.2.3. Endoplasmic Reticulum (ER) Stress Reducers
2.3. Address the Clinical Picture
2.3.1. Neonatal Period
2.3.2. Beyond the Neonatal Period
Fertility Preservation
Neurological and Cognitive Complications
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| AR | Aldose reductase |
| BBB | Blood–brain barrier |
| BBC | Babble Boot Camp |
| Cas13 | CRISPR-associated protein |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DHEA | Dehydroepiandrosterone |
| Dpf | Days post fertilization |
| eIF2α | Eukaryotic initiation factor 2α |
| ER | Endoplasmic reticulum |
| Gal-1-P | Galactose-1-phosphate |
| GALE | UDP-galactose 4-epimerase |
| GALK1 | Galactokinase 1 |
| GALM | Galactose mutarotase |
| GalNet | Galactosemias Network |
| GALT | Galactose 1-phosphate uridylyltransferase |
| GDH | Galactose dehydrogenase |
| GHMP | Galacto-, homoserine-, mevalonate- and phosphomevalonate kinase |
| Glc-1-P | Glucose-1-phosphate |
| hGALT | human GALT |
| HTS | High throughput screening |
| LNP | Lipid nanoparticle |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NBS | Newborn screening |
| NIBS | Non-invasive brain stimulation |
| PI3K/Akt | Phosphoinositide 3-kinase/protein kinase B |
| POI | Primary ovarian insufficiency |
| ROS | Reactive oxygen species |
| tACS | Transcranial alternating current stimulation |
| UGP | UDP-glucose pyrophosphorylase |
| UDP | Uridine diphosphate |
| UDP-Gal | Uridine diphosphate-galactose |
| UDP-Glc | Uridine diphosphate-glucose |
| UDP-GalNAc | UDP-N-acetylgalactosamine |
| UDP-GlcNAc | UDP-N-acetylglucosamine |
| UPR | Unfolded protein response |
References
- Coelho, A.I.; Berry, G.T.; Rubio-Gozalbo, M.E. Galactose metabolism and health. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 422–427. [Google Scholar] [CrossRef]
- Coelho, A.I.; Rubio-Gozalbo, M.E.; Vicente, J.B.; Rivera, I. Sweet and sour: An update on classic galactosemia. J. Inherit. Metab. Dis. 2017, 40, 325–342. [Google Scholar] [CrossRef]
- Demirbas, D.; Coelho, A.I.; Rubio-Gozalbo, M.E.; Berry, G.T. Hereditary galactosemia. Metab. Clin. Exp. 2018, 83, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Haskovic, M.; Coelho, A.I.; Bierau, J.; Vanoevelen, J.M.; Steinbusch, L.K.M.; Zimmermann, L.J.I.; Villamor-Martinez, E.; Berry, G.T.; Rubio-Gozalbo, M.E. Pathophysiology and targets for treatment in hereditary galactosemia: A systematic review of animal and cellular models. J. Inherit. Metab. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- McCorvie, T.J.; Kopec, J.; Pey, A.L.; Fitzpatrick, F.; Patel, D.; Chalk, R.; Shrestha, L.; Yue, W.W. Molecular basis of classic galactosemia from the structure of human galactose 1-phosphate uridylyltransferase. Hum. Mol. Genet. 2016, 25, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gozalbo, M.E.; Haskovic, M.; Bosch, A.M.; Burnyte, B.; Coelho, A.I.; Cassiman, D.; Couce, M.L.; Dawson, C.; Demirbas, D.; Derks, T.; et al. The natural history of classic galactosemia: Lessons from the GalNet registry. Orphanet J. Rare Dis. 2019, 14, 86. [Google Scholar] [CrossRef]
- Timson, D.J. The molecular basis of galactosemia—Past, present and future. Gene 2016, 589, 133–141. [Google Scholar] [CrossRef]
- Wada, Y.; Kikuchi, A.; Arai-Ichinoi, N.; Sakamoto, O.; Takezawa, Y.; Iwasawa, S.; Niihori, T.; Nyuzuki, H.; Nakajima, Y.; Ogawa, E.; et al. Biallelic GALM pathogenic variants cause a novel type of galactosemia. Genet. Med. Off. J. Am. Coll. Med. Genet. 2019, 21, 1286–1294. [Google Scholar] [CrossRef]
- Antshel, K.M.; Epstein, I.O.; Waisbren, S.E. Cognitive strengths and weaknesses in children and adolescents homozygous for the galactosemia Q188R mutation: A descriptive study. Neuropsychology 2004, 18, 658–664. [Google Scholar] [CrossRef]
- Bosch, A.M.; Grootenhuis, M.A.; Bakker, H.D.; Heijmans, H.S.; Wijburg, F.A.; Last, B.F. Living with classical galactosemia: Health-related quality of life consequences. Pediatrics 2004, 113, e423–e428. [Google Scholar] [CrossRef]
- Lambert, C.; Boneh, A. The impact of galactosaemia on quality of life—A pilot study. J. Inherit. Metab. Dis. 2004, 27, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Odejinmi, S.I.; Vankayalapati, H.; Wierenga, K.J.; Lai, K. Innovative therapy for Classic Galactosemia—Tale of two HTS. Mol. Genet. Metab. 2012, 105, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Waisbren, S.E.; Albers, S.; Amato, S.; Ampola, M.; Brewster, T.G.; Demmer, L.; Eaton, R.B.; Greenstein, R.; Korson, M.; Larson, C.; et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA 2003, 290, 2564–2572. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Rones, M.; Read, C.Y.; Marsden, D.; Levy, H.L. Brief report: Predictors of parenting stress among parents of children with biochemical genetic disorders. J. Pediatr. Psychol. 2004, 29, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gozalbo, M.E.; Bosch, A.M.; Burlina, A.; Berry, G.T.; Treacy, E.P. The galactosemia network (GalNet). J. Inherit. Metab. Dis. 2017, 40, 169–170. [Google Scholar] [CrossRef]
