rs6837671A>G in FAM13A Is a Trans-Ethnic Genetic Variant Interacting with Vitamin D Levels to Affect Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Demographic Clinical and Biological Data Collection
2.3. Genotyping Assays
2.4. Statistical Analysis
2.5. Meta-analysis with Chronic Obstructive Pulmonary Disease
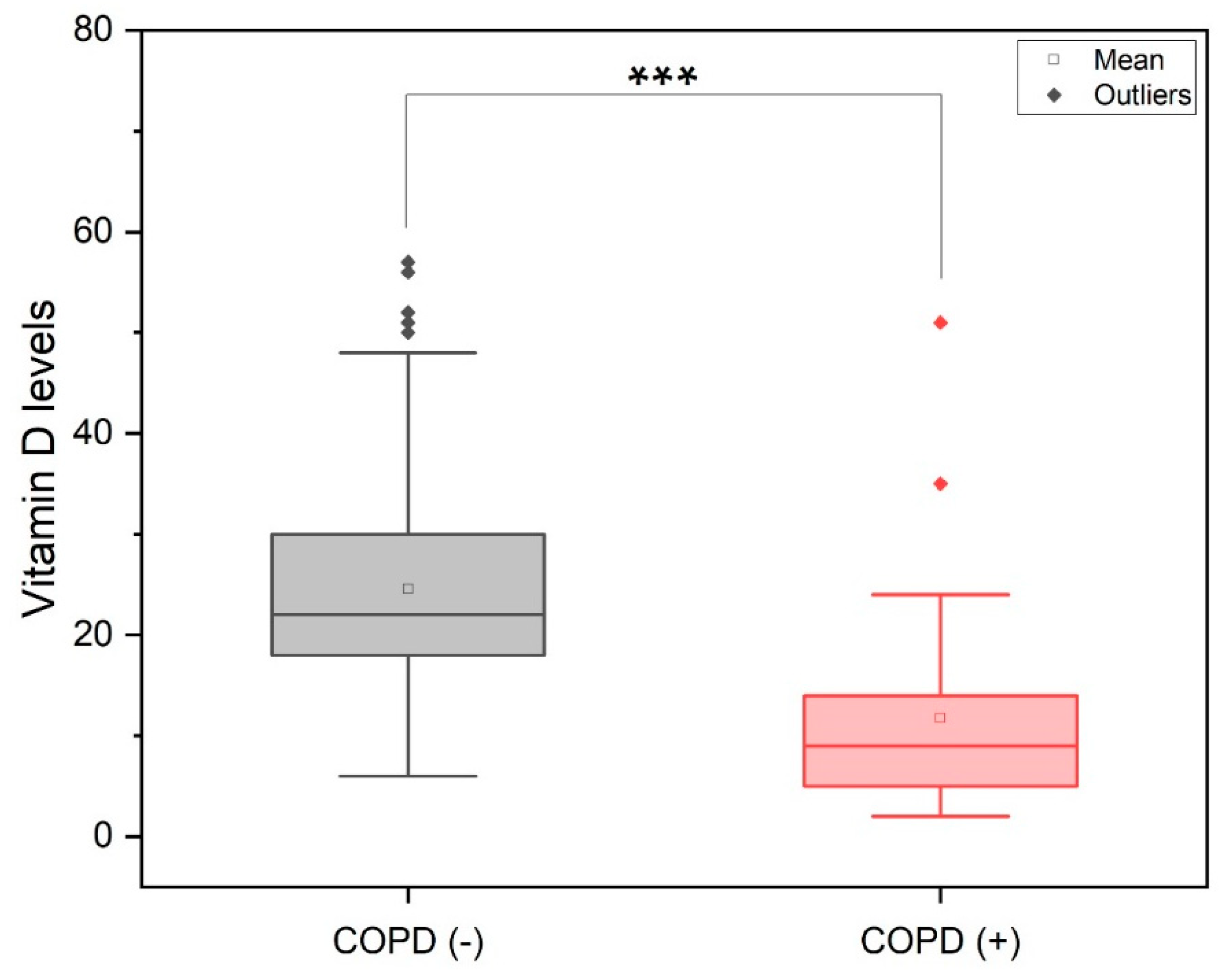
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| COPD | Chronic obstructive pulmonary disease |
| FAM13A | Family with sequence similarity 13, member A |
| FEV1 | Forced expiratory volume in one second |
| FVC | Forced vital capacity |
| GWAS | Genome-wide association studies |
| VitD | Vitamin D |
References
- Vestbo, J.; Hurd, S.S.; Agusti, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Report about Chronic Obstructive Pulmonary Disease (COPD). 1 December 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 18 January 2021).