- Pasquali, M.; Yu, C.; Coffee, B. Laboratory diagnosis of galactosemia: A technical standard and guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. Off. J. Am. Coll. Med. Genet. 2018, 20, 3–11. [Google Scholar] [CrossRef]
- Calderon, F.R.; Phansalkar, A.R.; Crockett, D.K.; Miller, M.; Mao, R. Mutation database for the galactose-1-phosphate uridyltransferase (GALT) gene. Hum. Mutat. 2007, 28, 939–943. [Google Scholar] [CrossRef]
- Coelho, A.I.; Trabuco, M.; Ramos, R.; Silva, M.J.; Tavares de Almeida, I.; Leandro, P.; Rivera, I.; Vicente, J.B. Functional and structural impact of the most prevalent missense mutations in classic galactosemia. Mol. Genet. Genom. Med. 2014, 2, 484–496. [Google Scholar] [CrossRef]
- Coelho, A.I.; Bierau, J.; Lindhout, M.; Achten, J.; Kramer, B.W.; Rubio-Gozalbo, M.E. Classic Galactosemia: Study on the Late Prenatal Development of GALT Specific Activity in a Sheep Model. Anat. Rec. (Hoboken) 2017, 300, 1570–1575. [Google Scholar] [CrossRef]
- Daenzer, J.M.; Jumbo-Lucioni, P.P.; Hopson, M.L.; Garza, K.R.; Ryan, E.L.; Fridovich-Keil, J.L. Acute and long-term outcomes in a Drosophila melanogaster model of classic galactosemia occur independently of galactose-1-phosphate accumulation. Dis. Model. Mech. 2016, 9, 1375–1382. [Google Scholar] [CrossRef]
- McCorvie, T.J.; Timson, D.J. Chapter 11—Galactosemia: Opportunities for novel therapies. In Protein Homeostasis Diseases; Pey, A.L., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 221–245. [Google Scholar] [CrossRef]
- Wehrli, S.L.; Berry, G.T.; Palmieri, M.; Mazur, A.; Elsas, L., III; Segal, S. Urinary galactonate in patients with galactosemia: Quantitation by nuclear magnetic resonance spectroscopy. Pediatr. Res. 1997, 42, 855–861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Machado, C.M.; De-Souza, E.A.; De-Queiroz, A.; Pimentel, F.S.A.; Silva, G.F.S.; Gomes, F.M.; Montero-Lomeli, M.; Masuda, C.A. The galactose-induced decrease in phosphate levels leads to toxicity in yeast models of galactosemia. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Kushner, R.F.; Ryan, E.L.; Sefton, J.M.; Sanders, R.D.; Lucioni, P.J.; Moberg, K.H.; Fridovich-Keil, J.L. A Drosophila melanogaster model of classic galactosemia. Dis. Model. Mech. 2010, 3, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Langley, S.D.; Khwaja, F.W.; Schmitt, E.W.; Elsas, L.J. GALT deficiency causes UDP-hexose deficit in human galactosemic cells. Glycobiology 2003, 13, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Reynolds, R.; Chen, J.; Yager, C.; Berry, G.T.; Leslie, N.; Segal, S. Galactose metabolism in mice with galactose-1-phosphate uridyltransferase deficiency: Sucklings and 7-week-old animals fed a high-galactose diet. Mol. Genet. Metab. 2001, 72, 306–315. [Google Scholar] [CrossRef]
- Ross, K.L.; Davis, C.N.; Fridovich-Keil, J.L. Differential roles of the Leloir pathway enzymes and metabolites in defining galactose sensitivity in yeast. Mol. Genet. Metab. 2004, 83, 103–116. [Google Scholar] [CrossRef]
- Ryan, E.L.; DuBoff, B.; Feany, M.B.; Fridovich-Keil, J.L. Mediators of a long-term movement abnormality in a Drosophila melanogaster model of classic galactosemia. Dis. Model. Mech. 2012, 5, 796–803. [Google Scholar] [CrossRef]
- Tang, M.; Siddiqi, A.; Witt, B.; Yuzyuk, T.; Johnson, B.; Fraser, N.; Chen, W.; Rascon, R.; Yin, X.; Goli, H.; et al. Subfertility and growth restriction in a new galactose-1 phosphate uridylyltransferase (GALT)—Deficient mouse model. Eur. J. Hum. Genet. 2014, 22, 1172–1179. [Google Scholar] [CrossRef]
- Yager, C.; Ning, C.; Reynolds, R.; Leslie, N.; Segal, S. Galactitol and galactonate accumulation in heart and skeletal muscle of mice with deficiency of galactose-1-phosphate uridyltransferase. Mol. Genet. Metab. 2004, 81, 105–111. [Google Scholar] [CrossRef]
- Haskovic, M.; Coelho, A.I.; Lindhout, M.; Zijlstra, F.; Veizaj, R.; Vos, R.; Vanoevelen, J.M.; Bierau, J.; Lefeber, D.J.; Rubio-Gozalbo, M.E. Nucleotide sugar profiles throughout development in wildtype and galt knockout zebrafish. J. Inherit. Metab. Dis. 2020, 43, 994–1001. [Google Scholar] [CrossRef]
- Coss, K.P.; Treacy, E.P.; Cotter, E.J.; Knerr, I.; Murray, D.W.; Shin, Y.S.; Doran, P.P. Systemic gene dysregulation in classical Galactosaemia: Is there a central mechanism? Mol. Genet. Metab. 2014, 113, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Dobbie, J.A.; Holton, J.B.; Clamp, J.R. Defective galactosylation of proteins in cultured skin fibroblasts from galactosaemic patients. Ann. Clin. Biochem. 1990, 27 Pt 3, 274–275. [Google Scholar] [CrossRef]
- Ornstein, K.S.; McGuire, E.J.; Berry, G.T.; Roth, S.; Segal, S. Abnormal galactosylation of complex carbohydrates in cultured fibroblasts from patients with galactose-1-phosphate uridyltransferase deficiency. Pediatr. Res. 1992, 31, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Petry, K.; Greinix, H.T.; Nudelman, E.; Eisen, H.; Hakomori, S.; Levy, H.L.; Reichardt, J.K. Characterization of a novel biochemical abnormality in galactosemia: Deficiency of glycolipids containing galactose or N-acetylgalactosamine and accumulation of precursors in brain and lymphocytes. Biochem. Med. Metab. Biol. 1991, 46, 93–104. [Google Scholar] [CrossRef]
- Staubach, S.; Müller, S.; Pekmez, M.; Hanisch, F.G. Classical Galactosemia: Insight into Molecular Pathomechanisms by Differential Membrane Proteomics of Fibroblasts under Galactose Stress. J. Proteome Res. 2017, 16, 516–527. [Google Scholar] [CrossRef]
- Van Erven, B. Classic galactosemia: A zebrafish model and new clinical insights. Ph.D Thesis, Maastricht University, Maastricht, The Netherlands, March 2017. [Google Scholar]
- Balakrishnan, B.; Chen, W.; Tang, M.; Huang, X.; Cakici, D.D.; Siddiqi, A.; Berry, G.; Lai, K. Galactose-1 phosphate uridylyltransferase (GalT) gene: A novel positive regulator of the PI3K/Akt signaling pathway in mouse fibroblasts. Biochem. Biophys. Res. Commun. 2016, 470, 205–212. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Nicholas, C.; Siddiqi, A.; Chen, W.; Bales, E.; Feng, M.; Johnson, J.; Lai, K. Reversal of aberrant PI3K/Akt signaling by Salubrinal in a GalT-deficient mouse model. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3286–3293. [Google Scholar] [CrossRef]
- De-Souza, E.A.; Pimentel, F.S.A.; Machado, C.M.; Martins, L.S.; da-Silva, W.S.; Montero-Lomelí, M.; Masuda, C.A. The unfolded protein response has a protective role in yeast models of classic galactosemia. Dis. Models Mech. 2014, 7, 55. [Google Scholar] [CrossRef]
- Slepak, T.; Tang, M.; Addo, F.; Lai, K. Intracellular galactose-1-phosphate accumulation leads to environmental stress response in yeast model. Mol. Genet. Metab. 2005, 86, 360–371. [Google Scholar] [CrossRef]
- Slepak, T.I.; Tang, M.; Slepak, V.Z.; Lai, K. Involvement of endoplasmic reticulum stress in a novel Classic Galactosemia model. Mol. Genet. Metab. 2007, 92, 78–87. [Google Scholar] [CrossRef]
- Jumbo-Lucioni, P.P.; Hopson, M.L.; Hang, D.; Liang, Y.; Jones, D.P.; Fridovich-Keil, J.L. Oxidative stress contributes to outcome severity in a Drosophila melanogaster model of classic galactosemia. Dis. Model. Mech. 2013, 6, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Jumbo-Lucioni, P.P.; Ryan, E.L.; Hopson, M.L.; Bishop, H.M.; Weitner, T.; Tovmasyan, A.; Spasojevic, I.; Batinic-Haberle, I.; Liang, Y.; Jones, D.P.; et al. Manganese-based superoxide dismutase mimics modify both acute and long-term outcome severity in a Drosophila melanogaster model of classic galactosemia. Antioxid. Redox Signal. 2014, 20, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Daenzer, J.M.I.; MacWilliams, J.A.; Head, S.T.; Williams, M.B.; Geurts, A.M.; Schroeder, J.P.; Weinshenker, D.; Fridovich-Keil, J.L. A galactose-1-phosphate uridylyltransferase-null rat model of classic galactosemia mimics relevant patient outcomes and reveals tissue-specific and longitudinal differences in galactose metabolism. J. Inherit. Metab. Dis. 2020, 43, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Vanoevelen, J.M.; van Erven, B.; Bierau, J.; Huang, X.; Berry, G.T.; Vos, R.; Coelho, A.I.; Rubio-Gozalbo, M.E. Impaired fertility and motor function in a zebrafish model for classic galactosemia. J. Inherit. Metab. Dis. 2018, 41, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Berry, G.T. Classic Galactosemia and Clinical Variant Galactosemia; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; GeneReviews®: Seattle, WA, USA, 1993. [Google Scholar]