- Waked, M.; Khayat, G.; Salameh, P. Chronic obstructive pulmonary disease prevalence in Lebanon: A cross-sectional descriptive study. Clin. Epidemiol. 2011, 3, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kalsheker, N.; Chappell, S. The new genetics and chronic obstructive pulmonary disease. COPD 2008, 5, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Laniado-Laborin, R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int. J. Environ. Res. Public Health 2009, 6, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, B.D.; de Jong, K.; Lamontagne, M.; Bosse, Y.; Shrine, N.; Artigas, M.S.; Wain, L.V.; Hall, I.P.; Jackson, V.E.; Wyss, A.B.; et al. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat. Genet. 2017, 49, 426–432. [Google Scholar] [CrossRef]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016, 4, 16041. [Google Scholar] [CrossRef]
- Herr, C.; Greulich, T.; Koczulla, R.A.; Meyer, S.; Zakharkina, T.; Branscheidt, M.; Eschmann, R.; Bals, R. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir. Res. 2011, 12, 31. [Google Scholar] [CrossRef]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 80–90. [Google Scholar] [CrossRef]
- Janssens, W.; Lehouck, A.; Carremans, C.; Bouillon, R.; Mathieu, C.; Decramer, M. Vitamin D beyond bones in chronic obstructive pulmonary disease: Time to act. Am. J. Respir. Crit. Care Med. 2009, 179, 630–636. [Google Scholar] [CrossRef]
- Sakornsakolpat, P.; Prokopenko, D.; Lamontagne, M.; Reeve, N.F.; Guyatt, A.L.; Jackson, V.E.; Shrine, N.; Qiao, D.; Bartz, T.M.; Kim, D.K.; et al. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat. Genet. 2019, 51, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, M.H.; Hersh, C.P.; McDonald, M.L.; Crapo, J.D.; Bakke, P.S.; Gulsvik, A.; Comellas, A.P.; Wendt, C.H.; Lomas, D.A.; et al. Genetic susceptibility for chronic bronchitis in chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ Clin. Res. Ed. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.J. Make no bones about it: Increasing epidemiologic evidence links vitamin D to pulmonary function and COPD. Chest 2005, 128, 3781–3783. [Google Scholar] [CrossRef][Green Version]
- Forli, L.; Halse, J.; Haug, E.; Bjortuft, O.; Vatn, M.; Kofstad, J.; Boe, J. Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J. Intern. Med. 2004, 256, 56–62. [Google Scholar] [CrossRef]
- Jiang, Z.; Lao, T.; Qiu, W.; Polverino, F.; Gupta, K.; Guo, F.; Mancini, J.D.; Naing, Z.Z.; Cho, M.H.; Castaldi, P.J.; et al. A Chronic Obstructive Pulmonary Disease Susceptibility Gene, FAM13A, Regulates Protein Stability of beta-Catenin. Am. J. Respir. Crit. Care Med. 2016, 194, 185–197. [Google Scholar] [CrossRef]
- Chi, J.T.; Wang, Z.; Nuyten, D.S.; Rodriguez, E.H.; Schaner, M.E.; Salim, A.; Wang, Y.; Kristensen, G.B.; Helland, A.; Borresen-Dale, A.L.; et al. Gene expression programs in response to hypoxia: Cell type specificity and prognostic significance in human cancers. PLoS Med. 2006, 3, e47. [Google Scholar] [CrossRef]
- Eisenhut, F.; Heim, L.; Trump, S.; Mittler, S.; Sopel, N.; Andreev, K.; Ferrazzi, F.; Ekici, A.B.; Rieker, R.; Springel, R.; et al. FAM13A is associated with non-small cell lung cancer (NSCLC) progression and controls tumor cell proliferation and survival. Oncoimmunology 2017, 6, e1256526. [Google Scholar] [CrossRef]
- Fingerlin, T.E.; Murphy, E.; Zhang, W.; Peljto, A.L.; Brown, K.K.; Steele, M.P.; Loyd, J.E.; Cosgrove, G.P.; Lynch, D.; Groshong, S.; et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat. Genet. 2013, 45, 613–620. [Google Scholar] [CrossRef]
- Dancer, R.C.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef]
- Dinh-Xuan, A.T.; Higenbottam, T.W.; Clelland, C.A.; Pepke-Zaba, J.; Cremona, G.; Butt, A.Y.; Large, S.R.; Wells, F.C.; Wallwork, J. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N. Engl. J. Med. 1991, 324, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 199–208. [Google Scholar]

| Participants (n = 631) | |