- Walter, J.; Fridovich-Keil, J. Galactosemia. In The Online Metabolic and Molecular Bases of Inherited Disease (OMMBID); Valle, D., Beaudet, A.L., Vogelstein, B., Kinzler, K.W., Antonarakis, S.E., Ballabio, A., Gibson, K., Mitchell, G., Eds.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Cring, M.R.; Sheffield, V.C. Gene therapy and gene correction: Targets, progress, and challenges for treating human diseases. Gene 2020. [Google Scholar] [CrossRef] [PubMed]
- Rutten, M.G.S.; Rots, M.G.; Oosterveer, M.H. Exploiting epigenetics for the treatment of inborn errors of metabolism. J. Inherit. Metab. Dis. 2020, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Schneller, J.L.; Lee, C.M.; Bao, G.; Venditti, C.P. Genome editing for inborn errors of metabolism: Advancing towards the clinic. BMC Med. 2017, 15, 43. [Google Scholar] [CrossRef]
- Chandler, R.J.; Venditti, C.P. Gene Therapy for Metabolic Diseases. Transl Sci. Rare Dis. 2016, 1, 73–89. [Google Scholar] [CrossRef]
- Yilmaz, B.S.; Gurung, S.; Perocheau, D.; Counsell, J.; Baruteau, J. Gene Therapy for Inherited Metabolic Diseases. J. Mother Child. 2020. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Daenzer, J.M.I.; Fridovich-Keil, J.L. A pilot study of neonatal GALT gene replacement using AAV9 dramatically lowers galactose metabolites in blood, liver, and brain and minimizes cataracts in GALT-null rat pups. J. Inherit. Metab. Dis. 2020. [Google Scholar] [CrossRef]
- Loiler, S.A. Gene therapy for the treatment of galactosemia. U.S. Patent WO2020047472A1, 30 August 2019. [Google Scholar]
- Brophy, M.L.; Chen, T.-W.; Le, K.; Tabet, R.; Ahn, Y.; Murphy, J.E.; Bell, R.D. AAV-Mediated Gene Therapy Rescues GALT Activity and Reduces ER Stress in Classic Galactosemia. Mol. Ther. 2020, 28, 303. [Google Scholar]
- Pan, X.; Sands, S.A.; Yue, Y.; Zhang, K.; LeVine, S.M.; Duan, D. An Engineered Galactosylceramidase Construct Improves AAV Gene Therapy for Krabbe Disease in Twitcher Mice. Hum. Gene 2019, 30, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Rabinowitz, J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008, 16, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Molecular 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Martini, P.G.V.; Guey, L.T. A New Era for Rare Genetic Diseases: Messenger RNA Therapy. Hum. Gene 2019, 30, 1180–1189. [Google Scholar] [CrossRef]
- Timson, D.J. Therapies for galactosemia: A patent landscape. Pharm. Pat. Anal. 2020, 9, 45–51. [Google Scholar] [CrossRef]
- Zifu, Z.; Mc Cafferty, S.; Combes, F.; Huysmans, H.; De Temmerman, J.; Gitsels, A.; Vanrompay, D.; Portela Catani, J.; Sanders, N. mRNA therapeutics deliver a hopeful message. Nano Today 2018. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Molecular 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- DeRosa, F.; Guild, B.; Karve, S.; Smith, L.; Love, K.; Dorkin, J.R.; Kauffman, K.J.; Zhang, J.; Yahalom, B.; Anderson, D.G.; et al. Therapeutic efficacy in a hemophilia B model using a biosynthetic mRNA liver depot system. Gene Ther. 2016, 23, 699. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid 2018, 28, 146–157. [Google Scholar] [CrossRef]
- Novakowski, S.; Jiang, K.; Prakash, G.; Kastrup, C. Delivery of mRNA to platelets using lipid nanoparticles. Sci. Rep. 2019, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hou, X.; Vick, O.G.; Dong, Y. RNA delivery biomaterials for the treatment of genetic and rare diseases. Biomaterials 2019, 217, 119291. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; An, D.; Nguyen, V.; DeAntonis, C.; Martini, P.G.V.; Lai, K. Novel mRNA-Based Therapy Reduces Toxic Galactose Metabolites and Overcomes Galactose Sensitivity in a Mouse Model of Classic Galactosemia. Molecular 2020, 28, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Truong, B.; Allegri, G.; Liu, X.B.; Burke, K.E.; Zhu, X.; Cederbaum, S.D.; Haberle, J.; Martini, P.G.V.; Lipshutz, G.S. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc. Natl. Acad. Sci. USA 2019, 116, 21150–21159. [Google Scholar] [CrossRef]
- Berraondo, P.; Martini, P.G.V.; Avila, M.A.; Fontanellas, A. Messenger RNA therapy for rare genetic metabolic diseases. Gut 2019, 68, 1323–1330. [Google Scholar] [CrossRef]
- An, D.; Frassetto, A.; Jacquinet, E.; Eybye, M.; Milano, J.; DeAntonis, C.; Nguyen, V.; Laureano, R.; Milton, J.; Sabnis, S.; et al. Long-term efficacy and safety of mRNA therapy in two murine models of methylmalonic acidemia. EBioMedicine 2019, 45, 519–528. [Google Scholar] [CrossRef]
- An, D.; Schneller, J.L.; Frassetto, A.; Liang, S.; Zhu, X.; Park, J.S.; Theisen, M.; Hong, S.J.; Zhou, J.; Rajendran, R.; et al. Systemic Messenger RNA Therapy as a Treatment for Methylmalonic Acidemia. Cell Rep. 2018, 24, 2520. [Google Scholar] [CrossRef]
- Jiang, L.; Berraondo, P.; Jerico, D.; Guey, L.T.; Sampedro, A.; Frassetto, A.; Benenato, K.E.; Burke, K.; Santamaria, E.; Alegre, M.; et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 2018, 24, 1899–1909. [Google Scholar] [CrossRef]
- Prieve, M.G.; Harvie, P.; Monahan, S.D.; Roy, D.; Li, A.G.; Blevins, T.L.; Paschal, A.E.; Waldheim, M.; Bell, E.C.; Galperin, A.; et al. Targeted mRNA Therapy for Ornithine Transcarbamylase Deficiency. Molecular 2018, 26, 801–813. [Google Scholar] [CrossRef]
- Roseman, D.S.; Khan, T.; Rajas, F.; Jun, L.S.; Asrani, K.H.; Isaacs, C.; Farelli, J.D.; Subramanian, R.R. G6PC mRNA Therapy Positively Regulates Fasting Blood Glucose and Decreases Liver Abnormalities in a Mouse Model of Glycogen Storage Disease 1a. Molecular 2018, 26, 814–821. [Google Scholar] [CrossRef]
- Haskovic, M.; Delnoy, B.; Bierau, J.; Lindhout, M.; Zimmermann, L.J.; Vanoevelen, J.M.; Coelho, A.I.; Rubio-Gozalbo, M.E. The promise of mRNA therapy as a treatment for classic galactosemia in Classic galactosemia: Natural histroy and new treatment approaches. Ph.D Thesis, Maastricht University, Maastricht, The Netherlands, October 2020; p. 17. [Google Scholar]
- Fan, J.Q. A counterintuitive approach to treat enzyme deficiencies: Use of enzyme inhibitors for restoring mutant enzyme activity. Biol. Chem. 2008, 389, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muntau, A.C.; Leandro, J.; Staudigl, M.; Mayer, F.; Gersting, S.W. Innovative strategies to treat protein misfolding in inborn errors of metabolism: Pharmacological chaperones and proteostasis regulators. J. Inherit. Metab. Dis. 2014, 37, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Aguirre, A.; Janovick, J.A.; Brothers, S.P.; Conn, P.M. Pharmacologic rescue of conformationally-defective proteins: Implications for the treatment of human disease. Traffic 2004, 5, 821–837. [Google Scholar] [CrossRef] [PubMed]
- McCorvie, T.J.; Gleason, T.J.; Fridovich-Keil, J.L.; Timson, D.J. Misfolding of galactose 1-phosphate uridylyltransferase can result in type I galactosemia. Biochim. Biophys. Acta 2013, 1832, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Facchiano, A.; Rachamadugu, R.; Calderon, F.; Mao, R.; Milanesi, L.; Marabotti, A.; Lai, K. Correlation assessment among clinical phenotypes, expression analysis and molecular modeling of 14 novel variations in the human galactose-1-phosphate uridylyltransferase gene. Hum. Mutat. 2012, 33, 1107–1115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, S.; Sarkar, S.; Paul, S.S.; Roy, S.; Chattopadhyay, K. A small molecule chemical chaperone optimizes its unfolded state contraction and denaturant like properties. Sci. Rep. 2013, 3, 3525. [Google Scholar] [CrossRef]
- Coelho, A.I.; Trabuco, M.; Silva, M.J.; de Almeida, I.T.; Leandro, P.; Rivera, I.; Vicente, J.B. Arginine Functionally Improves Clinically Relevant Human Galactose-1-Phosphate Uridylyltransferase (GALT) Variants Expressed in a Prokaryotic Model. JIMD Rep. 2015, 23, 1–6. [Google Scholar] [CrossRef]
- Haskovic, M.; Derks, B.; van der Ploeg, L.; Trommelen, J.; Nyakayiru, J.; van Loon, L.J.C.; Mackinnon, S.; Yue, W.W.; Peake, R.W.A.; Zha, L.; et al. Arginine does not rescue p.Q188R mutation deleterious effect in classic galactosemia. Orphanet J. Rare Dis. 2018, 13, 212. [Google Scholar] [CrossRef]
- Timson, D.J. Purple sweet potato colour—A potential therapy for galactosemia? Int. J. Food Sci. Nutr. 2014, 65, 391–393. [Google Scholar] [CrossRef]
- Bosch, A.M.; Bakker, H.D.; van Gennip, A.H.; van Kempen, J.V.; Wanders, R.J.; Wijburg, F.A. Clinical features of galactokinase deficiency: A review of the literature. J. Inherit. Metab. Dis. 2002, 25, 629–634. [Google Scholar] [CrossRef]
- Hennermann, J.B.; Schadewaldt, P.; Vetter, B.; Shin, Y.S.; Monch, E.; Klein, J. Features and outcome of galactokinase deficiency in children diagnosed by newborn screening. J. Inherit. Metab. Dis. 2011, 34, 399–407. [Google Scholar] [CrossRef]
- Rubio-Gozalbo, M.E.; Derks, B.; Das, A.M.; Meyer, U.; Moslinger, D.; Couce, M.L.; Empain, A.; Ficicioglu, C.; Julia Palacios, N.; De Los Santos De Pelegrin, M.M.; et al. Galactokinase deficiency: Lessons from the GalNet registry. Genet. Med. Off. J. Am. Coll. Med. Genet. 2020. [Google Scholar] [CrossRef]