|---|---|
| COPD status n(%) | 172 (27.5) |
| Age (years) | 46.75 ± 17.07 |
| Gender (n = 631) | |
| Female n(%) | 375 (59.4) |
| Male n(%) | 256 (40.6) |
| BMI (kg/m2) (n = 625) | |
| Normal weight n(%) | 311 (49.8) |
| Obesity n(%) | 314 (50.2) |
| Vitamin D (n = 489) | |
| Normal n(%) | 124 (19.7) |
| Low n(%) | 365 (57.8) |
| Smoking (n = 630) | |
| Non-smoker | 396 (62.8) |
| Smoker | 234 (37.1) |
| MAF | |
| rs17486278A>C in CHRNA5 | 0.41 |
| rs7733088G>A in HTR4 | 0.42 |
| rs9399401T>C in ADGRG6 | 0.26 |
| rs1441358T>G in THSD4 | 0.41 |
| rs6837671A>G in FAM13A | 0.25 |
| rs11727735A>G INTS12-GSTCD | 0.08 |
| rs2047409C>T in TET2 | 0.45 |
| rs2955083A>T in EEFSEC | 0.12 |
| COPD | |||
|---|---|---|---|
| OR | CI (95%) | p | |
| Age | 1.12 | 1.10–1.15 | < 0.001 |
| Gender | |||
| Female | 1 | - | - |
| Male | 1.02 | 0.61–1.70 | 0.946 |
| BMI | |||
| Normal weight | 1 | - | - |
| Obese | 2.66 | 1.56–4.55 | < 0.001 |
| Vitamin D levels | |||
| Normal | 1 | - | - |
| Low | 3.10 | 1.82–5.27 | < 0.001 |
| Smoking | |||
| Non-smoker | 1 | - | - |
| Smoker | 2.64 | 1.23–5.69 | 0.013 |
| rs17486278A>C in CHRNA5 | |||
| AA | 1 | - | - |
| CA | 0.84 | 0.48–1.47 | 0.553 |
| CC | 0.63 | 0.29–1.37 | 0.241 |
| rs7733088G>A in HTR4 | |||
| GG | 1 | - | - |
| GA | 1.16 | 0.67–2.01 | 0.604 |
| AA | 1.22 | 0.59–2.50 | 0.590 |
| rs9399401T>C in ADGRG6 | |||
| TT | 1 | - | - |
| TC | 1.69 | 1.01–2.84 | 0.048 |
| CC | 2.27 | 0.91–5.65 | 0.079 |
| rs1441358T>G in THSD4 | |||
| TT | 1 | - | - |
| TG | 1.25 | 0.73–2.17 | 0.417 |
| GG | 1.04 | 0.49–2.23 | 0.911 |
| rs6837671A>G in FAM13A | |||
| AA | 1 | - | - |
| GA | 1.53 | 0.90–2.59 | 0.117 |
| GG | 3.99 | 1.39–11.47 | 0.010 |
| rs11727735A>G INTS12-GSTCD | |||
| AA | 1 | - | - |
| GA | 0.49 | 0.23–1.06 | 0.071 |
| GG | 0.59 | 0.04–8.35 | 0.697 |
| rs2047409C>T in TET2 | |||
| CC | 1 | - | - |
| TC | 0.76 | 0.44–1.32 | 0.326 |
| TT | 0.51 | 0.24–1.08 | 0.079 |
| rs2955083A>T in EEFSEC | |||
| AA | 1 | - | - |
| TA | 0.47 | 0.25–0.91 | 0.025 |
| TT | 6.25 | 0.93–41.81 | 0.059 |
| COPD | |||
|---|---|---|---|
| OR | CI (95%) | p | |
| Age | 1.15 | 1.09–1.21 | < 0.001 |
| Gender | |||
| Female | 1 | ||
| Male | 0.63 | 0.21–1.91 | 0.413 |
| BMI | |||
| Normal weight | 1 | - | - |
| Obese | 2.04 | 0.69–6.02 | 0.197 |
| Smoking | |||
| Non-smoker | 1 | - | - |
| Smoker | 2.77 | 2.06–3.71 | < 0.001 |
| rs6837671A>G, FAM13A x Vitamin D levels | 3.35 | 1.73–6.49 | < 0.001 |
| Low Vitamin D | Normal Vitamin D | ||||||
|---|---|---|---|---|---|---|---|
| COPD (-) | COPD (+) | p | COPD (-) | COPD (+) | p | ||
| Frequency A Allele | 0.56 | 0.43 | 0.039 | 0.68 | 0.31 | 0.264 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Shamieh, S.; Salami, A.; Fawaz, M.; Jounblat, R.; Waked, M.; Fakhoury, R. rs6837671A>G in FAM13A Is a Trans-Ethnic Genetic Variant Interacting with Vitamin D Levels to Affect Chronic Obstructive Pulmonary Disease. J. Pers. Med. 2021, 11, 84. https://doi.org/10.3390/jpm11020084
El Shamieh S, Salami A, Fawaz M, Jounblat R, Waked M, Fakhoury R. rs6837671A>G in FAM13A Is a Trans-Ethnic Genetic Variant Interacting with Vitamin D Levels to Affect Chronic Obstructive Pulmonary Disease. Journal of Personalized Medicine. 2021; 11(2):84. https://doi.org/10.3390/jpm11020084
Chicago/Turabian StyleEl Shamieh, Said, Ali Salami, Mirna Fawaz, Rania Jounblat, Mirna Waked, and Rajaa Fakhoury. 2021. "rs6837671A>G in FAM13A Is a Trans-Ethnic Genetic Variant Interacting with Vitamin D Levels to Affect Chronic Obstructive Pulmonary Disease" Journal of Personalized Medicine 11, no. 2: 84. https://doi.org/10.3390/jpm11020084
APA StyleEl Shamieh, S., Salami, A., Fawaz, M., Jounblat, R., Waked, M., & Fakhoury, R. (2021). rs6837671A>G in FAM13A Is a Trans-Ethnic Genetic Variant Interacting with Vitamin D Levels to Affect Chronic Obstructive Pulmonary Disease. Journal of Personalized Medicine, 11(2), 84. https://doi.org/10.3390/jpm11020084