- Odejinmi, S.; Rascon, R.; Tang, M.; Vankayalapati, H.; Lai, K. Structure-activity analysis and cell-based optimization of human galactokinase inhibitors. ACS Med. Chem. Lett. 2011, 2, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wierenga, K.; Elsas, L.J.; Lai, K. Molecular and biochemical characterization of human galactokinase and its small molecule inhibitors. Chem. Biol. Interact. 2010, 188, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.Q.; Lee, O.W.; Liu, L.; Tang, M.; Lai, K.; Boxer, M.B.; Hall, M.D.; Shen, M. Discovery of novel inhibitors of human galactokinase by virtual screening. J. Comput Aided Mol. Des. 2019, 33, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, M.; Walsh, M.J.; Brimacombe, K.R.; Pragani, R.; Tanega, C.; Rohde, J.M.; Baker, H.L.; Fernandez, E.; Blackman, B.; et al. Structure activity relationships of human galactokinase inhibitors. Bioorg Med. Chem. Lett. 2015, 25, 721–727. [Google Scholar] [CrossRef]
- McAuley, M.; Mesa-Torres, N.; McFall, A.; Morris, S.; Huang, M.; Pey, A.L.; Timson, D.J. Improving the Activity and Stability of Human Galactokinase for Therapeutic and Biotechnological Applications. ChemBioChem 2018, 19, 1088–1095. [Google Scholar] [CrossRef]
- Wierenga, K.J.; Lai, K.; Buchwald, P.; Tang, M. High-throughput screening for human galactokinase inhibitors. J. Biomol. Screen 2008, 13, 415–423. [Google Scholar] [CrossRef]
- Lai, K.; Boxer, M.B.; Marabotti, A. GALK inhibitors for classic galactosemia. Future Med. Chem. 2014, 6, 1003–1015. [Google Scholar] [CrossRef]
- De Jongh, W.A.; Bro, C.; Ostergaard, S.; Regenberg, B.; Olsson, L.; Nielsen, J. The roles of galactitol, galactose-1-phosphate, and phosphoglucomutase in galactose-induced toxicity in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2008, 101, 317–326. [Google Scholar] [CrossRef]
- Lai, K.; Elsas, L.J.; Wierenga, K.J. Galactose toxicity in animals. IUBMB Life 2009, 61, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Pintor, J. Sugars, the crystalline lens and the development of cataracts. Biochem. Pharm. 2012, 1, 1–3. [Google Scholar] [CrossRef]
- Ai, Y.; Zheng, Z.; O’Brien-Jenkins, A.; Bernard, D.J.; Wynshaw-Boris, T.; Ning, C.; Reynolds, R.; Segal, S.; Huang, K.; Stambolian, D. A mouse model of galactose-induced cataracts. Hum. Mol. Genet. 2000, 9, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, M.; Basso, M.; Cherian, P.V.; Hohman, T.C.; Sima, A.A. Galactosemia produces ARI-preventable nodal changes similar to those of diabetic neuropathy. Diabetes Res. Clin. Pr. 1994, 25, 117–129. [Google Scholar] [CrossRef][Green Version]
- Berry, G.T.; Hunter, J.V.; Wang, Z.; Dreha, S.; Mazur, A.; Brooks, D.G.; Ning, C.; Zimmerman, R.A.; Segal, S. In vivo evidence of brain galactitol accumulation in an infant with galactosemia and encephalopathy. J. Pediatr. 2001, 138, 260–262. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Hillman, R.E.; Hsia, Y.E. Pseudotumor cerebri in galactosemia. J. Pediatr. 1970, 76, 902–905. [Google Scholar] [CrossRef]
- Lou, M.F.; Dickerson, J.E., Jr.; Chandler, M.L.; Brazzell, R.K.; York, B.M., Jr. The prevention of biochemical changes in lens, retina, and nerve of galactosemic dogs by the aldose reductase inhibitor AL01576. J. Ocul. Pharm. 1989, 5, 233–240. [Google Scholar] [CrossRef]
- Obrosova, I.; Faller, A.; Burgan, J.; Ostrow, E.; Williamson, J.R. Glycolytic pathway, redox state of NAD(P)-couples and energy metabolism in lens in galactose-fed rats: Effect of an aldose reductase inhibitor. Curr. Eye Res. 1997, 16, 34–43. [Google Scholar] [CrossRef]
- Mulhern, M.L.; Madson, C.J.; Danford, A.; Ikesugi, K.; Kador, P.F.; Shinohara, T. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3951–3959. [Google Scholar] [CrossRef]
- Mizisin, A.P.; Myers, R.R.; Powell, H.C. Endoneurial sodium accumulation in galactosemic rat nerves. Muscle Nerve 1986, 9, 440–444. [Google Scholar] [CrossRef]
- Mizisin, A.P.; Powell, H.C. Schwann cell injury is attenuated by aldose reductase inhibition in galactose intoxication. J. Neuropathol. Exp. Neurol. 1993, 52, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Mizisin, A.P.; Powell, H.C.; Myers, R.R. Edema and increased endoneurial sodium in galactose neuropathy. Reversal with an aldose reductase inhibitor. J. Neurol. Sci. 1986, 74, 35–43. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT04117711, Safety and Pharmacokinetics of AT-007 in Healthy Subjects and in Adult Subjects With Classic Galactosemia; 2019 Oct 07. Available online: https://clinicaltrials.gov/ct2/show/NCT04117711 (accessed on 27 January 2021).
- Balakrishnan, B.; Siddiqi, A.; Mella, J.; Lupo, A.; Li, E.; Hollien, J.; Johnson, J.; Lai, K. Salubrinal enhances eIF2α phosphorylation and improves fertility in a mouse model of Classic Galactosemia. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165516. [Google Scholar] [CrossRef] [PubMed]
- Welling, L.; Bernstein, L.E.; Berry, G.T.; Burlina, A.B.; Eyskens, F.; Gautschi, M.; Grunewald, S.; Gubbels, C.S.; Knerr, I.; Labrune, P.; et al. International clinical guideline for the management of classical galactosemia: Diagnosis, treatment, and follow-up. J. Inherit. Metab. Dis. 2017, 40, 171–176. [Google Scholar] [CrossRef]
- Pollitt, R.J.; Green, A.; McCabe, C.J.; Booth, A.; Cooper, N.J.; Leonard, J.V.; Nicholl, J.; Nicholson, P.; Tunaley, J.R.; Virdi, N.K. Neonatal screening for inborn errors of metabolism: Cost, yield and outcome. Health Technol. Assess. 1997, 1, i–iv. [Google Scholar]
- Schweitzer-Krantz, S. Early diagnosis of inherited metabolic disorders towards improving outcome: The controversial issue of galactosaemia. Eur. J. Pediatr. 2003, 162 (Suppl. S1), S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Friedman, S.; Moore, A.M.; Platt, B.A.; Feigenbaum, A.S. Selective screening for neonatal galactosemia: An alternative approach. Acta Paediatr. 2001, 90, 948–949. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Read, C.Y.; Ampola, M.; Brewster, T.G.; Demmer, L.; Greenstein, R.; Ingham, C.L.; Korson, M.; Msall, M.; Pueschel, S.; et al. Newborn screening compared to clinical identification of biochemical genetic disorders. J. Inherit. Metab. Dis. 2002, 25, 599–600. [Google Scholar] [CrossRef]
- Walter, J.H. Arguments for early screening: A clinician’s perspective. Eur. J. Pediatr. 2003, 162 (Suppl. S1), S2–S4. [Google Scholar] [CrossRef]
- Abidin, Z.; Treacy, E.P. Insights into the Pathophysiology of Infertility in Females with Classical Galactosaemia. Int. J. Mol. Sci. 2019, 20, 5236. [Google Scholar] [CrossRef]
- Gubbels, C.S.; Land, J.A.; Rubio-Gozalbo, M.E. Fertility and impact of pregnancies on the mother and child in classic galactosemia. Obs. Gynecol Surv. 2008, 63, 334–343. [Google Scholar] [CrossRef]
- Kaufman, F.; Kogut, M.D.; Donnell, G.N.; Koch, R.; Goebelsmann, U. Ovarian failure in galactosæmia. Lancet 1979, 314, 737–738. [Google Scholar] [CrossRef]
- Rubio-Gozalbo, M.E.; Gubbels, C.S.; Bakker, J.A.; Menheere, P.P.; Wodzig, W.K.; Land, J.A. Gonadal function in male and female patients with classic galactosemia. Hum. Reprod. Update 2010, 16, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, D.D.; Buist, N.R.; Donnell, G.N. Long-term prognosis in galactosaemia: Results of a survey of 350 cases. J. Inherit. Metab. Dis. 1990, 13, 802–818. [Google Scholar] [CrossRef] [PubMed]
- Mamsen, L.S.; Kelsey, T.W.; Ernst, E.; Macklon, K.T.; Lund, A.M.; Andersen, C.Y. Cryopreservation of ovarian tissue may be considered in young girls with galactosemia. J. Assist. Reprod Genet. 2018, 35, 1209–1217. [Google Scholar] [CrossRef]
- Sozen, B.; Ozekinci, M.; Erman, M.; Gunduz, T.; Demir, N.; Akouri, R. Dehydroepiandrosterone supplementation attenuates ovarian ageing in a galactose-induced primary ovarian insufficiency rat model. J. Assist. Reprod Genet. 2019, 36, 2181–2189. [Google Scholar] [CrossRef]
- Thakur, M.; Feldman, G.; Puscheck, E.E. Primary ovarian insufficiency in classic galactosemia: Current understanding and future research opportunities. J. Assist. Reprod Genet. 2018, 35, 3–16. [Google Scholar] [CrossRef]
- Gubbels, C.S.; Land, J.A.; Evers, J.L.; Bierau, J.; Menheere, P.P.; Robben, S.G.; Rubio-Gozalbo, M.E. Primary ovarian insufficiency in classic galactosemia: Role of FSH dysfunction and timing of the lesion. J. Inherit. Metab. Dis. 2013, 36, 29–34. [Google Scholar] [CrossRef]
- Sanders, R.D.; Spencer, J.B.; Epstein, M.P.; Pollak, S.V.; Vardhana, P.A.; Lustbader, J.W.; Fridovich-Keil, J.L. Biomarkers of ovarian function in girls and women with classic galactosemia. Fertil. Steril. 2009, 92, 344–351. [Google Scholar] [CrossRef]
- Spencer, J.B.; Badik, J.R.; Ryan, E.L.; Gleason, T.J.; Broadaway, K.A.; Epstein, M.P.; Fridovich-Keil, J.L. Modifiers of ovarian function in girls and women with classic galactosemia. J. Clin. Endocrinol. Metab. 2013, 98, E1257–E1265. [Google Scholar] [CrossRef]
- Van Erven, B.; Berry, G.T.; Cassiman, D.; Connolly, G.; Forga, M.; Gautschi, M.; Gubbels, C.S.; Hollak, C.E.M.; Janssen, M.C.; Knerr, I.; et al. Fertility in adult women with classic galactosemia and primary ovarian insufficiency. Fertil. Steril. 2017, 108, 168–174. [Google Scholar] [CrossRef]
- Van Erven, B.; Gubbels, C.S.; van Golde, R.J.; Dunselman, G.A.; Derhaag, J.G.; de Wert, G.; Geraedts, J.P.; Bosch, A.M.; Treacy, E.P.; Welt, C.K.; et al. Fertility preservation in female classic galactosemia patients. Orphanet J. Rare Dis. 2013, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Gamzatova, Z.; Komlichenko, E.; Kostareva, A.; Galagudza, M.; Ulrikh, E.; Zubareva, T.; Sheveleva, T.; Nezhentseva, E.; Kalinina, E. Autotransplantation of cryopreserved ovarian tissue—Effective method of fertility preservation in cancer patients. Gynecol. Endocrinol. 2014, 30 (Suppl. S1), 43–47. [Google Scholar] [CrossRef] [PubMed]
- Rivas Leonel, E.C.; Lucci, C.M.; Amorim, C.A. Cryopreservation of Human Ovarian Tissue: A Review. Transfus. Med. Hemother. 2019, 46, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.W.; Dittrich, R.; Lotz, L.; van der Ven, K.; van der Ven, H.H.; Liebenthron, J.; Korell, M.; Frambach, T.; Sutterlin, M.; Schwab, R.; et al. Fertility protection: Complications of surgery and results of removal and transplantation of ovarian tissue. Reprod. Biomed. Online 2018, 36, 188–196. [Google Scholar] [CrossRef]
- Haskovic, M.; Poot, W.J.; van Golde, R.J.T.; Benneheij, S.H.; Oussoren, E.; de Wert, G.; Krumeich, A.; Rubio-Gozalbo, M.E. Intrafamilial oocyte donation in classic galactosemia: Ethical and societal aspects. J. Inherit. Metab. Dis. 2018, 41, 791–797. [Google Scholar] [CrossRef]
- Timmers, I.; Jansma, B.M.; Rubio-Gozalbo, M.E. From mind to mouth: Event related potentials of sentence production in classic galactosemia. PLoS ONE 2012, 7, e52826. [Google Scholar] [CrossRef]
- Timmers, I.; van den Hurk, J.; Hofman, P.A.; Zimmermann, L.J.; Uludag, K.; Jansma, B.M.; Rubio-Gozalbo, M.E. Affected functional networks associated with sentence production in classic galactosemia. Brain Res. 2015, 1616, 166–176. [Google Scholar] [CrossRef]
- Timmers, I.; van der Korput, L.D.; Jansma, B.M.; Rubio-Gozalbo, M.E. Grey matter density decreases as well as increases in patients with classic galactosemia: A voxel-based morphometry study. Brain Res. 2016, 1648, 339–344. [Google Scholar] [CrossRef]
- Van Erven, B.; Jansma, B.M.; Rubio-Gozalbo, M.E.; Timmers, I. Exploration of the Brain in Rest: Resting-State Functional MRI Abnormalities in Patients with Classic Galactosemia. Sci. Rep. 2017, 7, 9095. [Google Scholar] [CrossRef]
- Welsink-Karssies, M.M.; Oostrom, K.J.; Hermans, M.E.; Hollak, C.E.M.; Janssen, M.C.H.; Langendonk, J.G.; Oussoren, E.; Rubio Gozalbo, M.E.; de Vries, M.; Geurtsen, G.J.; et al. Classical galactosemia: Neuropsychological and psychosocial functioning beyond intellectual abilities. Orphanet J. Rare Dis. 2020, 15, 42. [Google Scholar] [CrossRef]
- Welsink-Karssies, M.M.; Schrantee, A.; Caan, M.W.A.; Hollak, C.E.M.; Janssen, M.C.H.; Oussoren, E.; de Vries, M.C.; Roosendaal, S.D.; Engelen, M.; Bosch, A.M. Gray and white matter are both affected in classical galactosemia: An explorative study on the association between neuroimaging and clinical outcome. Mol. Genet. Metab. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.E.; Welsink-Karssies, M.M.; Bosch, A.M.; Oostrom, K.J.; Geurtsen, G.J. Cognitive functioning in patients with classical galactosemia: A systematic review. Orphanet J. Rare Dis. 2019, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Del Felice, A.; Castiglia, L.; Formaggio, E.; Cattelan, M.; Scarpa, B.; Manganotti, P.; Tenconi, E.; Masiero, S. Personalized transcranial alternating current stimulation (tACS) and physical therapy to treat motor and cognitive symptoms in Parkinson’s disease: A randomized cross-over trial. Neuroimage Clin. 2019, 22, 101768. [Google Scholar] [CrossRef] [PubMed]
- Elyamany, O.; Leicht, G.; Herrmann, C.S.; Mulert, C. Transcranial alternating current stimulation (tACS): From basic mechanisms towards first applications in psychiatry. Eur. Arch. Psychiatry Clin. Neurosci. 2020. [Google Scholar] [CrossRef]
- Riddle, J.; Rubinow, D.R.; Frohlich, F. A case study of weekly tACS for the treatment of major depressive disorder. Brain Stimul. 2020, 13, 576–577. [Google Scholar] [CrossRef]
- Rufener, K.S.; Krauel, K.; Meyer, M.; Heinze, H.J.; Zaehle, T. Transcranial electrical stimulation improves phoneme processing in developmental dyslexia. Brain Stimul. 2019, 12, 930–937. [Google Scholar] [CrossRef]
- Tavakoli, A.V.; Yun, K. Transcranial Alternating Current Stimulation (tACS) Mechanisms and Protocols. Front. Cell Neurosci. 2017, 11, 214. [Google Scholar] [CrossRef]
- Peter, B.; Potter, N.; Davis, J.; Donenfeld-Peled, I.; Finestack, L.; Stoel-Gammon, C.; Lien, K.; Bruce, L.; Vose, C.; Eng, L.; et al. Toward a paradigm shift from deficit-based to proactive speech and language treatment: Randomized pilot trial of the Babble Boot Camp in infants with classic galactosemia. F1000Research 2019, 8, 271. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delnoy, B.; Coelho, A.I.; Rubio-Gozalbo, M.E. Current and Future Treatments for Classic Galactosemia. J. Pers. Med. 2021, 11, 75. https://doi.org/10.3390/jpm11020075
Delnoy B, Coelho AI, Rubio-Gozalbo ME. Current and Future Treatments for Classic Galactosemia. Journal of Personalized Medicine. 2021; 11(2):75. https://doi.org/10.3390/jpm11020075
Chicago/Turabian StyleDelnoy, Britt, Ana I. Coelho, and Maria Estela Rubio-Gozalbo. 2021. "Current and Future Treatments for Classic Galactosemia" Journal of Personalized Medicine 11, no. 2: 75. https://doi.org/10.3390/jpm11020075
APA StyleDelnoy, B., Coelho, A. I., & Rubio-Gozalbo, M. E. (2021). Current and Future Treatments for Classic Galactosemia. Journal of Personalized Medicine, 11(2), 75. https://doi.org/10.3390/jpm11020075




